Treatment Patterns, Clinical Outcomes and Quality of Life in BRCA1/2-Associated Breast Cancer Patients: A Retrospective Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Statistical Analyses
3. Results
3.1. Cohort Description
3.2. Histopathological Characteristics
3.3. Treatment Regimens
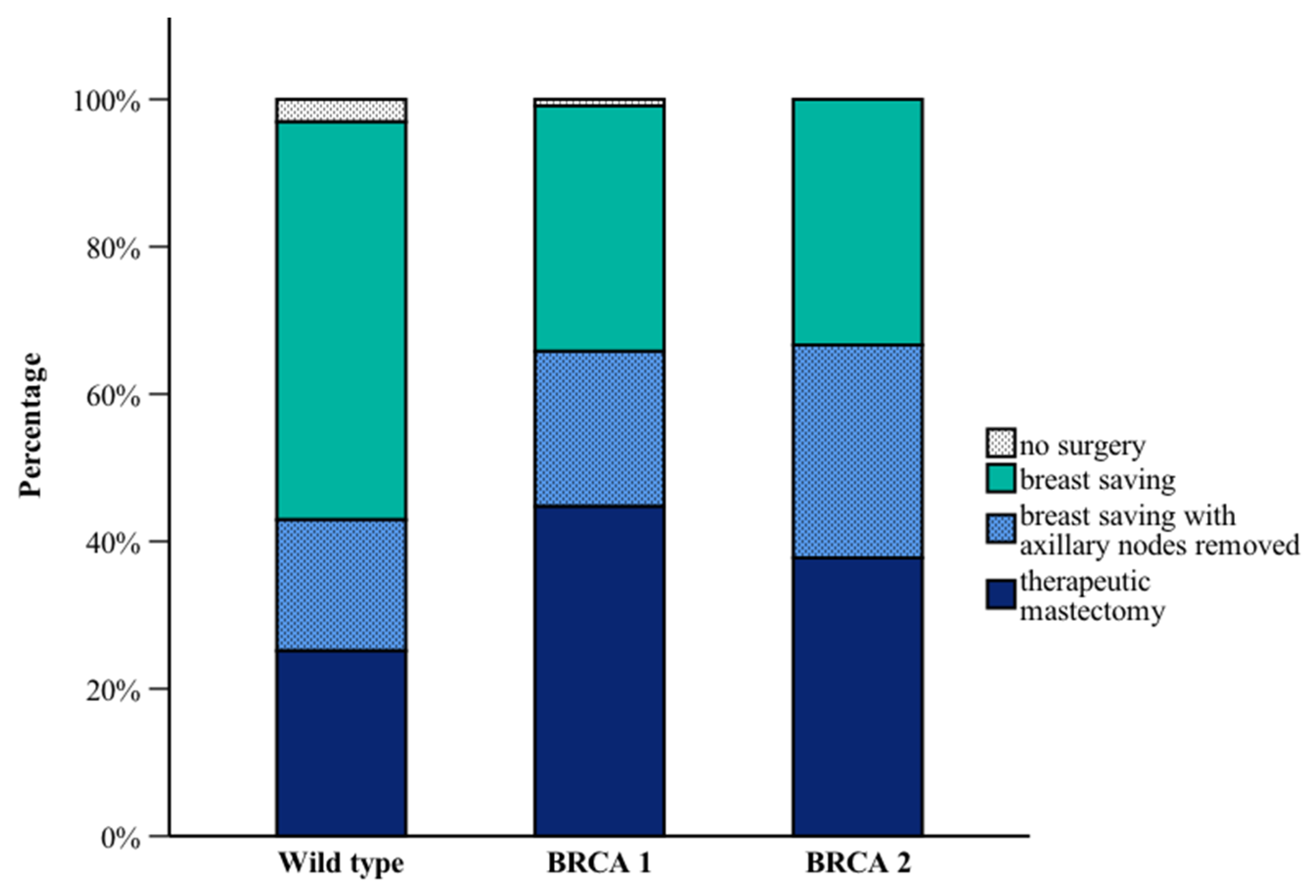
3.4. Surgical Interventions
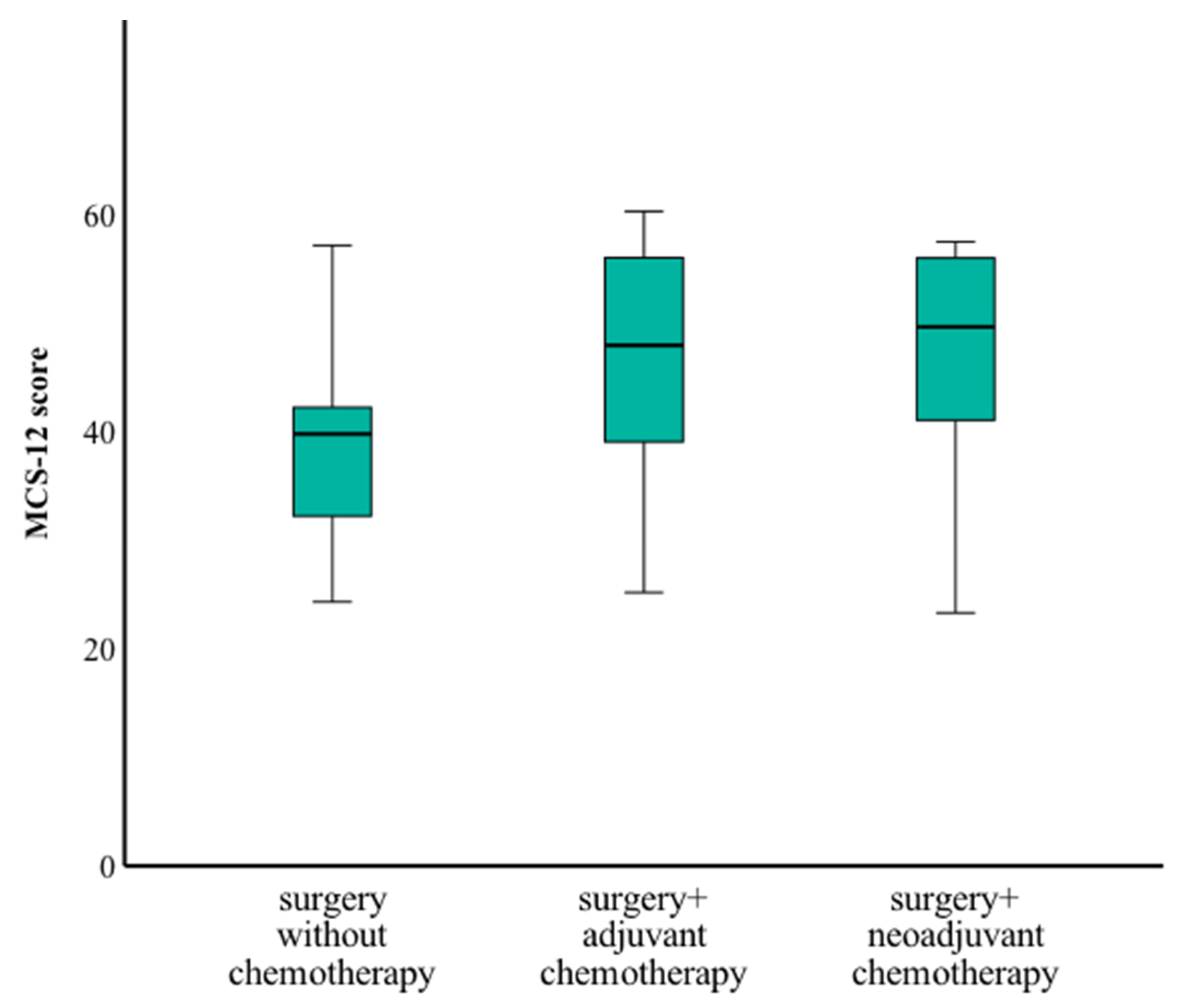
3.5. Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Risk Genes—Association Analysis in More than 113,000 Women. N. Engl. J. Med. 2021, 384, 428–439. [CrossRef]
- Byrski, T.; Gronwald, J.; Huzarski, T.; Grzybowska, E.; Budryk, M.; Stawicka, M.; Mierzwa, T.; Szwiec, M.; Wisniowski, R.; Siolek, M.; et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J. Clin. Oncol. 2010, 28, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Rennert, G.; Bisland-Naggan, S.; Barnett-Griness, O.; Bar-Joseph, N.; Zhang, S.; Rennert, H.S.; Narod, S.A. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N. Engl. J. Med. 2007, 357, 115–123. [Google Scholar] [CrossRef]
- Schmidt, M.K.; van den Broek, A.J.; Tollenaar, R.A.; Smit, V.T.; Westenend, P.J.; Brinkhuis, M.; Oosterhuis, W.J.; Wesseling, J.; Janssen-Heijnen, M.L.; Jobsen, J.J.; et al. Breast Cancer Survival of BRCA1/BRCA2 Mutation Carriers in a Hospital-Based Cohort of Young Women. J. Natl. Cancer Inst. 2017, 109, djw329. [Google Scholar] [CrossRef] [PubMed]
- Huzarski, T.; Byrski, T.; Gronwald, J.; Gorski, B.; Domagala, P.; Cybulski, C.; Oszurek, O.; Szwiec, M.; Gugala, K.; Stawicka, M.; et al. Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J. Clin. Oncol. 2013, 31, 3191–3196. [Google Scholar] [CrossRef]
