Mechanistic Insights and Future Directions for Enfortumab Vedotin in Urothelial Carcinoma: Highlights from the 10th Annual Leo & Anne Albert Institute for Bladder Cancer Care and Research Symposium
Abstract
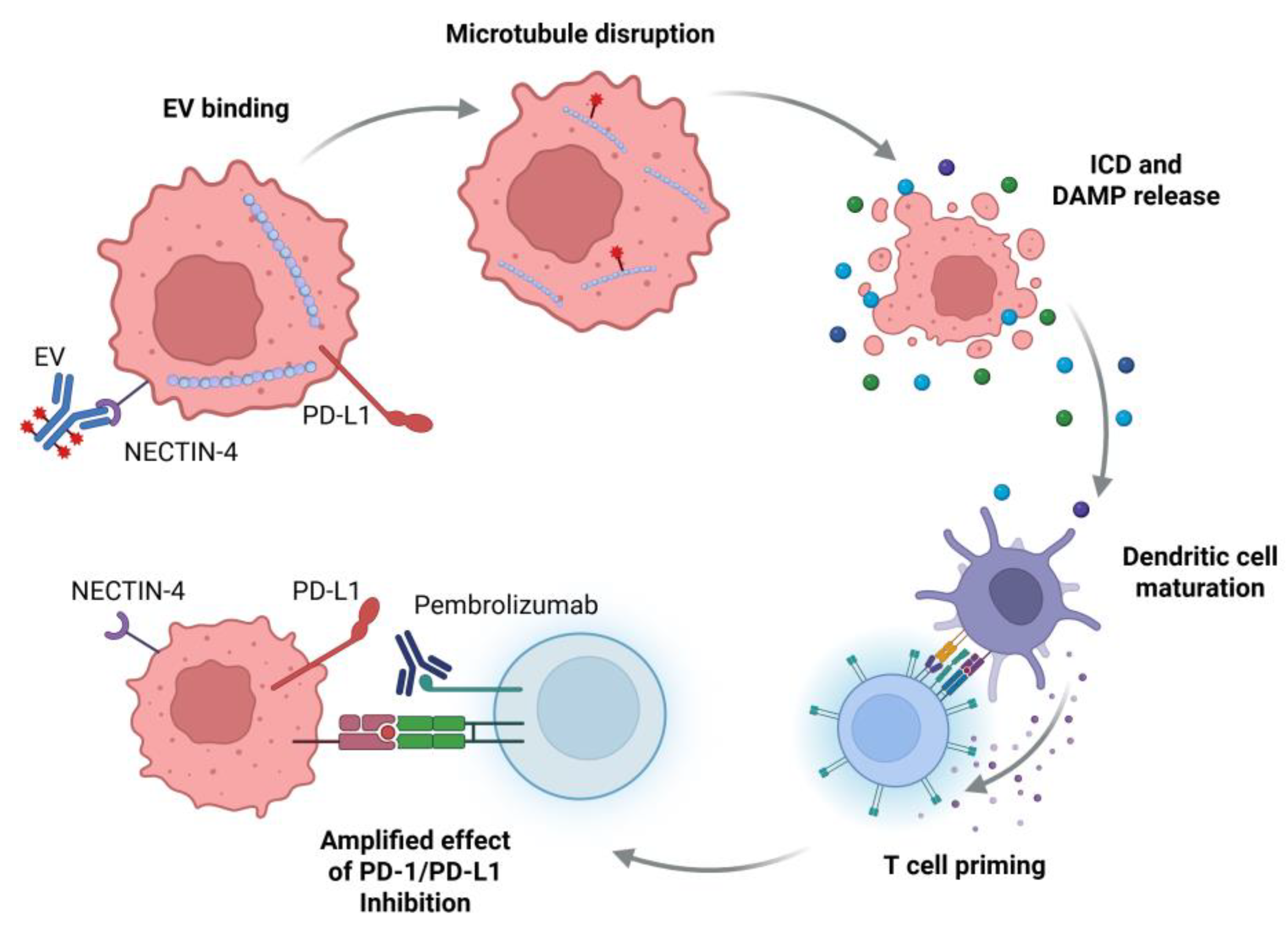
1. Current Role for EV in mUC
2. EV Mechanism of Action
3. Predictive Biomarkers for EV Response
4. Potential for EV in Other Clinical Disease States: MIBC
5. Potential for EV in Other Clinical Disease States: Non-Muscle Invasive Bladder Cancer and Upper Tract Urothelial Carcinoma
6. Novel NECTIN4 Targeting Agents
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EV | Enfortumab vedotin |
| mUC | Metastatic urothelial carcinoma |
| CI | Confidence Interval |
| EV + P | Enfortumab vedotin in combination with pembrolizumab |
| ADC | Antibody drug conjugate |
| MMAE | Monomethyl auristatin E |
| EMT | Epithelial–mesenchymal transition |
| PD-1 | Programed cell death-1 |
| ICD | Immunogenic cell death |
| DAMPs | Damage-associated molecular patterns |
| OS | Overall Survival |
| PFS | Progression free survival |
| ORR | Overall response rate |
| MIBC | Muscle invasive bladder cancer |
| pCR | Pathologic complete response |
| pDS | Pathologic downstaging |
| EAU | European Association of Urology |
| ESMO | European Society for Medical Oncology |
| ASCO | American Society for Clinical Oncology |
| NMIBC | Non-muscle invasive bladder cancer |
| CAB | Conditionally active biologic |
| BTC | Bicyclic toxin conjugate |
References
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.-L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef]
- O’Donnell, P.H.; Milowsky, M.I.; Petrylak, D.P.; Hoimes, C.J.; Flaig, T.W.; Mar, N.; Moon, H.H.; Friedlander, T.W.; McKay, R.R.; Bilen, M.A.; et al. Enfortumab Vedotin with or Without Pembrolizumab in Cisplatin-Ineligible Patients with Previously Untreated Locally Advanced or Metastatic Urothelial Cancer. J. Clin. Oncol. 2023, 41, 4107–4117. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Valderrama, B.P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J.; et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N. Engl. J. Med. 2024, 390, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Van Der Heijden, M.S.; Loriot, Y.; Bedke, J.; Valderrama, B.P.; Iyer, G.; Kikuchi, E.; Hoffman-Censits, J.; Vulsteke, C.; Drakaki, A.; et al. EV-302: Updated Analysis from the Phase 3 Global Study of Enfortumab Vedotin in Combination with Pembrolizumab (EV+P) vs Chemotherapy (Chemo) in Previously Untreated Locally Advanced or Metastatic Urothelial Carcinoma (La/mUC). J. Clin. Oncol. 2025, 43, 664. [Google Scholar] [CrossRef]
- EAU Guidelines—Uroweb. Available online: https://uroweb.org/guidelines/muscle-invasive-and-metastatic-bladder-cancer (accessed on 6 November 2024).
