Combination of Osimertinib and Brigatinib in the Treatment of EGFR Triple-Mutated Lung Adenocarcinoma: A Case Report
Abstract
1. Introduction
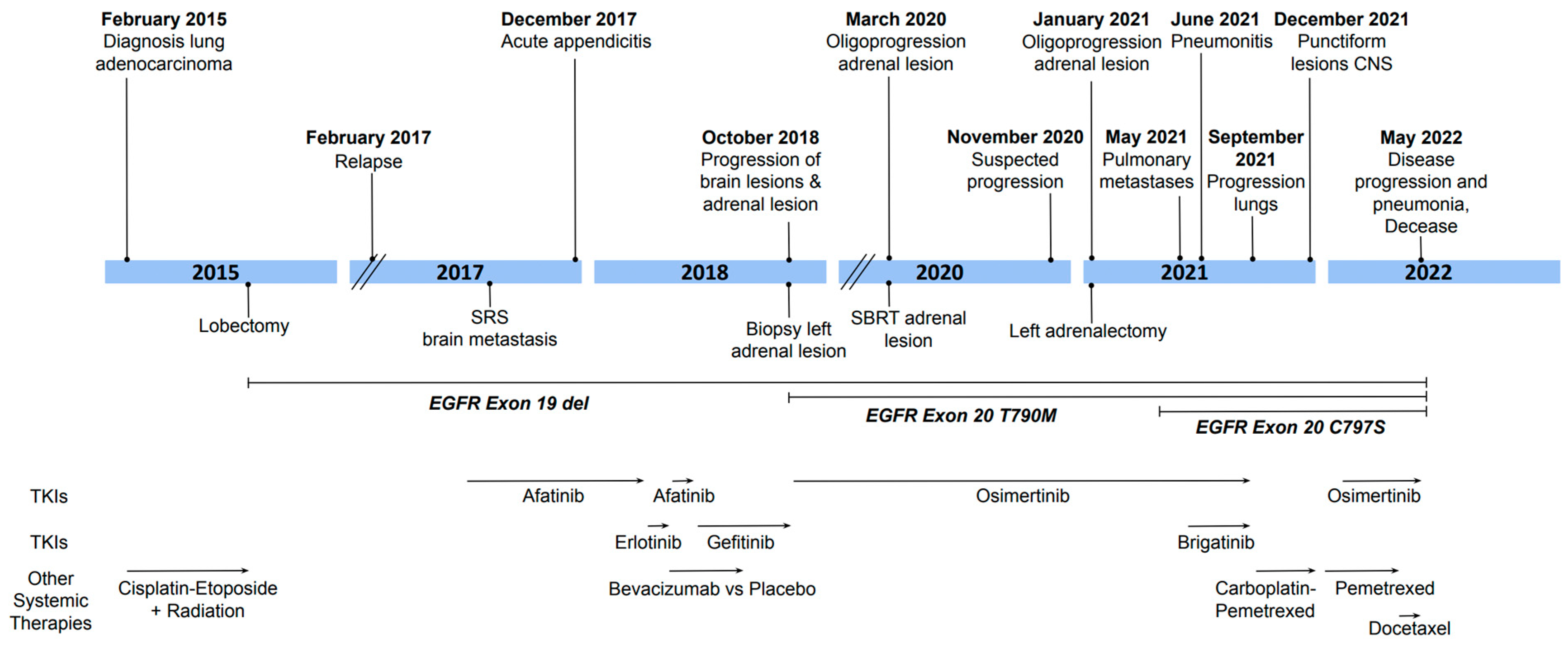
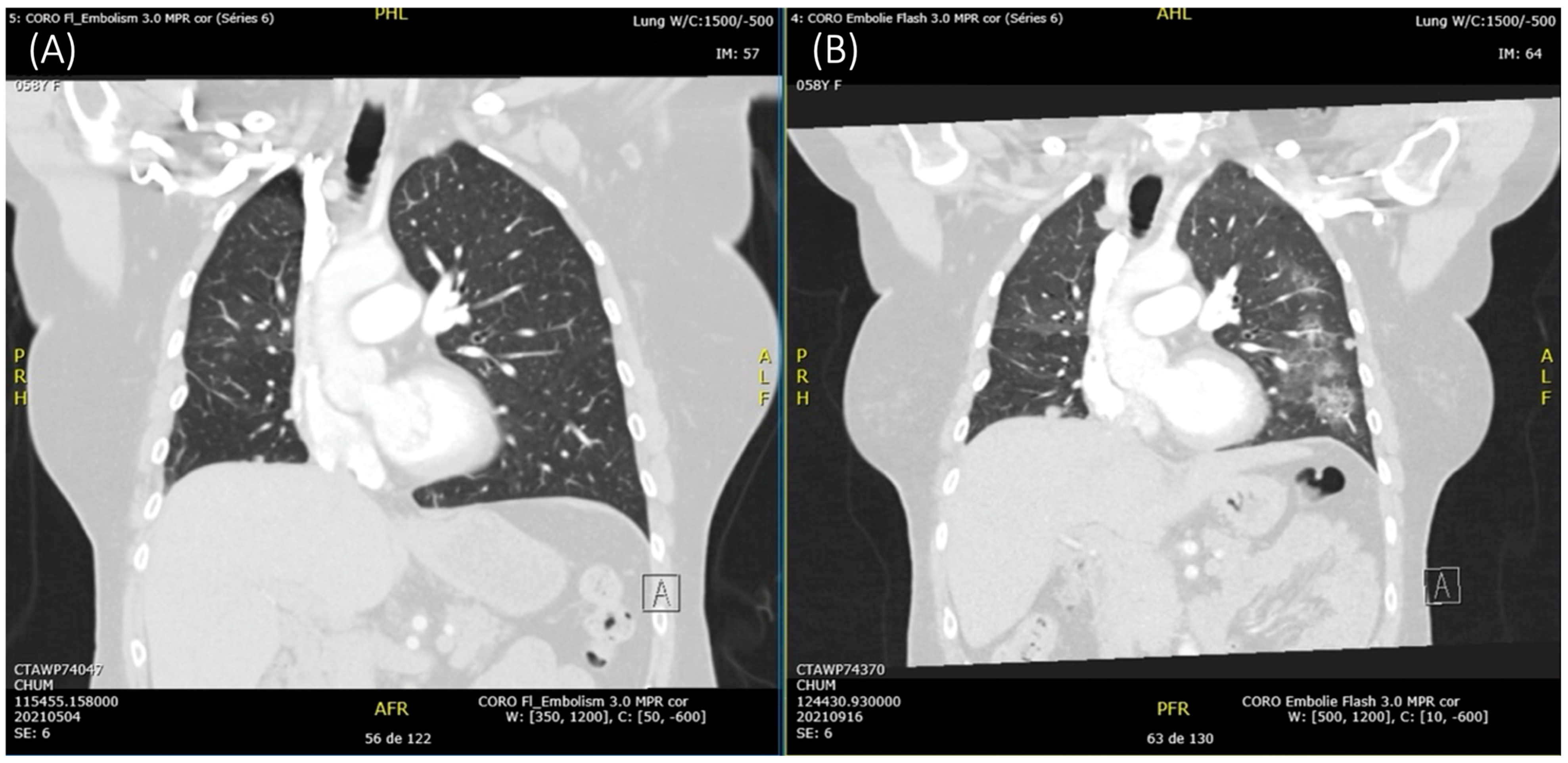
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statistics Canada. Lung Cancer Is the Leading Cause of Cancer Death in Canada. 2022. Available online: https://www.statcan.gc.ca/o1/en/plus/238-lung-cancer-leading-cause-cancer-death-canada (accessed on 28 January 2024).
- Tan, A.C.; Tan, D.S.W. Targeted Therapies for Lung Cancer Patients With Oncogenic Driver Molecular Alterations. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.L.; Thongprasert, S.; Yang, C.H.; Chu, D.T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Mitsudomi, T.; Morita, S.; Yatabe, Y.; Negoro, S.; Okamoto, I.; Tsurutani, J.; Seto, T.; Satouchi, M.; Tada, H.; Hirashima, T.; et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010, 11, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Leighl, N.B.; Wu, Y.L.; Zhong, W.Z. Emerging therapies for non-small cell lung cancer. J. Hematol. Oncol. 2019, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.; Kumarakulasinghe, N.B.; Huang, Y.Q.; Ang, Y.L.E.; Choo, J.R.; Goh, B.C.; Soo, R.A. Third generation EGFR TKIs: Current data and future directions. Mol. Cancer 