Predicting the Risk for Pathological Fracture in Bone Metastases
Abstract
:1. Introduction
2. Methods
3. The Prediction of Pathological Fractures Using Imaging Findings Alone
4. The Prediction of Pathological Fractures Using Imaging Findings and Clinical Symptoms
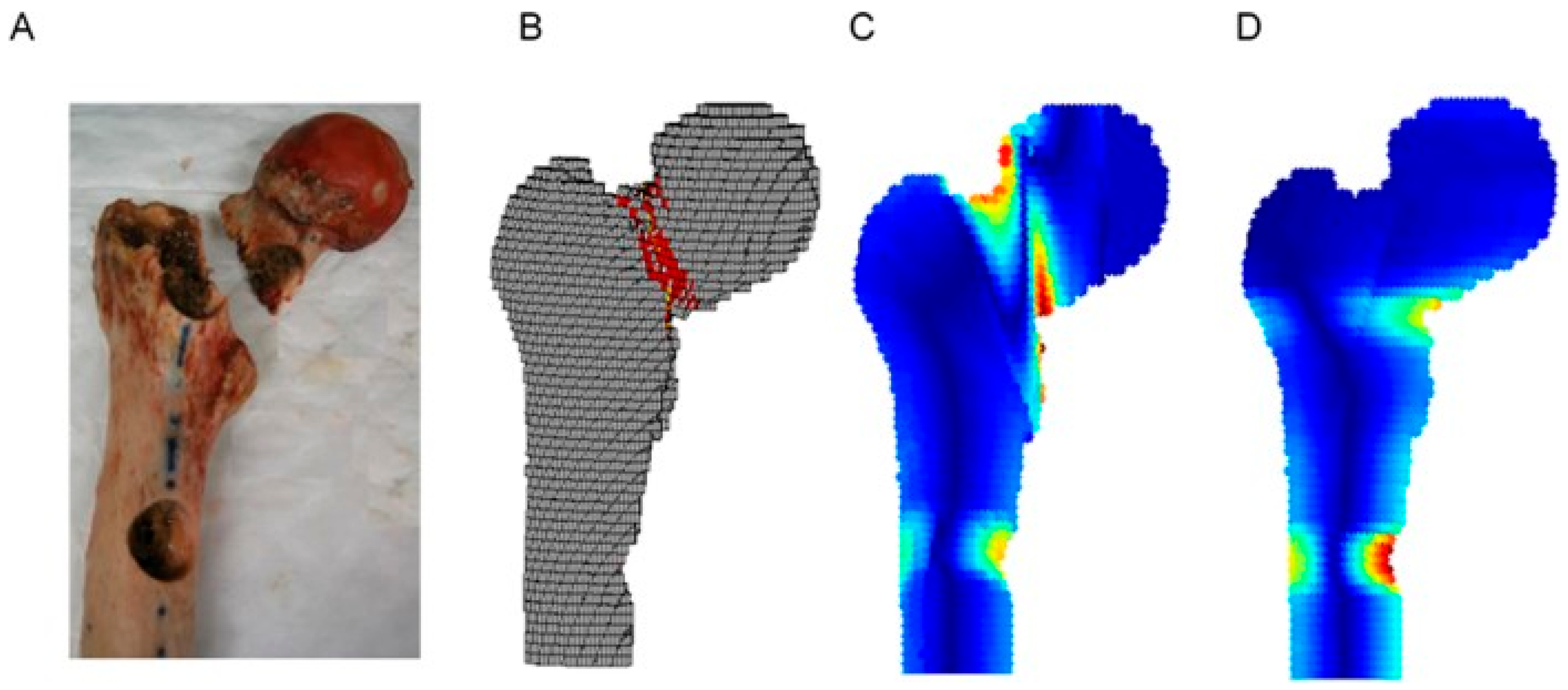
5. Finite Element Analysis
6. CT-Based Structural Rigidity Analyses
7. Conclusions
8. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Tsukamoto, S.; Kido, A.; Tanaka, Y.; Facchini, G.; Peta, G.; Rossi, G.; Mavrogenis, A.F. Current Overview of Treatment for Metastatic Bone Disease. Curr. Oncol. 2021, 28, 3347–3372. [Google Scholar] [CrossRef] [PubMed]
- Errani, C.; Mavrogenis, A.F.; Cevolani, L.; Spinelli, S.; Piccioli, A.; Maccauro, G.; Baldini, N.; Donati, D. Treatment for Long Bone Metastases Based on a Systematic Literature Review. Eur. J. Orthop. Surg. Traumatol. 2017, 27, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Costa, A.; Senkus, E.; Aapro, M.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.; Biganzoli, L.; Cardoso, M.J.; et al. 3rd ESO–ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Breast 2017, 31, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Fidler, M. Incidence of Fracture through Metastases in Long Bones. Acta Orthop. Scand. 1981, 52, 623–627. [Google Scholar] [CrossRef]
- Van der Linden, Y.M.; Dijkstra, P.D.S.; Kroon, H.M.; Lok, J.J.; Noordijk, E.M.; Leer, J.W.H.; Marijnen, C.A.M. Comparative Analysis of Risk Factors for Pathological Fracture with Femoral Metastases. J. Bone Jt. Surg. Br. 2004, 86, 566–573. [Google Scholar] [CrossRef]
- van der Wal, C.W.P.G.; Eggermont, F.; Fiocco, M.; Kroon, H.M.; Ayu, O.; Slot, A.; Snyers, A.; Rozema, T.; Verdonschot, N.J.J.; Dijkstra, P.D.S.; et al. Axial Cortical Involvement of Metastatic Lesions to Identify Impending Femoral Fractures; a Clinical Validation Study. Radiother. Oncol. 2020, 144, 59–64. [Google Scholar] [CrossRef]
- van der Linden, Y.M.; Kroon, H.M.; Dijkstra, S.P.D.S.; Lok, J.J.; Noordijk, E.M.; Leer, J.W.H.; Marijnen, C.A.M. Dutch Bone Metastasis Study Group Simple Radiographic Parameter Predicts Fracturing in Metastatic Femoral Bone Lesions: Results from a Randomised Trial. Radiother. Oncol. 2003, 69, 21–31. [Google Scholar] [CrossRef]