- Flaig, T.W.; Spiess, P.E.; Abern, M.; Agarwal, N.; Bangs, R.; Buyyounouski, M.K.; Chan, K.; Chang, S.S.; Chang, P.; Friedlander, T.; et al. NCCN Guidelines® Insights: Bladder Cancer, Version 3.2024: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Canc. Netw. 2024, 22, 216–225. [Google Scholar] [CrossRef]
- Powles, T.; Bellmunt, J.; Comperat, E.; Santis, M.D.; Huddart, R.; Loriot, Y.; Necchi, A.; Valderrama, B.P.; Ravaud, A.; Shariat, S.F.; et al. ESMO Clinical Practice Guideline Interim Update on First-Line Therapy in Advanced Urothelial Carcinoma. Ann. Oncol. 2024, 35, 485–490. [Google Scholar] [CrossRef]
- Bouleftour, W.; Guillot, A.; Magne, N. The Anti-Nectin 4: A Promising Tumor Cells Target. A Systematic Review. Mol. Cancer Ther. 2022, 21, 493–501. [Google Scholar] [CrossRef]
- Challita-Eid, P.M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D.R.; et al. Enfortumab Vedotin Antibody–Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016, 76, 3003–3013. [Google Scholar] [CrossRef]
- Olson, D.; Younan, P.; Liu, B.; Blahnik-Fagan, G.; Gosink, J.; Snead, K.; Tenn, E.; Hensley, K.; Sahetya, D.; Nesterova, A.; et al. 1187 Enfortumab Vedotin Induces Immunogenic Cell Death, Elicits Antitumor Immune Memory, and Shows Enhanced Preclinical Activity in Combination with Immune Checkpoint Inhibitors. J. Immunother. Cancer 2022, 10. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus Guidelines for the Definition, Detection and Interpretation of Immunogenic Cell Death. J. Immunother. Cancer 2020, 8, e000337. [Google Scholar] [CrossRef]
- Garg, A.D.; Galluzzi, L.; Apetoh, L.; Baert, T.; Birge, R.B.; Bravo-San Pedro, J.M.; Breckpot, K.; Brough, D.; Chaurio, R.; Cirone, M.; et al. Molecular and Translational Classifications of DAMPs in Immunogenic Cell Death. Front. Immunol. 2015, 6, 588. [Google Scholar] [CrossRef] [PubMed]
- Best, R.L.; LaPointe, N.E.; Azarenko, O.; Miller, H.; Genualdi, C.; Chih, S.; Shen, B.-Q.; Jordan, M.A.; Wilson, L.; Feinstein, S.C.; et al. Microtubule and Tubulin Binding and Regulation of Microtubule Dynamics by the Antibody Drug Conjugate (ADC) Payload, Monomethyl Auristatin E (MMAE): Mechanistic Insights into MMAE ADC Peripheral Neuropathy. Toxicol. Appl. Pharmacol. 2021, 421, 115534. [Google Scholar] [CrossRef] [PubMed]
- Klussman, K.; Tenn, E.-M.; Higgins, S.; Mazahreh, R.; Snead, K.; Hamilton, J.; Grogan, B.; Sigurjonsson, J.; Cao, A.; Gardai, S.; et al. 618 Vedotin ADCs Induce ER Stress and Elicit Hallmarks of ICD across Multiple Cancer Indications. J. Immunother. Cancer 2020, 8, A654. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, J.; Wang, A.; Wei, J.; Peng, Z.; Wang, X.; Zhou, J.; Qi, C.; Liu, D.; Li, J.; et al. Disitamab Vedotin (RC48) plus Toripalimab for HER2-Expressing Advanced Gastric or Gastroesophageal Junction and Other Solid Tumours: A Multicentre, Open Label, Dose Escalation and Expansion Phase 1 Trial. eClinicalMedicine 2024, 68, 102415. [Google Scholar] [CrossRef]
