Evaluating Treatment Plan Modifications from Surgeons’ Initial Recommendations to Multidisciplinary Tumor Board Consensus for Cancer Care in a Resource-Limited Setting
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Sample Size and Inclusion and Exclusion Criteria
2.3. Data Analysis
3. Results
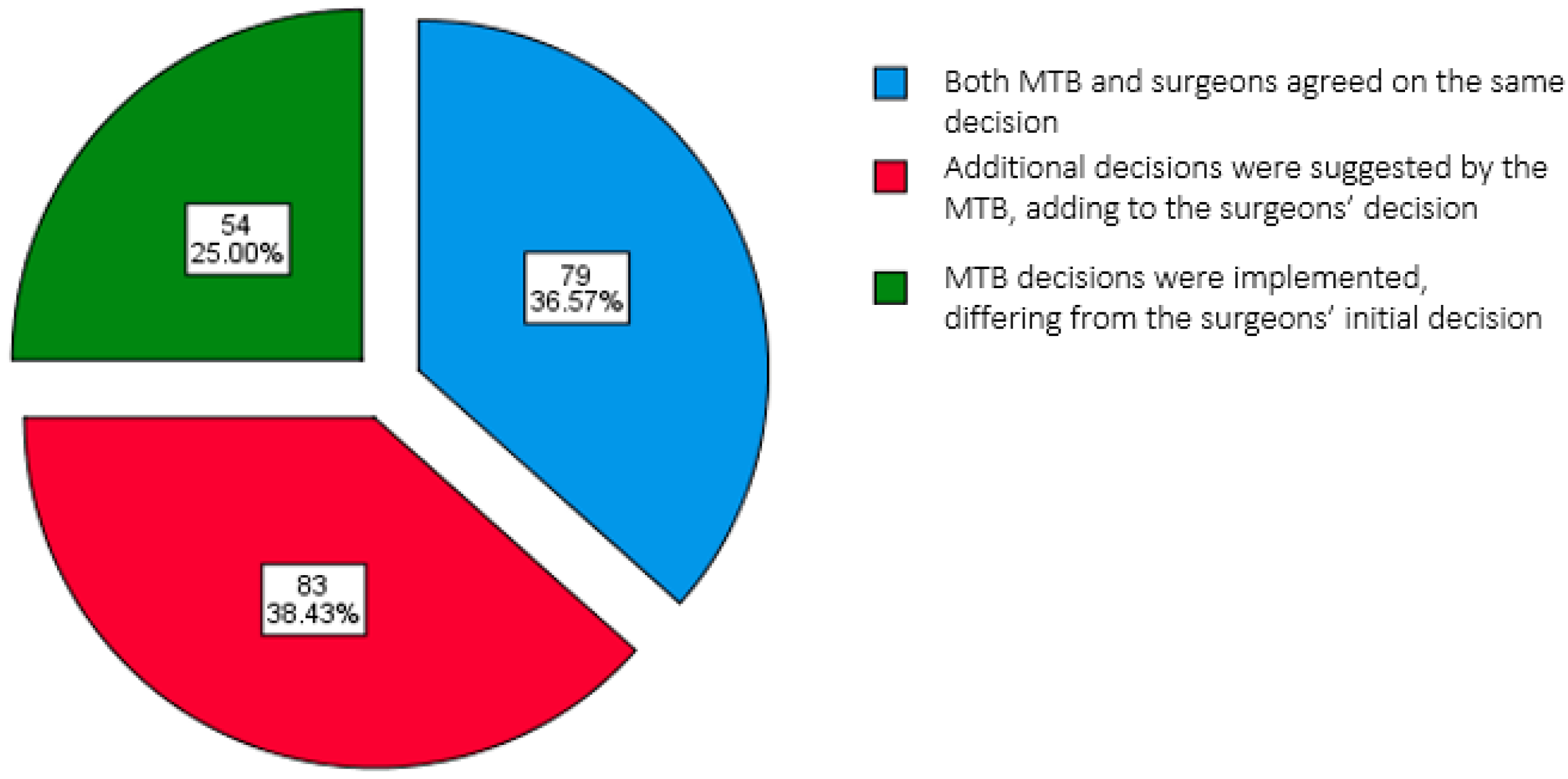
3.1. Patient Demographics, Tumor Characteristics, and Treatment Decision Outcomes
3.2. Agreement and Shifts in Curative vs. Palliative Decisions Between Surgeons and the MTB
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Kaifi, J.T.; Gusani, N.J.; Jiang, Y.; Mackley, H.B.; Dye, C.E.; Mathew, A.; Kimchi, E.T.; Reed, M.F.; Staveley-O’Carroll, K.F. Multidisciplinary management of early and locally advanced esophageal cancer. J. Clin. Gastroenterol. 2011, 45, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Jalil, R.; Akhter, W.; Lamb, B.W.; Taylor, C.; Harris, J.; Green, J.S.; Sevdalis, N. Validation of team performance assessment of multidisciplinary tumor boards. J. Urol. 2014, 192, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Association of Breast Surgery at Baso 2009. Surgical guidelines for the management of breast cancer. Eur. J. Surg. Oncol. (EJSO) 2009, 35 (Suppl. 1), S1–S22. [Google Scholar] [CrossRef]
- Stephens, M.R.; Lewis, W.G.; Brewster, A.; Lord, I.; Blackshaw, G.; Hodzovic, I.; Thomas, G.; Roberts, S.; Crosby, T.; Gent, C. Multidisciplinary team management is associated with improved outcomes after surgery for esophageal cancer. Dis. Esophagus 2006, 19, 164–171. [Google Scholar] [CrossRef]
- Davies, A.; Deans, D.; Penman, I.; Plevris, J.; Fletcher, J.; Wall, L.; Phillips, H.; Gilmour, H.; Patel, D.; De Beaux, A. The multidisciplinary team meeting improves staging accuracy and treatment selection for gastro-esophageal cancer. Dis. Esophagus 2006, 19, 496–503. [Google Scholar] [CrossRef]
- Junor, E.; Hole, D.; Gillis, C. Management of ovarian cancer: Referral to a multidisciplinary team matters. Br. J. Cancer 1994, 70, 363–370. [Google Scholar] [CrossRef]
- Shah, S.; Arora, S.; Atkin, G.; Glynne-Jones, R.; Mathur, P.; Darzi, A.; Sevdalis, N. Decision-making in colorectal cancer tumor board meetings: Results of a prospective observational assessment. Surg. Endosc. 2014, 28, 2783–2788. [Google Scholar] [CrossRef]
- Dew, K.; Stubbe, M.; Signal, L.; Stairmand, J.; Dennett, E.; Koea, J.; Simpson, A.; Sarfati, D.; Cunningham, C.; Batten, L. Cancer care decision making in multidisciplinary meetings. Qual. Health Res. 2015, 25, 397–407. [Google Scholar] [CrossRef]
