Real-World Treatment Patterns and Survival Outcomes of Patients with Relapsed/Refractory Multiple Myeloma Treated with a Selinexor-Containing Triplet-Based Regimen
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
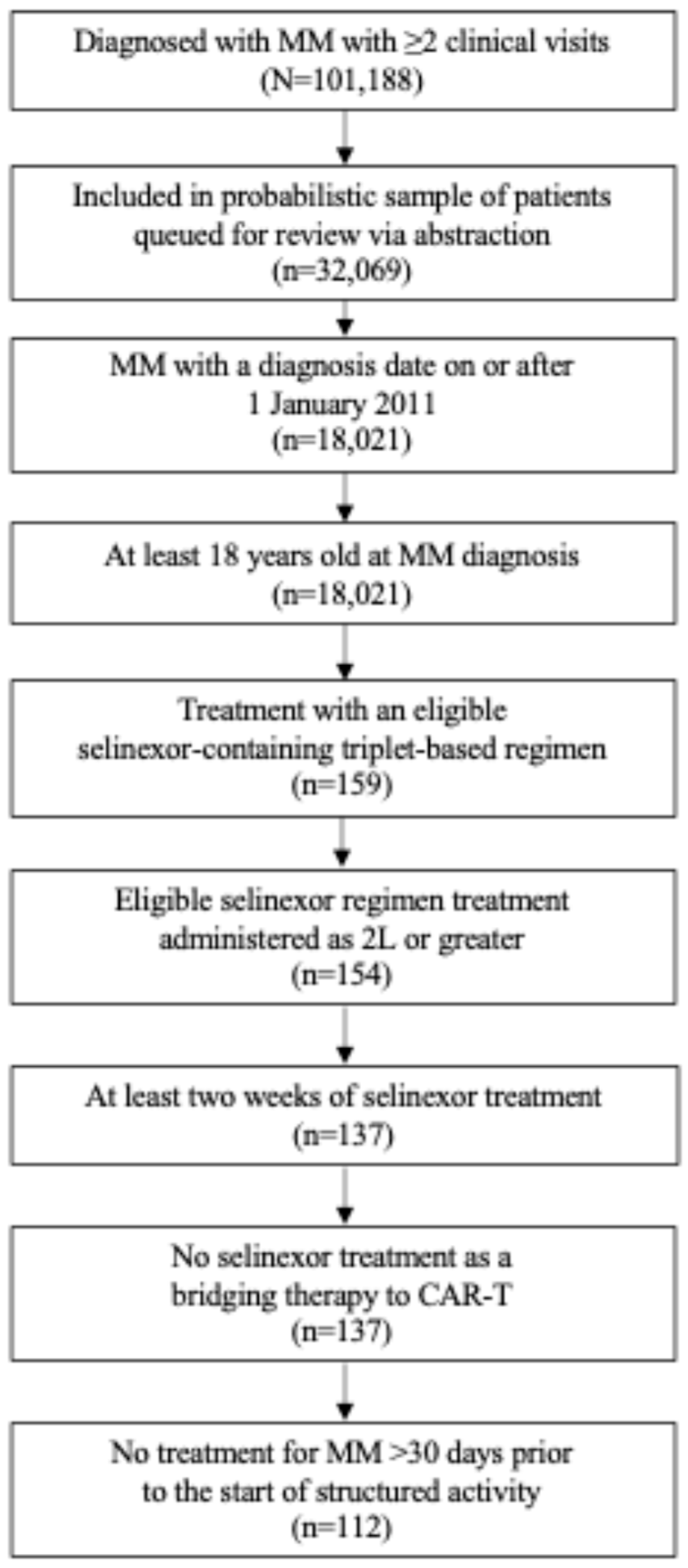
2.2. Eligibility
2.3. Data Source
2.4. Endpoints and Assessments
2.5. Statistical Analysis
3. Results
3.1. Patients
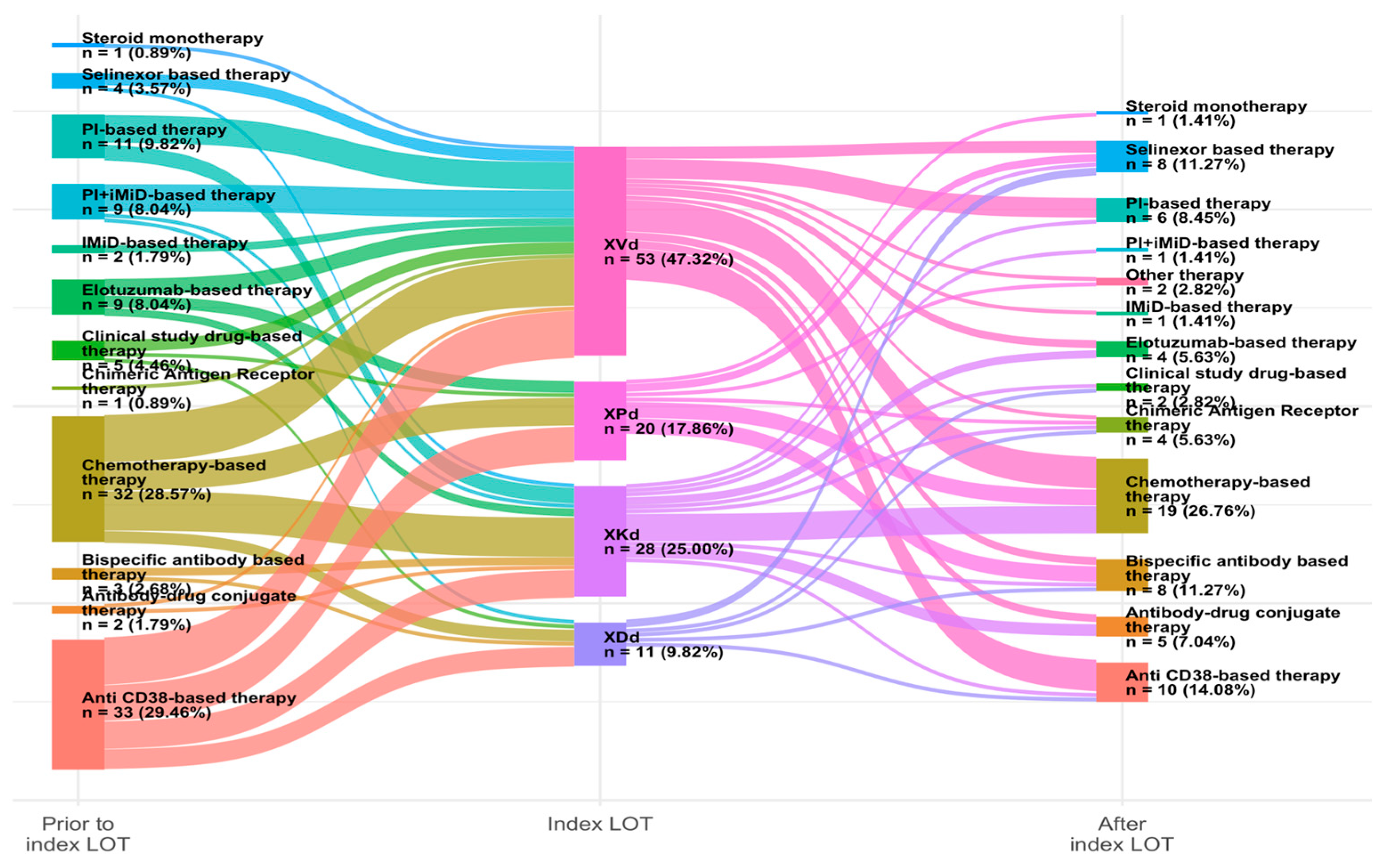
3.2. Treatment Regimens
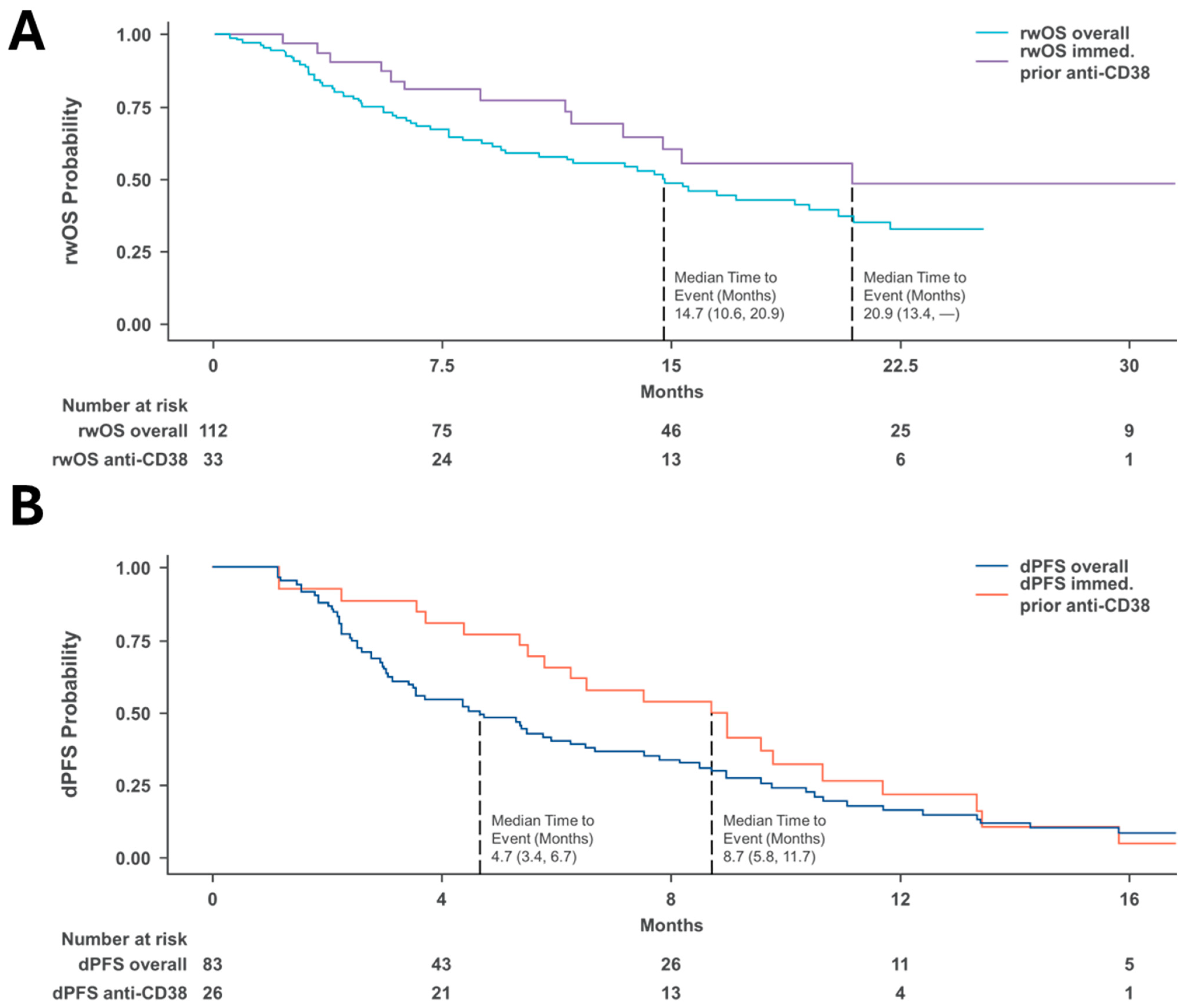
3.3. Survival Outcomes in Real-World Clinical Settings
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.K.; Callander, N.S.; Adekola, K.; Anderson, L.D.; Baljevic, M.; Baz, R.; Campagnaro, E.; Castillo, J.J.; Costello, C.; D’Angelo, C.; et al. Multiple Myeloma, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. JNCCN J. Natl. Compr. Cancer Netw. 2023, 21, 1281–1301. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, K.; Gay, F.; Weisel, K.; Zweegman, S.; Mateos, M.V.; Richardson, P. Improving outcomes for patients with relapsed multiple myeloma. Blood Rev. 2021, 49, 10080. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Barlogie, B.; Berenson, J.; Singhal, S.; Jagannath, S.; Irwin, D.; Rajkumar, S.V.; Srkalovic, G.; Alsina, M.; Alexanian, R.; et al. A Phase 2 Study of Bortezomib in Relapsed, Refractory Myeloma. N. Engl. J. Med. 2003, 348, 2609–2617. [Google Scholar] [CrossRef]
- Siegel, D.S.; Martin, T.; Wang, M.; Vij, R.; Jakubowiak, A.J.; Lonial, S.; Trudel, S.; Kukreti, V.; Bahlis, N.; Alsina, M.; et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood 2012, 120, 2817–2825. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; Jackson, S.R.; et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 374, 1621–1634. [Google Scholar] [CrossRef]