- Lee, H.; Flinn, I.W.; Melear, J.; Ramchandren, R.; Friedman, J.; Burke, J.M.; Linhares, Y.; Gonzales, P.A.; Raval, M.; Chintapatla, R.; et al. Brentuximab Vedotin, Nivolumab, Doxorubicin, and Dacarbazine (AN+AD) for Advanced Stage Classic Hodgkin Lymphoma: Updated Efficacy and Safety Results from the Single-Arm Phase 2 Study (SGN35-027 Part B). Blood 2022, 140, 763–765. [Google Scholar] [CrossRef]
- Vergote, I.; Van Nieuwenhuysen, E.; O’Cearbhaill, R.E.; Westermann, A.; Lorusso, D.; Ghamande, S.; Collins, D.C.; Banerjee, S.; Mathews, C.A.; Gennigens, C.; et al. Tisotumab Vedotin in Combination With Carboplatin, Pembrolizumab, or Bevacizumab in Recurrent or Metastatic Cervical Cancer: Results From the innovaTV 205/GOG-3024/ENGOT-Cx8 Study. J. Clin. Oncol. 2023, 41, 5536–5549. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, K.W.; Zhang, S.; Yan, X.Q.; Li, S.M.; Xu, H.Y.; Li, J.; Liu, Y.Q.; Tang, B.X.; Chi, Z.H.; et al. Disitamab Vedotin plus Toripalimab in Patients with Locally Advanced or Metastatic Urothelial Carcinoma (RC48-C014): A Phase 1b/2 Dose-Escalation and Dose-Expansion Study. Ann. Oncol. 2024, 36, 331–339. [Google Scholar] [CrossRef]
- Galsky, M.D.; Del Conte, G.; Foti, S.; Yu, E.Y.; Machiels, J.-P.H.; Doger, B.; Necchi, A.; De Braud, F.G.; Hamilton, E.P.; Hennequin, A.; et al. Primary Analysis from DS8201-A-U105: A Phase 1b, Two-Part, Open-Label Study of Trastuzumab Deruxtecan (T-DXd) with Nivolumab (Nivo) in Patients (Pts) with HER2-Expressing Urothelial Carcinoma (UC). J. Clin. Oncol. 2022, 40, 438. [Google Scholar] [CrossRef]
- Vlachou, E.; Matoso, A.; McConkey, D.; Jing, Y.; Johnson, B.A.; Hahn, N.M.; Hoffman-Censits, J. Enfortumab Vedotin-Related Cutaneous Toxicity and Radiographic Response in Patients with Urothelial Cancer: A Single-Center Experience and Review of the Literature. Eur. Urol. Open Sci. 2023, 49, 100–103. [Google Scholar] [CrossRef]
- Vlachou, E.; Johnson, B.A.; McConkey, D.; Jing, Y.; Matoso, A.; Hahn, N.M.; Hoffman-Censits, J. Enfortumab Vedotin-Related Cutaneous Toxicity Correlates with Overall Survival in Patients with Urothelial Cancer: A Retrospective Experience. Front. Oncol. 2024, 14, 1377842. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Patel, A.B.; Rosenberg, J.E.; O’Donnell, P.H. Management of Dermatologic Events Associated With the Nectin-4-Directed Antibody-Drug Conjugate Enfortumab Vedotin. Oncologist 2022, 27, e223–e232. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.E.; Sjöström, M.; Egusa, E.A.; Gibb, E.A.; Badura, M.L.; Zhu, J.; Koshkin, V.S.; Stohr, B.A.; Meng, M.V.; Pruthi, R.S.; et al. Heterogeneity in NECTIN4 Expression Across Molecular Subtypes of Urothelial Cancer Mediates Sensitivity to Enfortumab Vedotin. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 5123–5130. [Google Scholar] [CrossRef]