- Eggermont, F.; van der Wal, G.; Westhoff, P.; Laar, A.; de Jong, M.; Rozema, T.; Kroon, H.M.; Ayu, O.; Derikx, L.; Dijkstra, S.; et al. Patient-Specific Finite Element Computer Models Improve Fracture Risk Assessments in Cancer Patients with Femoral Bone Metastases Compared to Clinical Guidelines. Bone 2020, 130, 115101. [Google Scholar] [CrossRef]
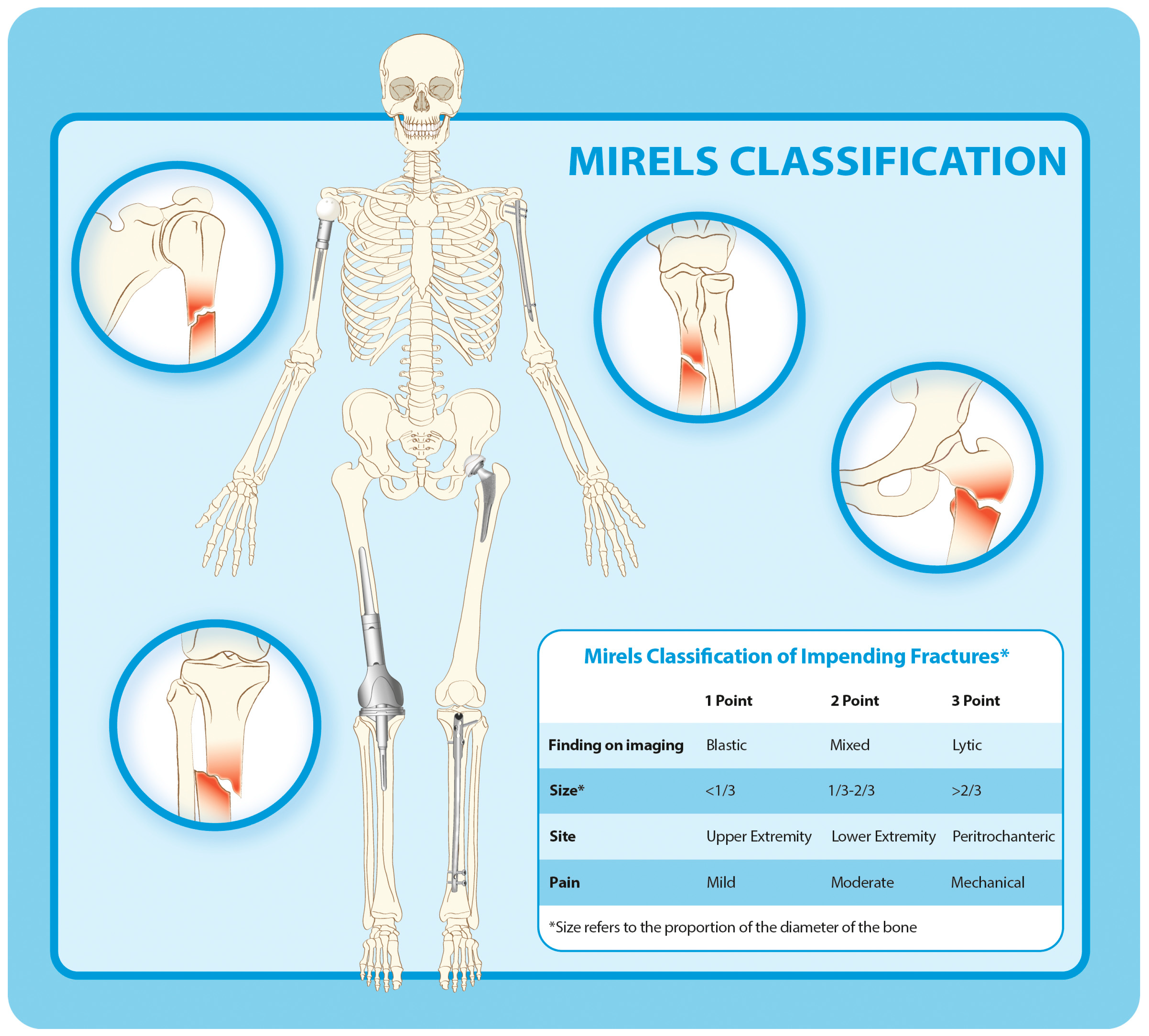
- Mirels, H. Metastatic Disease in Long Bones. A Proposed Scoring System for Diagnosing Impending Pathologic Fractures. Clin. Orthop. Relat. Res. 1989, 256–264. [Google Scholar] [CrossRef]
- Hoban, K.A.; Downie, S.; Adamson, D.J.A.; MacLean, J.G.; Cool, P.; Jariwala, A.C. Mirels’ Score for Upper Limb Metastatic Lesions: Do We Need a Different Cutoff for Recommending Prophylactic Fixation? JSES Int. 2022, 6, 675–681. [Google Scholar] [CrossRef]
- Goodheart, J.R.; Cleary, R.J.; Damron, T.A.; Mann, K.A. Simulating Activities of Daily Living with Finite Element Analysis Improves Fracture Prediction for Patients with Metastatic Femoral Lesions. J. Orthop. Res. 2015, 33, 1226–1234. [Google Scholar] [CrossRef]
- Damron, T.A.; Nazarian, A.; Entezari, V.; Brown, C.; Grant, W.; Calderon, N.; Zurakowski, D.; Terek, R.M.; Anderson, M.E.; Cheng, E.Y.; et al. CT-Based Structural Rigidity Analysis Is More Accurate Than Mirels Scoring for Fracture Prediction in Metastatic Femoral Lesions. Clin. Orthop. Relat. Res. 2016, 474, 643–651. [Google Scholar] [CrossRef]
- Sternheim, A.; Traub, F.; Trabelsi, N.; Dadia, S.; Gortzak, Y.; Snir, N.; Gorfine, M.; Yosibash, Z. When and Where Do Patients with Bone Metastases Actually Break Their Femurs? Bone Jt. J. 2020, 102, 638–645. [Google Scholar] [CrossRef]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 1067–1096. [Google Scholar] [CrossRef]
- Eggermont, F.; Derikx, L.C.; Verdonschot, N.; van der Geest, I.C.M.; de Jong, M.A.A.; Snyers, A.; van der Linden, Y.M.; Tanck, E. Can Patient-Specific Finite Element Models Better Predict Fractures in Metastatic Bone Disease than Experienced Clinicians?: Towards Computational Modelling in Daily Clinical Practice. Bone Jt. Res. 2018, 7, 430–439. [Google Scholar] [CrossRef]
- Sas, A.; Tanck, E.; Wafa, H.; van der Linden, Y.; Sermon, A.; van Lenthe, G.H. Fracture Risk Assessment and Evaluation of Femoroplasty in Metastatic Proximal Femurs. An in Vivo CT-Based Finite Element Study. J. Orthop. Res. 2023, 41, 225–234. [Google Scholar] [CrossRef]