- Bordeleau, L.; Panchal, S.; Goodwin, P. Prognosis of BRCA-associated breast cancer: A summary of evidence. Breast Cancer Res. Treat. 2010, 119, 13–24. [Google Scholar] [CrossRef]
- van den Broek, A.J.; Schmidt, M.K.; van ‘t Veer, L.J.; Tollenaar, R.A.; van Leeuwen, F.E. Worse breast cancer prognosis of BRCA1/BRCA2 mutation carriers: What’s the evidence? A systematic review with meta-analysis. PLoS ONE 2015, 10, e0120189. [Google Scholar] [CrossRef]
- Baretta, Z.; Mocellin, S.; Goldin, E.; Olopade, O.I.; Huo, D. Effect of BRCA germline mutations on breast cancer prognosis: A systematic review and meta-analysis. Medicine 2016, 95, e4975. [Google Scholar] [CrossRef]
- Stenehjem, D.D.; Telford, C.; Unni, S.K.; Bauer, H.; Sainski, A.; Deka, R.; Schauerhamer, M.B.; Ye, X.; Tak, C.R.; Ma, J.; et al. BRCA testing and outcomes in women with breast cancer. Breast Cancer Res. Treat. 2021, 186, 839–850. [Google Scholar] [CrossRef]
- Tutt, A.; Tovey, H.; Cheang, M.C.U.; Kernaghan, S.; Kilburn, L.; Gazinska, P.; Owen, J.; Abraham, J.; Barrett, S.; Barrett-Lee, P.; et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: The TNT Trial. Nat. Med. 2018, 24, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Copson, E.R.; Maishman, T.C.; Tapper, W.J.; Cutress, R.I.; Greville-Heygate, S.; Altman, D.G.; Eccles, B.; Gerty, S.; Durcan, L.T.; Jones, L.; et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): A prospective cohort study. Lancet Oncol. 2018, 19, 169–180. [Google Scholar] [CrossRef]
- Friedlander, M.; Gebski, V.; Gibbs, E.; Davies, L.; Bloomfield, R.; Hilpert, F.; Wenzel, L.B.; Eek, D.; Rodrigues, M.; Clamp, A.; et al. Health-related quality of life and patient-centred outcomes with olaparib maintenance after chemotherapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT Ov-21): A placebo-controlled, phase 3 randomised trial. Lancet Oncol. 2018, 19, 1126–1134. [Google Scholar] [CrossRef]