- Rosenberg, J.; Sridhar, S.S.; Zhang, J.; Smith, D.; Ruether, D.; Flaig, T.W.; Baranda, J.; Lang, J.; Plimack, E.R.; Sangha, R.; et al. EV-101: A Phase I Study of Single-Agent Enfortumab Vedotin in Patients With Nectin-4–Positive Solid Tumors, Including Metastatic Urothelial Carcinoma. J. Clin. Oncol. 2020, 38, 1041. [Google Scholar] [CrossRef] [PubMed]
- Klümper, N.; Ralser, D.J.; Ellinger, J.; Roghmann, F.; Albrecht, J.; Below, E.; Alajati, A.; Sikic, D.; Breyer, J.; Bolenz, C.; et al. Membranous NECTIN-4 Expression Frequently Decreases during Metastatic Spread of Urothelial Carcinoma and Is Associated with Enfortumab Vedotin Resistance. Clin. Cancer Res. 2023, 29, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Klümper, N.; Tran, N.K.; Zschäbitz, S.; Hahn, O.; Büttner, T.; Roghmann, F.; Bolenz, C.; Zengerling, F.; Schwab, C.; Nagy, D.; et al. NECTIN4 Amplification Is Frequent in Solid Tumors and Predicts Enfortumab Vedotin Response in Metastatic Urothelial Cancer. J. Clin. Oncol. 2024, 42, 2446–2455. [Google Scholar] [CrossRef]
- Flaig, T.W.; Rosenberg, J.E.; Hoimes, C.J.; O’Donnell, P.H.; Mar, N.; Gourdin, T.S.; Henry, S.; Bilen, M.A.; George, S.; Barata, P.C.; et al. Study EV-103: Neoadjuvant Treatment with Enfortumab Vedotin Monotherapy in Cisplatin-Ineligible Patients (Pts) with Muscle Invasive Bladder Cancer (MIBC): Updated Results for Cohort H. J. Clin. Oncol. 2023, 41, 4595. [Google Scholar] [CrossRef]
- Sridhar, S.; O’Donnell, P.H.; Flaig, T.W.; Rosenberg, J.E.; Hoimes, C.J.; Milowsky, M.I.; Srinivas, S.; George, S.; McKay, R.R.; Petrylak, D.P.; et al. 2365MO Study EV-103 Cohort L: Perioperative Treatment w/Enfortumab Vedotin (EV) Monotherapy in Cisplatin (Cis)-Ineligible Patients (Pts) w/Muscle Invasive Bladder Cancer (MIBC). Ann. Oncol. 2023, 34, S1203. [Google Scholar] [CrossRef]
- Hoimes, C.J.; Loriot, Y.; Bedke, J.; Nishiyama, H.; Kataria, R.S.; Homet Moreno, B.; Galsky, M.D. Perioperative Enfortumab Vedotin (EV) plus Pembrolizumab (Pembro) versus Chemotherapy in Cisplatin-Eligible Patients (Pts) with Muscle-Invasive Bladder Cancer (MIBC): Phase 3 KEYNOTE-B15/EV-304. J. Clin. Oncol. 2023, 41, TPS588. [Google Scholar] [CrossRef]
- Powles, T.; Catto, J.W.F.; Galsky, M.D.; Al-Ahmadie, H.; Meeks, J.J.; Nishiyama, H.; Vu, T.Q.; Antonuzzo, L.; Wiechno, P.; Atduev, V.; et al. Perioperative Durvalumab with Neoadjuvant Chemotherapy in Operable Bladder Cancer. N. Engl. J. Med. 2024, 391, 1773–1786. [Google Scholar] [CrossRef]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef]
- A Phase 3, Randomized, Open-Label Study to Evaluate Perioperative Enfortumab Vedotin Plus Pembrolizumab (MK-3475) Versus Neoadjuvant Gemcitabine and Cisplatin in Cisplatin-Eligible Participants with Muscle-Invasive Bladder Cancer (MK-3475-B15/KEYNOTE-B15/EV-304). Available online: https://clinicaltrials.gov/study/NCT04700124 (accessed on 2 May 2025).