- Abbasi, A.N. Tumor board saves lives–more evidence is emerging for the mandatory development of site specific multi-disciplinary teams. Natl. J. Health Sci. 2019, 4, 46–48. [Google Scholar] [CrossRef]
- Jiang, D.; Wu, Y.; Liu, L.; Shen, Y.; Li, T.; Lu, Y.; Wang, P.; Sun, C.; Wang, K.; Wang, K. Burden of Gastrointestinal Tumors in Asian Countries, 1990–2021: An Analysis for the Global Burden of Disease Study 2021. Clin. Epidemiol. 2024, 16, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, S.; Abbasi, W.A.; Jalil, H.A.; Mughal, S.; Quraishy, M.S. The First Comprehensive Evaluation of Immuno-Inflammatory Markers for Prognosis in Esophageal Cancer Patients: A South Asian Perspective. Clin. Pract. 2024, 14, 2071–2079. [Google Scholar] [CrossRef]
- Pervez, S.; Jabbar, A.A.; Haider, G.; Qureshi, M.A.; Ashraf, S.; Lateef, F.; Khurshid, M.; Bashir, I.; Zaidi, M.; Mushtaq, N. Karachi Cancer Registry (KCR): Consolidated Data of 5-years 2017–2021. J. Coll. Physicians Surg. Pak. 2023, 33, 560–565. [Google Scholar]
- van Hagen, P.; Spaander, M.C.; van der Gaast, A.; van Rij, C.M.; Tilanus, H.W.; van Lanschot, J.J.B.; Wijnhoven, B.P. Impact of a multidisciplinary tumour board meeting for upper-GI malignancies on clinical decision making: A prospective cohort study. Int. J. Clin. Oncol. 2013, 18, 214–219. [Google Scholar] [CrossRef]
- Orlowski, C.; Lai, J.; Vereker, M.; Antill, Y.; Richardson, G.; White, M.; Gregory, P.; Kemp, S.; Morgan, J.; Ooi, C. Impact of multidisciplinary team meetings on the management of patients with breast cancer in a large private healthcare facility. Asia-Pac. J. Clin. Oncol. 2024, 20, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Francisse, S.; Gkolfakis, P.; Viesca, M.F.Y.; Mans, L.; Demols, A.; Pezzullo, M.; Loi, P.; Navez, J.; Closset, J.; Bali, M.A. The impact of a multidisciplinary team approach on the management of focal pancreatic lesions: A single tertiary center experience. Ann. Gastroenterol. 2023, 36, 580. [Google Scholar] [CrossRef] [PubMed]
- Anghelone, A.; Bensi, M.; Barbaro, B.; Calegari, M.A.; Cina, C.; Menghi, R.; Lorenzon, L.; Pozzo, C.; Basso, M.; Schietroma, F. The impact of the multidisciplinary team (MDT) in the management of colorectal cancer (CRC). In Proceedings of the 2022 ASCO Annual Meeting, Chicago, IL, USA, 3–7 June 2022. [Google Scholar]