- O’Rourke, D.; Johnson, L.J.; Jagiello, J.; Taylor, M. Examining Agreement between Finite Element Modelling Methodologies in Predicting Pathological Fracture Risk in Proximal Femurs with Bone Metastases. Clin. Biomech. 2023, 104, 105931. [Google Scholar] [CrossRef]
- Knowles, N.K.; Reeves, J.M.; Ferreira, L.M. Quantitative Computed Tomography (QCT) Derived Bone Mineral Density (BMD) in Finite Element Studies: A Review of the Literature. J. Exp. Orthop. 2016, 3, 36. [Google Scholar] [CrossRef]
- Fleps, I.; Bahaloo, H.; Zysset, P.K.; Ferguson, S.J.; Pálsson, H.; Helgason, B. Empirical Relationships between Bone Density and Ultimate Strength: A Literature Review. J. Mech. Behav. Biomed. Mater. 2020, 110, 103866. [Google Scholar] [CrossRef]
- Oftadeh, R.; Karimi, Z.; Villa-Camacho, J.; Tanck, E.; Verdonschot, N.; Goebel, R.; Snyder, B.D.; Hashemi, H.N.; Vaziri, A.; Nazarian, A. Curved Beam Computed Tomography Based Structural Rigidity Analysis of Bones with Simulated Lytic Defect: A Comparative Study with Finite Element Analysis. Sci. Rep. 2016, 6, 32397. [Google Scholar] [CrossRef]
- Keyak, J.H.; Skinner, H.B. Three-Dimensional Finite Element Modelling of Bone: Effects of Element Size. J. Biomed. Eng. 1992, 14, 483–489. [Google Scholar] [CrossRef]
- Burkhart, T.A.; Andrews, D.M.; Dunning, C.E. Finite Element Modeling Mesh Quality, Energy Balance and Validation Methods: A Review with Recommendations Associated with the Modeling of Bone Tissue. J. Biomech. 2013, 46, 1477–1488. [Google Scholar] [CrossRef]
- Knowles, N.K.; Ip, K.; Ferreira, L.M. The Effect of Material Heterogeneity, Element Type, and Down-Sampling on Trabecular Stiffness in Micro Finite Element Models. Ann. Biomed. Eng. 2019, 47, 615–623. [Google Scholar] [CrossRef]
- Imai, K.; Ohnishi, I.; Bessho, M.; Nakamura, K. Nonlinear Finite Element Model Predicts Vertebral Bone Strength and Fracture Site. Spine 2006, 31, 1789–1794. [Google Scholar] [CrossRef]
- Zysset, P.K.; Dall’ara, E.; Varga, P.; Pahr, D.H. Finite Element Analysis for Prediction of Bone Strength. BoneKEy Rep. 2013, 2, 386. [Google Scholar] [CrossRef]