- Robson, M.; Ruddy, K.J.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Li, W.; Tung, N.; Armstrong, A.; et al. Patient-reported outcomes in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer receiving olaparib versus chemotherapy in the OlympiAD trial. Eur. J. Cancer 2019, 120, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Dibble, K.E.; Donorfio, L.K.M.; Britner, P.A.; Bellizzi, K.M. Stress, anxiety, and health-related quality of life in BRCA1/2-positive women with and without cancer: A comparison of four US female samples. Gynecol. Oncol. Rep. 2022, 42, 101033. [Google Scholar] [CrossRef]
- Ettl, J.; Quek, R.G.W.; Lee, K.H.; Rugo, H.S.; Hurvitz, S.; Goncalves, A.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Quality of life with talazoparib versus physician’s choice of chemotherapy in patients with advanced breast cancer and germline BRCA1/2 mutation: Patient-reported outcomes from the EMBRACA phase III trial. Ann. Oncol. 2018, 29, 1939–1947. [Google Scholar] [CrossRef]
- D’Alonzo, M.; Piva, E.; Pecchio, S.; Liberale, V.; Modaffari, P.; Ponzone, R.; Biglia, N. Satisfaction and Impact on Quality of Life of Clinical and Instrumental Surveillance and Prophylactic Surgery in BRCA-mutation Carriers. Clin. Breast Cancer 2018, 18, e1361–e1366. [Google Scholar] [CrossRef] [PubMed]
- Howard-Anderson, J.; Ganz, P.A.; Bower, J.E.; Stanton, A.L. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: A systematic review. J. Natl. Cancer Inst. 2012, 104, 386–405. [Google Scholar] [CrossRef] [PubMed]