- Galsky, M.D.; Necchi, A.; Shore, N.D.; Plimack, E.R.; Jia, C.; Sbar, E.; Homet Moreno, B.; Witjes, J.A. KEYNOTE-905/EV-303: Perioperative Pembrolizumab or Pembrolizumab plus Enfortumab Vedotin (EV) and Cystectomy Compared to Cystectomy Alone in Cisplatin-Ineligible Patients with Muscle-Invasive Bladder Cancer (MIBC). J. Clin. Oncol. 2021, 39, TPS507. [Google Scholar] [CrossRef]
- A Randomized Phase 3 Study Evaluating Cystectomy With Perioperative Pembrolizumab and Cystectomy With Perioperative Enfortumab Vedotin and Pembrolizumab Versus Cystectomy Alone in Participants Who Are Cisplatin-Ineligible or Decline Cisplatin with Muscle-Invasive Bladder Cancer (KEYNOTE-905/EV-303). Available online: https://clinicaltrials.gov/study/NCT03924895 (accessed on 2 May 2025).
- Powles, T.; Drakaki, A.; Teoh, J.Y.-C.; Grande, E.; Fontes-Sousa, M.; Porta, C.; Wu, E.; Goluboff, E.T.; Ho, S.; Hois, S.; et al. A Phase 3, Randomized, Open-Label, Multicenter, Global Study of the Efficacy and Safety of Durvalumab (D) + Tremelimumab (T) + Enfortumab Vedotin (EV) or D + EV for Neoadjuvant Treatment in Cisplatin-Ineligible Muscle-Invasive Bladder Cancer (MIBC) (VOLGA). J. Clin. Oncol. 2022, 40, TPS579. [Google Scholar] [CrossRef]
- A Phase III Randomized, Open-Label, Multicenter Study to Determine the Efficacy and Safety of Durvalumab in Combination With Tremelimumab and Enfortumab Vedotin or Durvalumab in Combination With Enfortumab Vedotin for Perioperative Treatment in Patients Ineligible for Cisplatin or Who Refuse Cisplatin Undergoing Radical Cystectomy for Muscle Invasive Bladder Cancer (VOLGA). Available online: https://clinicaltrials.gov/study/NCT04960709 (accessed on 2 May 2025).
- Enfortumab Vedotin in Combination with Pembrolizumab for Locally Advanced and/or Node Positive Urothelial Carcinoma Prior to Surgery (EV-ECLIPSE). Available online: https://clinicaltrials.gov/study/NCT05239624 (accessed on 2 May 2025).
- A Phase 1/2 Study of V940 Plus Pembrolizumab with or Without Enfortumab Vedotin in Muscle-Invasive Urothelial Carcinoma (MIUC) (INTerpath-005). Available online: https://clinicaltrials.gov/study/NCT06305767 (accessed on 2 May 2025).
- Phase Ib/II Study of Enfortumab Vedotin and Pembrolizumab Combined with Radiotherapy as a Bladder-Sparing Trimodality Therapy in Muscle Invasive Bladder Cancer. Available online: https://clinicaltrials.gov/study/NCT06470282 (accessed on 15 November 2024).