2018, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, A.; Sharma, S.; Minari, R.; Perego, P.; Giovannetti, E.; Tiseo, M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer 2019, 121, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Uchibori, K.; Inase, N.; Araki, M.; Kamada, M.; Sato, S.; Okuno, Y.; Fujita, N.; Katayama, R. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat. Commun. 2017, 8, 14768. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, H.; Ma, L.; Yang, L.; Yang, G.; Zhang, S.; Ai, X.; Zhang, S.; Wang, Y. Possibility of brigatinib-based therapy, or chemotherapy plus anti-angiogenic treatment after resistance of osimertinib harboring EGFR T790M-cis-C797S mutations in lung adenocarcinoma patients. Cancer Med. 2021, 10, 8328–8337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zou, M.; Lv, J.; Han, Y.; Wang, G.; Wang, G. Effective treatment of pulmonary adenocarcinoma harboring triple EGFR mutations of L858R, T790M, and cis-C797S by osimertinib, bevacizumab, and brigatinib combination therapy: A case report. OncoTargets Ther. 2018, 11, 5545–5550. [Google Scholar] [CrossRef] [PubMed]
- Clinical Guidance Report for Brigatinib. 2021. Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2021/10230BrigatinibALK%2BNSCLC_fnCGR_NOREDACT_EC21Apr2021_final.pdf (accessed on 30 January 2024).