- Baljevic, M.; Orlowski, R.Z. Pharmacodynamics and pharmacokinetics of proteasome inhibitors for the treatment of multiple myeloma. Expert Opin. Drug Metab. Toxicol. 2019, 15, 459–473. [Google Scholar] [CrossRef]
- McCarthy, P.L.; Owzar, K.; Hofmeister, C.C.; Hurd, D.D.; Hassoun, H.; Richardson, P.G.; Giralt, S.; Stadtmauer, E.A.; Weisdorf, D.J.; Vij, R.; et al. Lenalidomide after Stem-Cell Transplantation for Multiple Myeloma. N. Engl. J. Med. 2012, 366, 1770–1781. [Google Scholar] [CrossRef]
- Richardson, P.G.; Siegel, D.S.; Vij, R.; Hofmeister, C.C.; Baz, R.; Jagannath, S.; Chen, C.; Lonial, S.; Jakubowiak, A.; Bahlis, N.; et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: A randomized phase 2 study. Blood 2014, 123, 1826–1832. [Google Scholar] [CrossRef]
- Miguel, J.S.; Weisel, K.; Moreau, P.; Lacy, M.; Song, K.; Delforge, M.; Karlin, L.; Goldschmidt, H.; Banos, A.; Oriol, A.; et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 1055–1066. [Google Scholar] [CrossRef]
- Holstein, S.A.; McCarthy, P.L. Immunomodulatory Drugs in Multiple Myeloma: Mechanisms of Action and Clinical Experience. Drugs 2017, 77, 505–520. [Google Scholar] [CrossRef]
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V.; et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 754–766. [Google Scholar] [CrossRef]
- Attal, M.; Richardson, P.G.; Rajkumar, S.V.; San-Miguel, J.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; Dimopoulos, M.A.; et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): A randomised, multicentre, open-label, phase 3 study. Lancet 2019, 394, 2096–2107. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Oriol, A.; Nahi, H.; San-Miguel, J.; Bahlis, N.J.; Usmani, S.Z.; Rabin, N.; Orlowski, R.Z.; Komarnicki, M.; Suzuki, K.; et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 1319–1331. [Google Scholar] [CrossRef]