- Kamat, A.M.; Steinberg, G.D.; Inman, B.A.; Kates, M.R.; Uchio, E.M.; Porten, S.P.; Roupret, M.; Redorta, J.; Catto, J.W.F.; Kulkarni, G.S.; et al. Study EV-104: Phase 1 Study of Intravesical Enfortumab Vedotin for Treatment of Patients with Non-Muscle Invasive Bladder Cancer (NMIBC)—Trial in Progress. J. Clin. Oncol. 2023, 41, TPS582. [Google Scholar] [CrossRef]
- Balar, A.V.; Kamat, A.M.; Kulkarni, G.S.; Uchio, E.M.; Boormans, J.L.; Roumiguié, M.; Krieger, L.E.M.; Singer, E.A.; Bajorin, D.F.; Grivas, P.; et al. Pembrolizumab Monotherapy for the Treatment of High-Risk Non-Muscle-Invasive Bladder Cancer Unresponsive to BCG (KEYNOTE-057): An Open-Label, Single-Arm, Multicentre, Phase 2 Study. Lancet Oncol. 2021, 22, 919–930. [Google Scholar] [CrossRef]
- Narayan, V.M.; Boorjian, S.A.; Alemozaffar, M.; Konety, B.R.; Shore, N.D.; Gomella, L.G.; Kamat, A.M.; Bivalacqua, T.J.; Montgomery, J.S.; Lerner, S.P.; et al. Efficacy of Intravesical Nadofaragene Firadenovec for Patients With Bacillus Calmette-Guérin-Unresponsive Nonmuscle-Invasive Bladder Cancer: 5-Year Follow-Up From a Phase 3 Trial. J. Urol. 2024, 212, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Chamie, K.; Chang, S.S.; Kramolowsky, E.; Gonzalgo, M.L.; Agarwal, P.K.; Bassett, J.C.; Bjurlin, M.; Cher, M.L.; Clark, W.; Cowan, B.E.; et al. IL-15 Superagonist NAI in BCG-Unresponsive Non–Muscle-Invasive Bladder Cancer. NEJM Evid. 2022, 2, EVIDoa2200167. [Google Scholar] [CrossRef]
- FDA Approves Pembrolizumab for BCG-Unresponsive, High-Risk Non-Muscle Invasive Bladder Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-bcg-unresponsive-high-risk-non-muscle-invasive-bladder-cancer (accessed on 4 May 2025).
- FDA Approves First Adenoviral Vector-Based Gene Therapy for High-Risk Bacillus Calmette-Guérin Unresponsive Non-Muscle Invasive Bladder Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-first-adenoviral-vector-based-gene-therapy-high-risk-bacillus-calmette-guerin (accessed on 4 May 2025).
- FDA Approves Nogapendekin Alfa Inbakicept-Pmln for BCG-Unresponsive Non-Muscle Invasive Bladder Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nogapendekin-alfa-inbakicept-pmln-bcg-unresponsive-non-muscle-invasive-bladder-cancer (accessed on 4 May 2025).
- A Phase II, Open-Label, Single-Arm, Multi-Center Study of Neoadjuvant Enfortumab Vedotin and Pembrolizumab in Cisplatin-Eligible Upper Tract Urothelial Cancer (NEPTUNE). Available online: https://clinicaltrials.gov/study/NCT06356155 (accessed on 27 March 2025).
- Neoadjuvant Enfortumab Vedotin in High-Grade Urothelial Carcinoma of the Upper Urinary Tract (Including Ureter and Renal Pelvis). Available online: https://clinicaltrials.gov/study/NCT05868265 (accessed on 27 March 2025).
- Li, K.; Zhou, Y.; Zang, M.; Jin, X.; Li, X. Therapeutic Prospects of Nectin-4 in Cancer: Applications and Value. Front. Oncol. 2024, 14, 1354543. [Google Scholar] [CrossRef]
- Khosravanian, M.J.; Mirzaei, Y.; Mer, A.H.; Keyhani-Khankahdani, M.; Abdinia, F.S.; Misamogooe, F.; Amirkhani, Z.; Bagheri, N.; Meyfour, A.; Jahandideh, S.; et al. Nectin-4-Directed Antibody-Drug Conjugates (ADCs): Spotlight on Preclinical and Clinical Evidence. Life Sci. 2024, 352, 122910. [Google Scholar] [CrossRef]