- Hendrickx, J.-J.; Mennega, T.; Uppelschoten, J.M.; Leemans, C.R. Changes in multidisciplinary team decisions in a high volume head and neck oncological center following those made in its preferred partner. Front. Oncol. 2023, 13, 1205224. [Google Scholar] [CrossRef]
- Basendowah, M.; Awlia, A.M.; Alamoudi, H.A.; Ali Kanawi, H.M.; Saleem, A.; Malibary, N.; Hijazi, H.; Alfawaz, M.; Alzahrani, A.H. Impact of optional multidisciplinary tumor board meeting on the mortality of patients with gastrointestinal cancer: A retrospective observational study. Cancer Rep. 2021, 4, e1373. [Google Scholar] [CrossRef]
- Freytag, M.; Herrlinger, U.; Hauser, S.; Bauernfeind, F.G.; Gonzalez-Carmona, M.A.; Landsberg, J.; Buermann, J.; Vatter, H.; Holderried, T.; Send, T. Higher number of multidisciplinary tumor board meetings per case leads to improved clinical outcome. BMC Cancer 2020, 20, 355. [Google Scholar] [CrossRef]
- Pillay, B.; Wootten, A.C.; Crowe, H.; Corcoran, N.; Tran, B.; Bowden, P.; Crowe, J.; Costello, A.J. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat. Rev. 2016, 42, 56–72. [Google Scholar] [CrossRef]
- van den Brink, L.; Ruiter, A.E.; Lagerveld, B.W.; Graafland, N.M.; Bex, A.; Beerlage, H.P.; van Moorselaar, J.R.; Zondervan, P.J. The Impact of a Multidisciplinary Tumor Board (MTB) on Treatment Decision Making for Patients With Renal Cell Carcinoma (RCC): 5-Year Data Analysis. Clin. Genitourin. Cancer 2024, 22, 610–617.e1. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.Y.; Blair, S.; Nah, S.A.; Ariffin, H.; Assanasen, C.; Soh, S.Y.; Jacobsen, A.S.; Lam, C.; Loh, A.H. Pediatric solid tumor care and multidisciplinary tumor boards in low-and middle-income countries in Southeast Asia. JCO Glob. Oncol. 2020, 6, 1328–1345. [Google Scholar] [CrossRef] [PubMed]
- Niyibizi, B.A.; Muhizi, E.; Rangira, D.; Ndoli, D.A.; Nzeyimana, I.N.; Muvunyi, J.; Irakoze, M.; Kazindu, M.; Rugamba, A.; Uwimana, K.; et al. Multidisciplinary approach to cancer care in Rwanda: The role of tumour board meetings. Ecancermedicalscience 2023, 17, 1515. [Google Scholar] [CrossRef] [PubMed]
- George, P.E.; Fahdil, G.; Luutu, I.; Bulamu, A.; Sekabira, J.; Kakembo, N.; Nabadda, S.; Kalungi, S.; Kambugu, J.B. Analysis of management decisions and outcomes of a weekly multidisciplinary pediatric tumor board meeting in Uganda. Future Sci. OA 2019, 5, FSO417. [Google Scholar] [CrossRef]