- Nooka, A.K.; Kaufman, J.L.; Hofmeister, C.C.; Joseph, N.S.; Heffner, T.L.; Gupta, V.A. Daratumumab in multiple myeloma. Cancer 2019, 125, 2364–2382. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Rajkumar, V.; Kyle, R.A.; Van Duin, M.; Sonneveld, P.; Mateos, M.V.; Gay, F.; Anderson, K.C. Multiple myeloma. Nat. Rev. Dis. Primers 2017, 3, 17046. [Google Scholar] [CrossRef] [PubMed]
- Stalker, M.E.; Mark, T.M. Clinical Management of Triple-Class Refractory Multiple Myeloma: A Review of Current Strategies and Emerging Therapies. Curr. Oncol. 2022, 29, 4464–4477. [Google Scholar] [CrossRef]
- Mikhael, J. Treatment Options for Triple-class Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2020, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Richardson, P.; Lonial, S. Treatment Options for Patients with Heavily Pretreated Relapsed and Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2022, 20, 1–7. [Google Scholar]
- Dang, A. Real-World Evidence: A Primer. Pharm. Med. Adis 2023, 37, 25–36. [Google Scholar] [CrossRef]
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of Patients with Multiple Myeloma Refractory to CD38-Targeted Monoclonal Antibody Therapy. Leukemia 2019, 33, 2266–2275. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6820050/ (accessed on 9 April 2025). [CrossRef]
- De Acha, O.P.; Reiman, L.; Jayabalan, D.S.; Walker, Z.J.; Bosma, G.; Keller, A.L.; Parzych, S.E.; Abbott, D.; Idler, B.M.; Ribadeneyra, D.; et al. CD38 antibody re-treatment in daratumumab-refractory multiple myeloma after time on other therapies. Blood Adv. 2023, 7, 6430–6440. [Google Scholar] [CrossRef] [PubMed]