- Zhou, W.; Fang, P.; Yu, D.; Ren, H.; You, M.; Yin, L.; Mei, F.; Zhu, H.; Wang, Z.; Xu, H.; et al. Preclinical Evaluation of 9MW2821, a Site-Specific Monomethyl Auristatin E–Based Antibody–Drug Conjugate for Treatment of Nectin-4–Expressing Cancers. Mol. Cancer Ther. 2023, 22, 913–925. [Google Scholar] [CrossRef]
- Ye, D.-W.; Zhang, J.; Yang, H.; Yang, J.; Zheng, T.; Sun, H.; Wan, X.; Lan, G.; Sun, G.; Zhang, X. Phase 1 Dose Escalation of SYS6002 (CRB-701), a next-Generation Nectin-4 Targeting Antibody Drug Conjugate (ADC). J. Clin. Oncol. 2024, 42, 622. [Google Scholar] [CrossRef]
- Rosenberg, J.; Sabatier, R.; Viceneux, A.; de Rouge, T.L.M.; Champiat, S.; Lebellec, L.; Barthélémy, P.; Sonpavde, G.; Gao, X.; Niglio, S.; et al. Abstract CT084: A Phase 1 Study of LY4101174 (ETx-22), an Antibody-Drug Conjugate Targeting Nectin-4, in Patients with Advanced or Metastatic Urothelial Cancer and Other Solid Tumors (Trial in Progress). Cancer Res. 2024, 84, CT084. [Google Scholar] [CrossRef]
- Jiang, L.; Song, Z.; Gong, Y.; Jin, J.; Ding, Y.; Tang, L.; Deng, X.; Li, X.; Li, S.; Cheng, X.; et al. A Phase 1 Study of BAT8007, an Anti-Nectin-4 Monoclonal Antibody-Exatecan Conjugate, in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2024, 42, e16567. [Google Scholar] [CrossRef]
- Shahmoradgoli, M.; Hau, A.; Lee, D.J.; Wang, A.; Challita, P.P.; Betancourt, O.; Sisson, W.; Kuo, M.M.; Zhang, K.; Goldson, A.; et al. Abstract 1902: ADRX-0706 Nectin-4 Antibody-Drug Conjugate PK/PD Characterization Elucidates Its Widened Therapeutic Window. Cancer Res. 2024, 84, 1902. [Google Scholar] [CrossRef]
- Sagar, D.; Srinivasan, M.; Lindquist, K.; Guo, Q.; Wong, W.; Lebron, M.B.; Sattler, R.M.; Zhou, J.; Helms, W.; Boyles, J.; et al. Abstract 1872: A next Generation Treatment for Nectin-4 Positive Cancers—Preclinical Characterization of LY4052031, an Anti-Nectin-4 Antibody, Conjugated to a Novel Camptothecin Payload. Cancer Res. 2024, 84, 1872. [Google Scholar] [CrossRef]
- Tang, B.; Sheng, X.; Guo, J.; Niu, H.; Shen, Y.; Jiang, S.; Fu, B.; Guo, J.; Wahafu, W.; Yao, K.; et al. Nectin-4 Targeted ADC, SHR-A2102, in Patients with Advanced or Metastatic Urothelial Carcinoma: A Phase 1 Study. J. Clin. Oncol. 2025, 43, 657. [Google Scholar] [CrossRef]
- Wang, J.; Xing, C.; Liu, H.; Cugnetti, A.P.G.; Wheeler, C.; Lucas, M.; Frey, G.; Chang, C.; Boyle, W.J.; Short, J.M. Abstract 4560: Conditionally Active Biologics (CAB): A Novel Class of Molecules Targeting Solid Tumors. Cancer Res. 2020, 80, 4560. [Google Scholar] [CrossRef]
- Frey, G.; Wang, J.; Johnson, K.; Liu, H.; Wheeler, C.; Xing, C.; Cugnetti, A.P.; McNeeley, P.; Joyner, S.; Chang, C.; et al. Abstract 1541: NextGen Conditionally Active Biologic (CAB) Anti-Nectin-4-ADC with Improved Stability and Safety. Cancer Res. 2023, 83, 1541. [Google Scholar] [CrossRef]
- BioAtla Announces FDA Clearance of Investigational New Drug Application for BA3361, a CAB-Nectin-4 Antibody Drug Conjugate for the Treatment of Multiple Tumors. Available online: https://www.globenewswire.com/news-release/2024/05/06/2875781/0/en/BioAtla-Announces-FDA-Clearance-of-Investigational-New-Drug-Application-for-BA3361-a-CAB-Nectin-4-Antibody-Drug-Conjugate-for-the-Treatment-of-Multiple-Tumors.html (accessed on 13 December 2024).