- Lamb, B.W.; Green, J.S.; Benn, J.; Brown, K.F.; Vincent, C.A.; Sevdalis, N. Improving decision making in multidisciplinary tumor boards: Prospective longitudinal evaluation of a multicomponent intervention for 1421 patients. J. Am. Coll. Surg. 2013, 217, 412–420. [Google Scholar] [CrossRef]


| Final Decision | ||||||
|---|---|---|---|---|---|---|
| Both the MTB and Surgeons Agreed on the Same Decision | Additional Decisions Were Suggested by the MTB, Adding to the Surgeons’ Decision | MTB Decisions Differing from the Initial Surgeons’ Decision Were Implemented | Total | p-Value | ||
| n = 79 (%) | n = 83 (%) | n = 54 (%) | n = 216(%) | |||
| Age, Mean ± SD (years) | 48.14 ± 14.15 | 48.17 ± 13.53 | 46.44 ± 12.62 | 47.73 ± 13.51 | 0.725 | |
| Gender | ||||||
| Male | 36 (45.6) | 45 (54.2) | 28 (51.9) | 109 (50.5) | 0.531 | |
| Female | 53 (54.4) | 36 (45.8) | 26 (48.1) | 107 (49.5) | ||
| Region of Cancer | ||||||
| Esophagus | 48 (60.8) | 41 (49.4) | 26 (48.1) | 115 (53.2) | N/A | |
| Colorectal | 10 (12.6) | 14 (16.9) | 7 (13.0) | 31 (14.4) | ||
| Stomach | 4 (5.1) | 8 (9.6) | 11 (20.3) | 23 (10.6) | ||
| Pancreas | 14 (17.6) | 3 (3.8) | 3 (5.6) | 20 (9.3) | ||
| Breast | 1 (1.3) | 9 (10.8) | 6 (11.1) | 16 (7.4) | ||
| Abdominal wall | 1 (1.3) | 3 (3.6) | 0 (0) | 4 (1.9) | ||
| Retroperitoneal | 0 (0) | 3 (3.5) | 0 (0) | 3 (1.4) | ||
| GE junction | 1 (1.3) | 1 (1.2) | 0 (0) | 2 (0.9) | ||
| Others | 0 (0) | 1 (1.2) | 1 (1.9) | 2 (0.9) | ||
| Categories of Histology | ||||||
| Carcinoma of esophagus | 50 (63.3) | 45 (54.2) | 26 (48.1) | 121 (56.0) | N/A | |
| Colorectal adenocarcinoma | 8 (10.1) | 10 (12.0) | 7 (13.0) | 25 (11.6) | ||
| Adenocarcinoma of stomach | 3 (3.8) | 8 (9.6) | 11 (20.4) | 22 (10.2) | ||
| Carcinoma of breast | 1 (1.3) | 9 (10.8) | 5 (9.3) | 15 (6.9) | ||
| Periampullary adenocarcinoma | 11 (13.9) | 2 (2.4) | 2 (3.7) | 15 (6.9) | ||
| Abdominal wall tumors | 1 (1.3) | 3 (3.6) | 0 (0) | 4 (1.9) | ||
| Pancreatic tumors | 4 (5.1) | 0 (0) | 0 (0) | 4 (1.9) | ||
| Tetroperitoneal tumors | 0 (0) | 2 (2.4) | 0 (0) | 2 (0.9) | ||
| Phyllodes tumor of breast | 0 (0) | 0 (0) | 2 (3.7) | 2 (0.9) | ||
| Pheochromocytoma | 0 (0) | 1 (1.2) | 0 (0) | 1 (0.5) | ||
| Cholangiocarcinoma | 0 (0) | 0 (0) | 1 (1.9) | 1 (0.5) | ||
| Grade of Differentiation | ||||||
| Well | 20 (25.3) | 13 (15.7) | 12 (22.2) | 45 (20.8) | 0.072 | |
| Moderate | 43 (54.4) | 46 (55.4) | 20 (37.0) | 109 (50.5) | ||
| Poor | 7 (8.9) | 16 (19.3) | 15 (27.8) | 38 (17.6) | ||