- Neuner, J.M.; Zokoe, N.; McGinley, E.L.; Pezzin, L.E.; Yen, T.W.; Schapira, M.M.; Nattinger, A.B. Quality of life among a population-based cohort of older patients with breast cancer. Breast 2014, 23, 609–616. [Google Scholar] [CrossRef]
- Ware, J.E.; Kosinski, M.; Turner-Bowker, D.M.; Gandek, B. How to Score Version 2 of the SF-12 Health Survey (with a Supplement Documenting Version 1); QualityMetric Incorporated: Lincoln, RI, USA, 2005. [Google Scholar]
- Bullinger, M.; Der, K.I. SF-36 Fragebogen zum Gesundheitszustand. Handbuch für die Deutschsprachige Fragebogenversion; Hogrefe Verlag für Psychologie: Göttingen, Germany, 1998. [Google Scholar]
- Friedenson, B. BRCA1 and BRCA2 pathways and the risk of cancers other than breast or ovarian. MedGenMed 2005, 7, 60. [Google Scholar]
- Verhoog, L.C.; Brekelmans, C.T.; Seynaeve, C.; van den Bosch, L.M.; Dahmen, G.; van Geel, A.N.; Tilanus-Linthorst, M.M.; Bartels, C.C.; Wagner, A.; van den Ouweland, A.; et al. Survival and tumour characteristics of breast-cancer patients with germline mutations of BRCA1. Lancet 1998, 351, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Singer, C.F.; Tan, Y.Y.; Muhr, D.; Rappaport, C.; Gschwantler-Kaulich, D.; Grimm, C.; Polterauer, S.; Pfeiler, G.; Berger, A.; Tea, M.M. Association between family history, mutation locations, and prevalence of BRCA1 or 2 mutations in ovarian cancer patients. Cancer Med. 2019, 8, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Blondeaux, E.; Tomasello, L.M.; Agostinetto, E.; Hamy, A.-S.; Kim, H.J.; Franzoi, M.A.B.; Bernstein-Molho, R.; Hilbers, F.; Pogoda, K.; et al. Clinical behavior of breast cancer in young BRCA carriers and prognostic impact of the timing of genetic testing: Results from an international cohort study. J. Clin. Oncol. 2024, 42, 10503. [Google Scholar] [CrossRef]