- Rigby, M.; Bennett, G.; Chen, L.; Mudd, G.E.; Harrison, H.; Beswick, P.J.; Van Rietschoten, K.; Watcham, S.M.; Scott, H.S.; Brown, A.N.; et al. BT8009; A Nectin-4 Targeting Bicycle Toxin Conjugate for Treatment of Solid Tumors. Mol. Cancer Ther. 2022, 21, 1747–1756. [Google Scholar] [CrossRef]
- Hurov, K.; Lahdenranta, J.; Upadhyaya, P.; Haines, E.; Cohen, H.; Repash, E.; Kanakia, D.; Ma, J.; Kristensson, J.; You, F.; et al. BT7480, a Novel Fully Synthetic Bicycle Tumor-Targeted Immune Cell AgonistTM (Bicycle TICATM) Induces Tumor Localized CD137 Agonism. J. Immunother. Cancer 2021, 9, e002883. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, G.; Luo, W.; Xu, M.; Peng, R.; Du, Z.; Liu, Y.; Bai, Z.; Xiao, X.; Qin, S. PROTAC Technology: From Drug Development to Probe Technology for Target Deconvolution. Eur. J. Med. Chem. 2024, 276, 116725. [Google Scholar] [CrossRef]
| Trial Name | Patient Eligibility | Neoadjuvant Treatment | Adjuvant Treatment | Primary Endpoint | Planned Completion |
|---|---|---|---|---|---|
| KEYNOTE-B15/EV-304 [29] NCT04700124 | Cisplatin eligible | EV + P versus gemcitabine + cisplatin | EV + P versus observation | EFS | December 2026 [32] |
| KEYNOTE-905/EV-303 [33] NCT03924895 | Cisplatin ineligible | Pembrolizumab versus EV + P versus none | Pembrolizumab versus EV + P versus none | EFS | December 2027 [34] |
| VOLGA [35] NCT04960709 | Cisplatin ineligible | Durvalumab + Tremelimumab + EV versus Durvalumab + EV versus none | Durvalumab + Tremelimumab versus Durvalumab versus none | EFS | September 2028 [36] |
| EV-ECLIPSE [37] NCT05239624 | Cisplatin eligible and ineligible, lymph node involvement | EV + P | Pembrolizumab | pCR | June 2026 [37] |
| INTerpath-005 [38] NCT06305767 Perioperative Cohort | Cisplatin ineligible | EV + P + V940 | EV + P + V490 | AE rate, treatment discontinuation | October 2031 [38] |
| Drug | Mechanism | Toxin | Company | Status |
|---|---|---|---|---|
| BT8009 | BTC | MMAE | Bicycle Therapeutics, Cambridge, UK | I/II/III |
| BT7480 | BTC | Bicycle Therapeutics, Cambridge, UK | I/II | |
| 9MW2821 | ADC | MMAE | Mabwell, Shanghai, China | I/II/III |
| LY4101174 | ADC | exatecan | Lilly, Indianapolis, IN, USA | I |
| BA3361 | CAB ADC | MMAE | BioAlta, San Diego, CA, USA | IND |
| BAT8007 | ADC | exatecan | Bio-Thera, Guangzhou, China | I |
| ADRX-0706 | ADC | AP052 | Adcentrx Therapeutics, San Diego, CA, USA | I |
| SYS6002/ CRB-701 | ADC | MMAE | Corbus Pharmaceuticals, Norwood, MA, USA | I/II |
| SHR-A2102 | ADC | Rezetecan | Jiangsu HengRui, Lianyungang, China | I/II/III |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahey, C.C.; Clark-Garvey, S.; Porten, S.; Kamat, A.M.; Flaig, T.W.; Taylor, J.A.; Kim, W.Y.; Milowsky, M.I. Mechanistic Insights and Future Directions for Enfortumab Vedotin in Urothelial Carcinoma: Highlights from the 10th Annual Leo & Anne Albert Institute for Bladder Cancer Care and Research Symposium. Curr. Oncol. 2025, 32, 278. https://doi.org/10.3390/curroncol32050278
Fahey CC, Clark-Garvey S, Porten S, Kamat AM, Flaig TW, Taylor JA, Kim WY, Milowsky MI. Mechanistic Insights and Future Directions for Enfortumab Vedotin in Urothelial Carcinoma: Highlights from the 10th Annual Leo & Anne Albert Institute for Bladder Cancer Care and Research Symposium. Current Oncology. 2025; 32(5):278. https://doi.org/10.3390/curroncol32050278
Chicago/Turabian StyleFahey, Catherine C., Sean Clark-Garvey, Sima Porten, Ashish M. Kamat, Thomas W. Flaig, John A. Taylor, William Y. Kim, and Matthew I. Milowsky. 2025. "Mechanistic Insights and Future Directions for Enfortumab Vedotin in Urothelial Carcinoma: Highlights from the 10th Annual Leo & Anne Albert Institute for Bladder Cancer Care and Research Symposium" Current Oncology 32, no. 5: 278. https://doi.org/10.3390/curroncol32050278
APA StyleFahey, C. C., Clark-Garvey, S., Porten, S., Kamat, A. M., Flaig, T. W., Taylor, J. A., Kim, W. Y., & Milowsky, M. I. (2025). Mechanistic Insights and Future Directions for Enfortumab Vedotin in Urothelial Carcinoma: Highlights from the 10th Annual Leo & Anne Albert Institute for Bladder Cancer Care and Research Symposium. Current Oncology, 32(5), 278. https://doi.org/10.3390/curroncol32050278