| Not specified | 9 (11.4) | 8 (9.6) | 7 (13.0) | 24 (11.1) | ||
| Pre-/Post-Neoadjuvant | ||||||
| Pre | 47 (59.5) | 55 (66.3) | 34 (63.0) | 136 (63.0) | 0.457 ^ | |
| Post | 32 (40.5) | 26 (31.3) | 20 (37.0) | 78 (36.1) | ||
| Not specified | 0 (0) | 2 (2.4) | 0 (0) | 2 (0.9) | ||
| T Stage (n = 206) | ||||||
| T0 | 2 (2.7) | 2 (2.5) | 1 (1.9) | 5 (2.4) | 0.997 ^ | |
| T1 | 5 (6.7) | 4 (5.1) | 2 (3.8) | 11 (5.3) | ||
| T2 | 12 (16.0) | 10 (12.7) | 6 (11.5) | 28 (13.6) | ||
| T3 | 33 (44.0) | 37 (46.8) | 25 (48.1) | 95 (46.2) | ||
| T4 | 23 (30.7) | 26 (32.9) | 18 (34.6) | 67 (32.5) | ||
| Node Stage (n = 206) | ||||||
| N0 | 23 (30.7) | 22 (27.8) | 16 (30.8) | 61 (29.6) | 0.894 ^ | |
| N1 | 38 (50.7) | 44 (55.7) | 25 (48.1) | 107 (51.9) | ||
| N2 | 13 (17.3) | 10 (12.7) | 9 (17.3) | 32 (15.6) | ||
| N3 | 1 (1.3) | 3 (3.8) | 2 (3.8) | 6 (2.9) | ||
| M: Metastasis (n = 198) | ||||||
| MO | 64 (91.4) | 66 (85.7) | 47 (95.9) | 177 (90.3) | 0.169 ^ | |
| M1 | 6 (8.6) | 11 (14.3) | 2 (4.1) | 19 (9.7) | ||
| Clinical Stage | ||||||
| Stage I | 27 (34.2) | 30 (36.1) | 19 (35.2) | 11 (5.1) | 0.754 ^ | |
| Stage II | 6 (7.6) | 3 (3.6) | 2 (3.7) | 28 (13.0) | ||
| Stage III | 8 (10.1) | 15 (18.1) | 5 (9.3) | 88 (40.7) | ||
| Stage IV | 32 (40.5) | 31 (37.3) | 25 (46.3) | 76 (35.2) | ||
| Not specified | 6 (7.6) | 4 (4.8) | 3 (5.6) | 13 (6.0) | ||
| MTB Decision | Concordance | Cohen’s Kappa | p-Value | |||
|---|---|---|---|---|---|---|
| Curative (n = 188) | Palliative (n = 28) | |||||
| Surgeons’ Decision | Curative (n = 191) | 187 (99.5) | 4 (14.3) | 0.97 | 0.89 | <0.001 * |
| Palliative (n = 25) | 1 (0.5) | 24 (85.7) | ||||
| Region of Malignancy | Surgeons’ Decision | MTB Decision | Concordance | Cohen’s Kappa | p-Value | |
|---|---|---|---|---|---|---|
| n (%) | n (%) | |||||
| Colorectal | Curative (n = 27) | Palliative (n = 4) | ||||
| Curative (n = 27) | 27 (100.0) | 0 (0) | 1.00 | 1.00 | <0.001 * | |
| Palliative (n = 4) | 0 (0) | 4 (100.0) | ||||
| Breast | Curative (n = 15) | Palliative (n = 1) | ||||
| Curative (n = 15) | 15 (100.0) | 0 (0) | 1.00 | 1.00 | <0.001 * | |
| Palliative (n = 1) | 0 (0) | 1 (100.0) | ||||
| Esophagus | Curative (n = 101) | Palliative (n = 14) | ||||
| Curative (n = 103) | 101 (100.0) | 2 (14.3) | 0.98 | 0.91 | <0.001 * | |
| Palliative (n = 12) | 0 (0) | 12 (85.7) | ||||
| GE Junction | Curative (n = 1) | Palliative (n = 1) | ||||