- Voutouri, C.; Stylianopoulos, T. Accumulation of Mechanical Forces in Tumors Is Related to Hyaluronan Content and Tissue Stiffness. PLoS ONE 2018, 13, e0193801. [Google Scholar] [CrossRef]
- Stadelmann, M.A.; Schenk, D.E.; Maquer, G.; Lenherr, C.; Buck, F.M.; Bosshardt, D.D.; Hoppe, S.; Theumann, N.; Alkalay, R.N.; Zysset, P.K. Conventional Finite Element Models Estimate the Strength of Metastatic Human Vertebrae despite Alterations of the Bone’s Tissue and Structure. Bone 2020, 141, 115598. [Google Scholar] [CrossRef]
- Kaneko, T.S.; Pejcic, M.R.; Tehranzadeh, J.; Keyak, J.H. Relationships between Material Properties and CT Scan Data of Cortical Bone with and without Metastatic Lesions. Med. Eng. Phys. 2003, 25, 445–454. [Google Scholar] [CrossRef]
- Keyak, J.H.; Kaneko, T.S.; Skinner, H.B.; Hoang, B.H. The Effect of Simulated Metastatic Lytic Lesions on Proximal Femoral Strength. Clin. Orthop. Relat. Res. 2007, 459, 139–145. [Google Scholar] [CrossRef]
- Keyak, J.H.; Kaneko, T.S.; Rossi, S.A.; Pejcic, M.R.; Tehranzadeh, J.; Skinner, H.B. Predicting the Strength of Femoral Shafts with and without Metastatic Lesions. Clin. Orthop. Relat. Res. 2005, 439, 161–170. [Google Scholar] [CrossRef]
- Hipp, J.A.; Springfield, D.S.; Hayes, W.C. Predicting Pathologic Fracture Risk in the Management of Metastatic Bone Defects. Clin. Orthop. Relat. Res. 1995, 312, 120–135. [Google Scholar]
- Bergmann, G.; Graichen, F.; Rohlmann, A. Hip Joint Loading during Walking and Running, Measured in Two Patients. J. Biomech. 1993, 26, 969–990. [Google Scholar] [CrossRef]
- Michaeli, D.A.; Inoue, K.; Hayes, W.C.; Hipp, J.A. Density Predicts the Activity-Dependent Failure Load of Proximal Femora with Defects. Skeletal. Radiol. 1999, 28, 90–95. [Google Scholar] [CrossRef]
- Anez-Bustillos, L.; Derikx, L.C.; Verdonschot, N.; Calderon, N.; Zurakowski, D.; Snyder, B.D.; Nazarian, A.; Tanck, E. Finite Element Analysis and CT-Based Structural Rigidity Analysis to Assess Failure Load in Bones with Simulated Lytic Defects. Bone 2014, 58, 160–167. [Google Scholar] [CrossRef]
- Cory, E.; Nazarian, A.; Entezari, V.; Vartanians, V.; Müller, R.; Snyder, B.D. Compressive Axial Mechanical Properties of Rat Bone as Functions of Bone Volume Fraction, Apparent Density and Micro-Ct Based Mineral Density. J. Biomech. 2010, 43, 953–960. [Google Scholar] [CrossRef]