- Yadav, S.; Boddicker, N.J.; Na, J.; Polley, E.C.; Hu, C.; Hart, S.N.; Gnanaolivu, R.D.; Larson, N.; Holtegaard, S.; Huang, H.; et al. Contralateral Breast Cancer Risk Among Carriers of Germline Pathogenic Variants in ATM, BRCA1, BRCA2, CHEK2, and PALB2. J. Clin. Oncol. 2023, 41, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Mylavarapu, S.; Das, A.; Roy, M. Role of BRCA Mutations in the Modulation of Response to Platinum Therapy. Front. Oncol. 2018, 8, 16. [Google Scholar] [CrossRef]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; Azambuja, E.d.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef]
- Geyer, C.E., Jr.; Garber, J.E.; Gelber, R.D.; Yothers, G.; Taboada, M.; Ross, L.; Rastogi, P.; Cui, K.; Arahmani, A.; Aktan, G.; et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann. Oncol. 2022, 33, 1250–1268. [Google Scholar] [CrossRef]
- Manning, A.T.; Wood, C.; Eaton, A.; Stempel, M.; Capko, D.; Pusic, A.; Morrow, M.; Sacchini, V. Nipple-sparing mastectomy in patients with BRCA1/2 mutations and variants of uncertain significance. Br. J. Surg. 2015, 102, 1354–1359. [Google Scholar] [CrossRef]
- Shubeck, S.; Sevilimedu, V.; Berger, E.; Robson, M.; Heerdt, A.S.; Pilewskie, M.L. Comparison of Outcomes Between BRCA Pathogenic Variant Carriers Undergoing Breast-Conserving Surgery Versus Mastectomy. Ann. Surg. Oncol. 2022, 29, 4706–4713. [Google Scholar] [CrossRef]
- Valachis, A.; Nearchou, A.D.; Lind, P. Surgical management of breast cancer in BRCA-mutation carriers: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2014, 144, 443–455. [Google Scholar] [CrossRef]