| Curative (n = 1) | 1 (100.0) | 0 (0) | 1.00 | 1.00 | 0.157 | |
| Palliative (n = 1) | 0 (0) | 1 (100.0) | ||||
| Stomach | Curative (n = 16) | Palliative (n = 7) | ||||
| Curative (n = 17) | 15 (93.3) | 2 (16.7) | 0.86 | 0.68 | 0.001 * | |
| Palliative (n = 6) | 1 (6.7) | 5 (83.3) | ||||
| Others | Curative (n = 1) | Palliative (n = 1) | ||||
| Curative (n = 1) | 1 (100.0) | 0 (0) | 1.00 | 1.00 | 0.157 | |
| Palliative (n = 1) | 0 (0) | 1 (100.0) | ||||
| Pancreas | Curative (n = 20) | Palliative (n = 0) | ||||
| Curative (n = 20) | 20 (100.0) | 0 (0) | N/A | N/A | N/A | |
| Retroperitoneum | Curative (n = 3) | Palliative (n = 0) | ||||
| Curative (n = 3) | 3 (100.0) | 0 (0) | N/A | N/A | N/A | |
| Abdominal Wall | Curative (n = 4) | Palliative (n = 0) | ||||
| Curative (n = 4) | 4 (100.0) | 0 (0) | N/A | N/A | N/A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qureshi, S.; Abbasi, W.A.; Jalil, H.A.; Ahmed, R.; Iqbal, M.; Saiyed, H.; Waseem, H.F.; Naimatullah, N.; Amin, S.R.; Quraishy, M.S. Evaluating Treatment Plan Modifications from Surgeons’ Initial Recommendations to Multidisciplinary Tumor Board Consensus for Cancer Care in a Resource-Limited Setting. Curr. Oncol. 2025, 32, 310. https://doi.org/10.3390/curroncol32060310
Qureshi S, Abbasi WA, Jalil HA, Ahmed R, Iqbal M, Saiyed H, Waseem HF, Naimatullah N, Amin SR, Quraishy MS. Evaluating Treatment Plan Modifications from Surgeons’ Initial Recommendations to Multidisciplinary Tumor Board Consensus for Cancer Care in a Resource-Limited Setting. Current Oncology. 2025; 32(6):310. https://doi.org/10.3390/curroncol32060310
Chicago/Turabian StyleQureshi, Sajida, Waqas Ahmad Abbasi, Hira Abdul Jalil, Raheel Ahmed, Mubashir Iqbal, Hanieya Saiyed, Hira Fatima Waseem, Najeeb Naimatullah, Syed Rashidul Amin, and Muhammad Saeed Quraishy. 2025. "Evaluating Treatment Plan Modifications from Surgeons’ Initial Recommendations to Multidisciplinary Tumor Board Consensus for Cancer Care in a Resource-Limited Setting" Current Oncology 32, no. 6: 310. https://doi.org/10.3390/curroncol32060310
APA StyleQureshi, S., Abbasi, W. A., Jalil, H. A., Ahmed, R., Iqbal, M., Saiyed, H., Waseem, H. F., Naimatullah, N., Amin, S. R., & Quraishy, M. S. (2025). Evaluating Treatment Plan Modifications from Surgeons’ Initial Recommendations to Multidisciplinary Tumor Board Consensus for Cancer Care in a Resource-Limited Setting. Current Oncology, 32(6), 310. https://doi.org/10.3390/curroncol32060310