- Sudalagunta, P.R.; Canevarolo, R.R.; Meads, M.B.; Silva, M.; Zhao, X.; Cubitt, C.L.; Sansil, S.S.; DeAvila, G.; Alugubelli, R.R.; Bishop, R.T.; et al. The Functional Transcriptomic Landscape Informs Therapeutic Strategies in Multiple Myeloma. Cancer Res. 2024, 85, 378–398. [Google Scholar] [CrossRef] [PubMed]
- Schiller, G.J.; Lipe, B.C.; Bahlis, N.J.; Tuchman, S.A.; Bensinger, W.I.; Sutherland, H.J.; Lentzsch, S.; Baljevic, M.; White, D.; Kotb, R.; et al. Selinexor-Based Triplet Regimens in Patients with Multiple Myeloma Previously Treated with Anti-CD38 Monoclon Antibodies. Clin. Lymphoma Myeloma Leuk. 2023, 23, e286–e296.e4. [Google Scholar] [CrossRef]
- Birnbaum, B.; Nussbaum, N.; Seidl-Rathkopf, K.; Agrawal, M.; Estevez, M.; Estola, E.; SHaimson, J.; He, L.; Larson, P.; Richardson, P. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv 2020, arXiv:2001.09765. [Google Scholar]
- Ma, X.; Long, L.; Moon, S.; Adamson, B.J.S.; Baxi, S.S. Comparison of Population Characteristics in Real-World Clinical Oncology Databases in the US: Flatiron Health, SEER, and NPCR. Medrxiv 2020. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Wang, J.R.; Hayden, J.; Yue, Y.; Meyer, C.S.; Gan, R.W.; Zhang, Y.; Ackerman, B.; Mohanty, P.; Roose, J.; Lund, J.L.; et al. Design and Methodological Considerations for Real World Data-Derived Progression-Free Survival in Multiple Myeloma. Blood 2024, 144, 3763. [Google Scholar] [CrossRef]
- Ackerman, B.; Gan, R.W.; Meyer, C.S.; Wang, J.R.; Zhang, Y.; Hayden, J.; Mahoney, G.; Lund, J.L.; Weberpals, J.; Schneeweiss, S.; et al. Measurement Error and Bias in Real-World Oncology Endpoints when Constructing External Control Arms. Front. Drug Saf. Regul. 2024, 4, 1423493. [Google Scholar] [CrossRef]