- Friedlander, M.; Lee, Y.C.; Tew, W.P. Managing Adverse Effects Associated with Poly (ADP-ribose) Polymerase Inhibitors in Ovarian Cancer: A Synthesis of Clinical Trial and Real-World Data. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e390876. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Quek, R.G.W.; Turner, N.C.; Telli, M.L.; Rugo, H.S.; Mailliez, A.; Ettl, J.; Grischke, E.; Mina, L.A.; Balmaña, J.; et al. Quality of life with talazoparib after platinum or multiple cytotoxic non-platinum regimens in patients with advanced breast cancer and germline BRCA1/2 mutations: Patient-reported outcomes from the ABRAZO phase 2 trial. Eur. J. Cancer 2018, 104, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.J.; Crawford-Williams, F.; Crichton, M.; Joseph, R.; Hart, N.H.; Milley, K.; Druce, P.; Zhang, J.; Jefford, M.; Lisy, K.; et al. Effectiveness and implementation of models of cancer survivorship care: An overview of systematic reviews. J. Cancer Surviv. 2023, 17, 197–221. [Google Scholar] [CrossRef] [PubMed]
- Vaz-Luis, I.; Masiero, M.; Cavaletti, G.; Cervantes, A.; Chlebowski, R.T.; Curigliano, G.; Felip, E.; Ferreira, A.R.; Ganz, P.A.; Hegarty, J.; et al. ESMO Expert Consensus Statements on Cancer Survivorship: Promoting high-quality survivorship care and research in Europe. Ann. Oncol. 2022, 33, 1119–1133. [Google Scholar] [CrossRef]

| BRCA-WT N (%) 171 (50.6%) | BRCA1 N (%) 120 (35.5%) | BRCA2 N (%) 47 (13.9%) | p-Value | ||
|---|---|---|---|---|---|
| Age at disease onset, median years | 48.0 | 41.5 | 46.0 | Post hoc: WT vs. BRCA1: <0.001 WT vs. BRCA2: 0.178 BRCA1 vs. 2: 0.080 | |
| Age at local recurrence, median years | 58.0 | 50.0 | 54.5 | 0.071 | |