- Nazarian, A.; Entezari, V.; Zurakowski, D.; Calderon, N.; Hipp, J.A.; Villa-Camacho, J.C.; Lin, P.P.; Cheung, F.H.; Aboulafia, A.J.; Turcotte, R.; et al. Treatment Planning and Fracture Prediction in Patients with Skeletal Metastasis with CT-Based Rigidity Analysis. Clin. Cancer Res. 2015, 21, 2514–2519. [Google Scholar] [CrossRef]
- Nazarian, A.; Entezari, V.; Villa-Camacho, J.C.; Zurakowski, D.; Katz, J.N.; Hochman, M.; Baldini, E.H.; Vartanians, V.; Rosen, M.P.; Gebhardt, M.C.; et al. Does CT-Based Rigidity Analysis Influence Clinical Decision-Making in Simulations of Metastatic Bone Disease? Clin. Orthop. Relat. Res. 2016, 474, 652–659. [Google Scholar] [CrossRef]
- Puchalski, C.M.; Sbrana, A.; Ferrell, B.; Jafari, N.; King, S.; Balboni, T.; Miccinesi, G.; Vandenhoeck, A.; Silbermann, M.; Balducci, L.; et al. Interprofessional Spiritual Care in Oncology: A Literature Review. ESMO Open 2019, 4, e000465. [Google Scholar] [CrossRef]
- Pavan Kalyan, B.; Kumar, L. 3D Printing: Applications in Tissue Engineering, Medical Devices, and Drug Delivery. AAPS PharmSciTech 2022, 23, 92. [Google Scholar] [CrossRef]
- Galbusera, F.; Casaroli, G.; Bassani, T. Artificial Intelligence and Machine Learning in Spine Research. JOR Spine 2019, 2, e1044. [Google Scholar] [CrossRef]
- Clézardin, P.; Coleman, R.; Puppo, M.; Ottewell, P.; Bonnelye, E.; Paycha, F.; Confavreux, C.B.; Holen, I. Bone Metastasis: Mechanisms, Therapies, and Biomarkers. Physiol. Rev. 2021, 101, 797–855. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef]
- Yuan, J.; Li, B.; Zhang, C.; Wang, J.; Huang, B.; Ma, L. Machine Learning-Based CT Radiomics Model to Predict the Risk of Hip Fragility Fracture. Acad. Radiol. 2025, 32, 2854–2862. [Google Scholar] [CrossRef]
- Coleman, R.; Brown, J.; Terpos, E.; Lipton, A.; Smith, M.R.; Cook, R.; Major, P. Bone Markers and Their Prognostic Value in Metastatic Bone Disease: Clinical Evidence and Future Directions. Cancer Treat. Rev. 2008, 34, 629–639. [Google Scholar] [CrossRef]

| Prediction Method | Imaging Method | Specialized Software | Analysis Time | Positive Predictive Value (%) | Negative Predictive Value (%) | Types of Loading | Anatomic/Modeling Limitations |
|---|---|---|---|---|---|---|---|
| Mirels’ | Plane radiographs | No | <5 min | 10–32 | 90–100 | Not applicable | None |