- Cornell, R.; Hari, P.; Tang, S.; Biran, N.; Callander, N.; Chari, A.; Chhabra, S.; Fiala, M.A.; Gahvari, Z.; Gandhi, U.; et al. Overall survival of patients with triple-class refractory multiple myeloma treated with selinexor plus dexamethasone vs standard of care in MAMMOTH. Am. J. Hematol. 2021, 96, E5–E8. [Google Scholar] [CrossRef]
- White, D.; Schiller, G.J.; Madan, S.; Lentzsch, S.; Chubar, E.; Lavi, N.; Van Domelen, D.R.; Bentur, O.S.; Baljevic, M. Efficacy and safety of once weekly selinexor 40 mg versus 60 mg with pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Front. Oncol. 2024, 14, 1352281. [Google Scholar] [CrossRef]
- Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Yee, A.J.; Huff, C.A.; Moreau, P.; Dingli, D.; Cole, C.; Lonial, S.; et al. Oral Selinexor–Dexamethasone for Triple-Class Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 381, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Binder, A.F.; Walker, C.J.; Mark, T.M.; Baljevic, M. Impacting T-cell fitness in multiple myeloma: Potential roles for selinexor and XPO1 inhibitors. Front. Immunol. 2023, 14, 1275329. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.J.; Banerjee, R.; Mian, H.; Weisel, K.; Bal, S.; Derman, B.A.; Htut, M.M.; Nagarajan, C.; Rodriguez, C.; Richter, J.; et al. International Myeloma Working Group Immunotherapy Committee Recommendation on Sequencing Immunotherapy for Treatment of Multiple Myeloma: Multiple myeloma, gammopathies. Leukemia 2025, 39, 543–554. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Characteristic, n (%) | Overall (n = 112) | Anti-CD38 mAb Treatment Immediately Prior to Selinexor-Triplet Regimen (n = 33) |
|---|---|---|
| Age at index (year), median (IQR) | 69 (64, 76) | 69 (68, 77) |
| Age, years * | ||
| <65 | 38 (34) | 8 (24) |
| 65–74 | 41 (37) | 10 (30) |
| ≥75 | 33 (29) | 15 (45) |