- Xing, P.; Hao, X.; Zhang, X.; Li, J. Efficacy and safety of brigatinib in ALK-positive non-small cell lung cancer treatment: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 920709. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, R.; Jiang, T.; Duan, D.; Wu, Y.; Li, C.; Li, Z.; Ni, R.; Li, L.; Liu, Y. Mechanism of Lethal Skin Toxicities Induced by Epidermal Growth Factor Receptor Inhibitors and Related Treatment Strategies. Front. Oncol. 2022, 12, 804212. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.-H.; Han, J.-Y.; Lee, J.-S.; Hochmair, M.J.; Li, J.Y.-C.; Chang, G.-C.; Lee, K.H.; et al. Brigatinib versus Crizotinib in ALK-Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.D.; Panchabhai, T.S. Pulmonary Toxicities of Targeted Therapy. In Handbook of Cancer Treatment-Related Symptoms and Toxicities, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 133–146. [Google Scholar] [CrossRef]
- Lee, B.J.; Lim, C.K.; Chang, H.T.; Zhang, J.H. Early-Onset Pulmonary Events with Combined Brigatinib and Afatinib Treatment of L858/cisT790M/cisC797S NSCLC: A Case Report. Am. J. Case Rep. 2022, 23, e937209. [Google Scholar] [CrossRef] [PubMed]
- LiverTox, Clinical and Research Information on Drug-Induced Liver Injury. 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548523/ (accessed on 15 November 2024).
- LiverTox, Clinical and Research Information on Drug-Induced Liver Injury. 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548495/ (accessed on 15 November 2024).
- Osimertinib. DrugBank. 2015. Available online: https://go.drugbank.com/ (accessed on 29 November 2023).
- Hendriks, L.E.; Schoenmaekers, J.; Zindler, J.D.; Eekers, D.B.; Hoeben, A.; De Ruysscher, D.K.; Dingemans, A.M. Safety of cranial radiotherapy concurrent with tyrosine kinase inhibitors in non-small cell lung cancer patients: A systematic review. Cancer Treat. Rev. 2015, 41, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Uchibori, K.; Oh-Hara, T.; Nomura, Y.; Suzuki, R.; Takemoto, A.; Araki, M.; Matsumoto, S.; Sagae, Y.; Kukimoto-Niino, M.; et al. A macrocyclic kinase inhibitor overcomes triple resistant mutations in EGFR-positive lung cancer. NPJ Precis. Oncol. 2024, 8, 46. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demers, D.; Florescu, M. Combination of Osimertinib and Brigatinib in the Treatment of EGFR Triple-Mutated Lung Adenocarcinoma: A Case Report. Curr. Oncol. 2025, 32, 270. https://doi.org/10.3390/curroncol32050270
Demers D, Florescu M. Combination of Osimertinib and Brigatinib in the Treatment of EGFR Triple-Mutated Lung Adenocarcinoma: A Case Report. Current Oncology. 2025; 32(5):270. https://doi.org/10.3390/curroncol32050270
Chicago/Turabian StyleDemers, Daphnée, and Marie Florescu. 2025. "Combination of Osimertinib and Brigatinib in the Treatment of EGFR Triple-Mutated Lung Adenocarcinoma: A Case Report" Current Oncology 32, no. 5: 270. https://doi.org/10.3390/curroncol32050270
APA StyleDemers, D., & Florescu, M. (2025). Combination of Osimertinib and Brigatinib in the Treatment of EGFR Triple-Mutated Lung Adenocarcinoma: A Case Report. Current Oncology, 32(5), 270. https://doi.org/10.3390/curroncol32050270