| Age at contralateral BC, median years | 61.0 | 47.0 | 52.5 | Post hoc: WT vs. BRCA1: 0.026 WT vs. BRCA2: 0.999 BRCA1 vs. 2: 0.993 | |
| Molecular subtype | TNBC | 56 (34.6) | 75 (78.1) | 12 (31.6) | Post hoc: WT vs. BRCA1: <0.001 WT vs. BRCA2: 0.999 BRCA1 vs. 2: <0.001 |
| ER/PR-pos, HER2 neg | 72 (44.5) | 16 (16.7) | 20 (52.6) | ||
| HER2-pos | 25 (15.4) | 5 (5.2) | 4 (10.5) | ||
| ER-pos/PR-neg/HER2-neg | 9 (5.5) | 0 | 2 (5.3) | ||
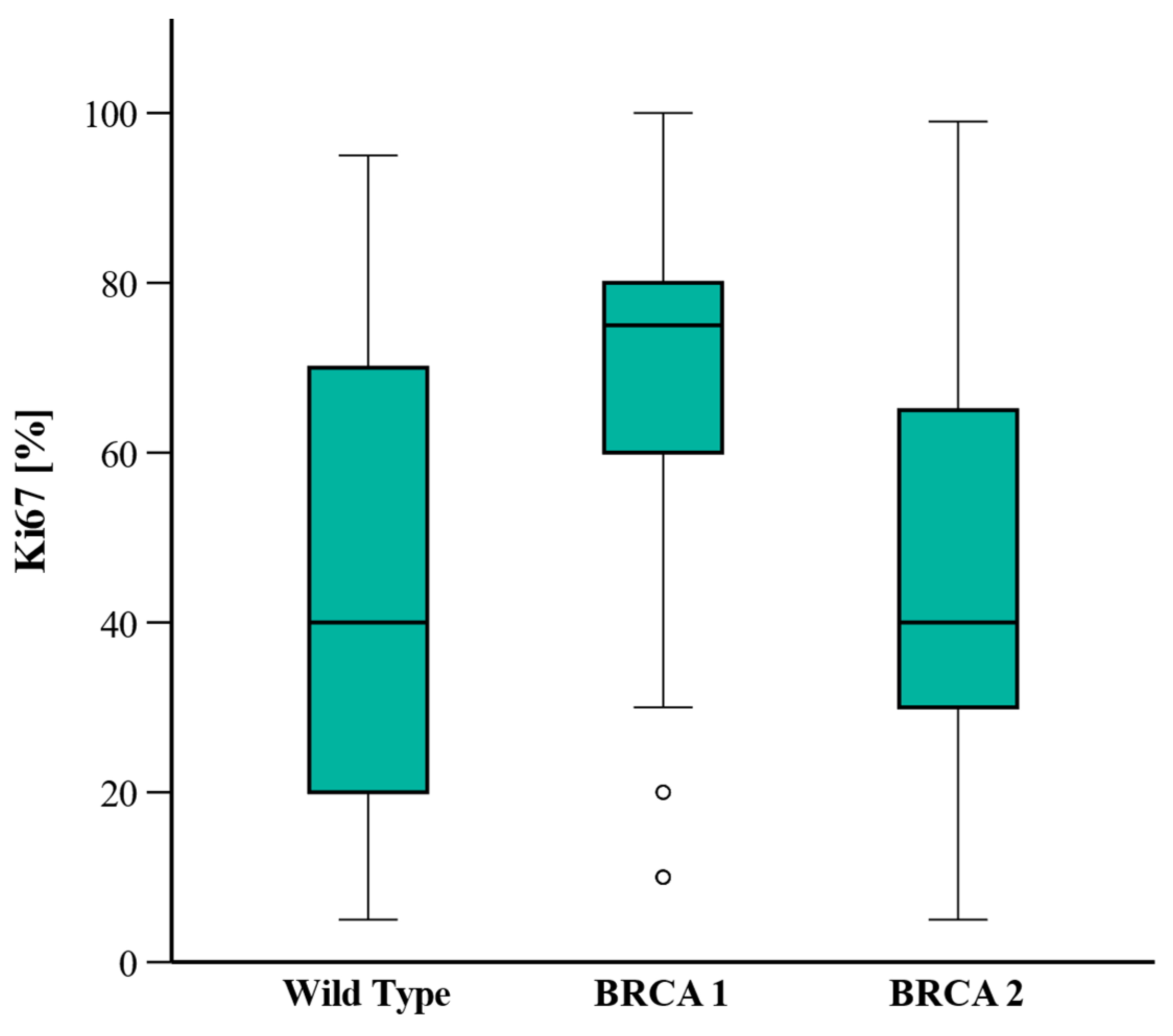
| Ki-67, percent (range) | 40 (20–70) | 75 (60–80) | 40 (30–70) | Post hoc: WT vs. BRCA1: <0.001 WT vs. BRCA2: 0.561 BRCA1 vs. 2: 0.591 | |
| Time to local recurrence, median months (range) | 130.0 (12–407) | 93.5 (16–258) | 97.0 (25–288) | 0.661 | |
| Time to contralateral BC, median months (range) | 132.0 (22–373) | 106.0 (25–186) | 124.5 (25–172) | 0.502 | |
| pCR | Complete response | 16 (29.6) | 19 (45.2) | 7 (41.2) | |
| Partial response | 28 (51.9) | 22 (52.3) | 6 (35.3) | 0.070 | |
| No response | 10 (18.5) | 1 (2.5) | 4 (23.5) | ||
| Deceased | 22 (12.9) | 16 (13.3) | 6 (12.8) | 0.992 | |
| BRCA-WT N (%) 171 (50.6%) | BRCA1 N (%) 120 (35.5%) | BRCA2 N (%) 47 (13.9%) | p-Value | ||
|---|---|---|---|---|---|
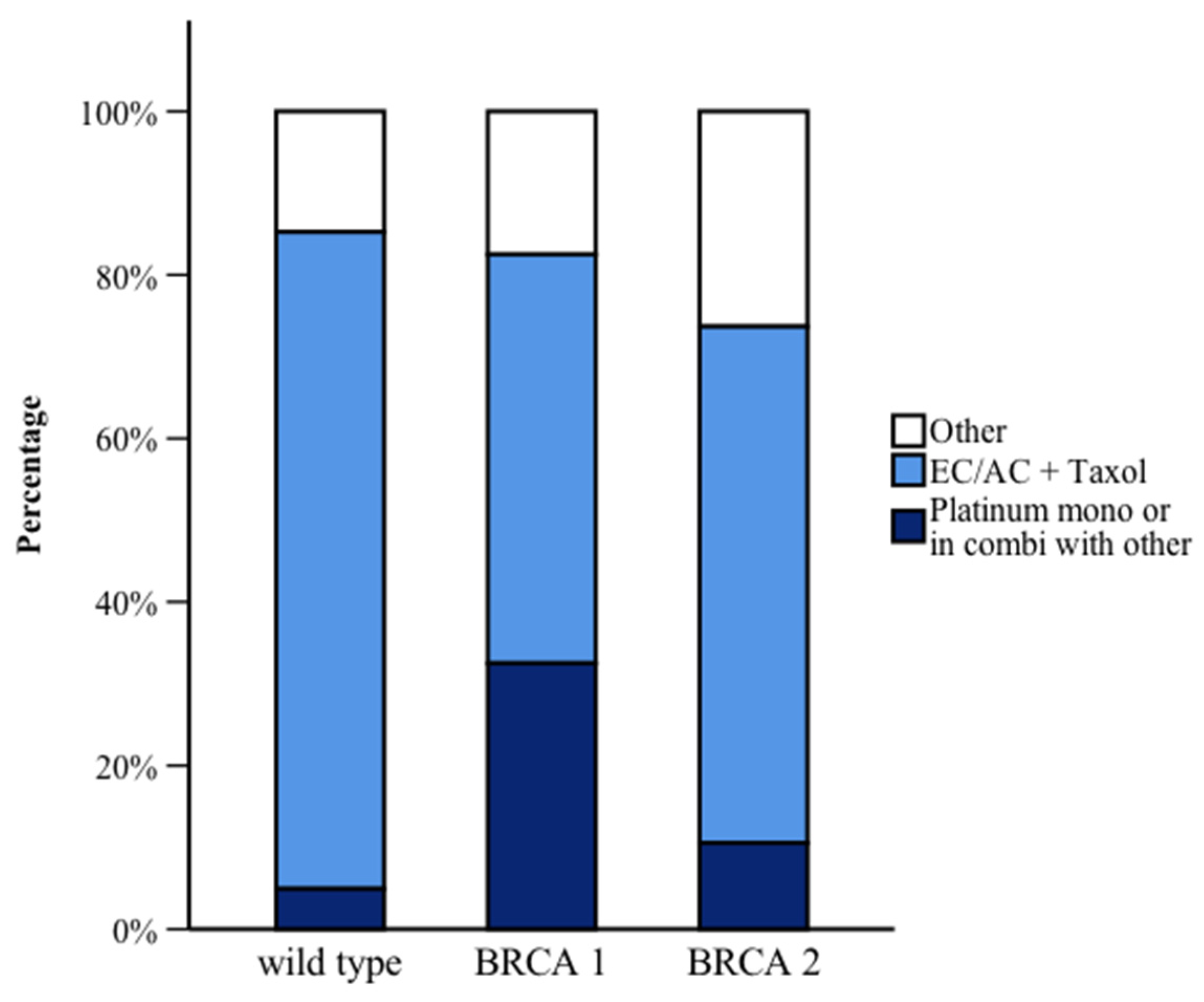
| Adjuvant chemotherapy | no | 135 (81.3) | 65 (59.1) | 29 (69.0) | Post hoc WT vs. BRCA1: <0.001 WT vs. BRCA2: 0.246 BRCA1 vs. BRCA2: 0.756 |
| yes | 31 (18.7) | 45 (40.9) | 13 (31.0) | ||
| Neoadjuvant chemotherapy | no | 98 (59.4) | 55 (51.9) | 20 (47.6) | 0.270 |
| yes | 67 (40.6) | 51 (48.1) | 22 (52.4) | ||
| PARP inhibitor 1 | no | - | 105 (95.5) | 33 (82.5) | BRCA1 vs. BRCA2: 0.016 |
| yes | - | 5 (4.5) | 7 (17.5) | ||