| FEA | Computed tomography | Yes, to build the model and run an analysis | 2–8 h, requiring engineering expertise | 29–75 | 96–100 | Functional loading (stance, gait, stair climbing, etc.) | Models and loading for the proximal femur different from those for the distal femur |
| CTRA | Computed tomography | Yes, to calculate section rigidities | <15 min, with custom software | 18–54 | 100 | Axial, bending, torsion | Errors associated with the ends of long bones |
| Discussion Topic | Key Insights |
|---|---|
| Importance of fracture risk prediction | Pathological fractures significantly impact quality of life, necessitating reliable prediction methods |
| Superiority of CTRA | CTRA shows greater positive predictive value compared to that of Mirels’ system, making it a more accurate tool for fracture risk assessments |
| Biomechanical integration | Unlike Mirels’ system, CTRA incorporates the mechanical properties of metastatic bone, improving the predictive accuracy |
| Clinical impact | Implementing CTRA in routine oncology practice may enhance early intervention, reducing unnecessary surgeries |
| Challenges & future directions | Further validation through multicenter trials is needed, along with integration of molecular biomarkers for personalized care |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altsitzioglou, P.; Tsukamoto, S.; Errani, C.; Tanaka, Y.; Mavrogenis, A.F. Predicting the Risk for Pathological Fracture in Bone Metastases. Curr. Oncol. 2025, 32, 309. https://doi.org/10.3390/curroncol32060309
Altsitzioglou P, Tsukamoto S, Errani C, Tanaka Y, Mavrogenis AF. Predicting the Risk for Pathological Fracture in Bone Metastases. Current Oncology. 2025; 32(6):309. https://doi.org/10.3390/curroncol32060309
Chicago/Turabian StyleAltsitzioglou, Pavlos, Shinji Tsukamoto, Costantino Errani, Yasuhito Tanaka, and Andreas F. Mavrogenis. 2025. "Predicting the Risk for Pathological Fracture in Bone Metastases" Current Oncology 32, no. 6: 309. https://doi.org/10.3390/curroncol32060309
APA StyleAltsitzioglou, P., Tsukamoto, S., Errani, C., Tanaka, Y., & Mavrogenis, A. F. (2025). Predicting the Risk for Pathological Fracture in Bone Metastases. Current Oncology, 32(6), 309. https://doi.org/10.3390/curroncol32060309