| Anti-hormonal treatment | no | 70 (45.2) | 88 (83.0) | 16 (43.2) | Post hoc WT vs. BRCA1: <0.001 WT vs. BRCA2: 1.000 BRCA1 vs. BRCA2: <0.001 |
| yes | 85 (54.8) | 18 (17.0) | 21 (56.8) | ||
| Trastuzumab | no | 140 (87.5) | 109 (98.2) | 37 (94.9) | Post hoc: WT vs. BRCA1: 0.006 WT vs. BRCA2: 0.777 BRCA1 vs. BRCA2: 0.831 |
| yes | 20 (12.5) | 2 (1.8) | 2 (5.1) | ||
| Number of adjuvant chemotherapy cycles | Median (range) | 6 (3–9) | 6 (2–9) | 8 (3–9) | 0.584 |
| Number of neoadjuvant chemotherapy cycles | Median (range) | 8 (2–8) | 7 (3–9) | 8 (3–9) | 0.178 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parger, A.-M.; Gebhart, P.; Muhr, D.; Singer, C.F.; Tan, Y.Y. Treatment Patterns, Clinical Outcomes and Quality of Life in BRCA1/2-Associated Breast Cancer Patients: A Retrospective Analysis. Curr. Oncol. 2025, 32, 269. https://doi.org/10.3390/curroncol32050269
Parger A-M, Gebhart P, Muhr D, Singer CF, Tan YY. Treatment Patterns, Clinical Outcomes and Quality of Life in BRCA1/2-Associated Breast Cancer Patients: A Retrospective Analysis. Current Oncology. 2025; 32(5):269. https://doi.org/10.3390/curroncol32050269
Chicago/Turabian StyleParger, Anna-Maria, Paulina Gebhart, Daniela Muhr, Christian F. Singer, and Yen Y. Tan. 2025. "Treatment Patterns, Clinical Outcomes and Quality of Life in BRCA1/2-Associated Breast Cancer Patients: A Retrospective Analysis" Current Oncology 32, no. 5: 269. https://doi.org/10.3390/curroncol32050269
APA StyleParger, A.-M., Gebhart, P., Muhr, D., Singer, C. F., & Tan, Y. Y. (2025). Treatment Patterns, Clinical Outcomes and Quality of Life in BRCA1/2-Associated Breast Cancer Patients: A Retrospective Analysis. Current Oncology, 32(5), 269. https://doi.org/10.3390/curroncol32050269