| Starting weekly dosage, mg | ||
| 100 | 24 (31) | 6 (27) ** |
| 80 | 22 (29) | 3 (14) ** |
| 60 | 27 (35) | 11 (50) ** |
| 40 | 4 (5.2) | 2 (9) ** |
| Gender | ||
| Male | 61 (54) | 17 (52) |
| Female | 51 (46) | 16 (48) |
| Geographic Region | ||
| Midwest | 11 (9.8) | 4 (12) |
| Northeast | 21 (19) | 9 (27) |
| South | 30 (27) | 10 (30) |
| West | 12 (11) | 5 (15) |
| Unknown/Masked | 38 (34) | 5 (15) |
| Race | ||
| Black or African American | 15 (15) | 3 (10) |
| Other Race | 11 (11) | 4 (13) |
| White | 76 (75) | 23 (77) |
| Unknown | 0 (0) | 0 (0) |
| Ethnicity | ||
| Hispanic or Latino/Unknown | 8 (8.4) | 1 (3) |
| Not Hispanic or Latino | 87 (92) | 31 (97) |
| Cytogenic risk | ||
| High | 29 (26) | 12 (38) |
| Standard | 39 (35) | 9 (28) |
| Unknown | 39 (35) | 11 (34) |
| Index LOT line number | ||
| ≤3L | 7 (6.3) | 5 (15) |
| 4L | 17 (15) | 9 (27) |
| 5L | 31 (28) | 14 (42) |
| 6L | 11 (9.8) | 2 (6) |
| 7L | 18 (16) | 1 (3) |
| ≥8L | 28 (25) | 2 (6) |
| Index LOT regimen | ||
| XVd | 53 (47) | 12 (36) |
| XKd | 28 (25) | 7 (21) |
| XDd | 11 (9.8) | 5 (15) |
| XPd | 20 (18) | 9 (27) |
| ECOG performance status | ||
| 0 | 30 (27) | 9 (27) |
| 1 | 50 (45) | 16 (48) |
| ≥2 | 17 (15) | 4 (12) |
| Unknown | 15 (13) | 4 (12) |
| ISS stage | ||
| I | 22 (20) | 6 (18) |
| II | 34 (30) | 7 (21) |
| III | 32 (29) | 10 (30) |
| Unknown/not documented | 30 (27) | 10 (30) |
| Previous therapy exposures | ||
| Bortezomib | 107 (96) | 28 (85) |
| Carfilzomib | 86 (77) | 23 (70) |
| Ixazomib | 31 (28) | 8 (24) |
| Daratumumab | 105 (94) | 32 (97) |
| Isatuximab | 5 (4.5) | 2 (6) |
| Elotuzumab | 27 (24) | 6 (18) |
| Lenalidomide | 109 (97) | 32 (97) |
| Pomalidomide | 93 (83) | 24 (73) |
| Thalidomide | 5 (4.5) | 0 (0) |
| Autologous Hematopoietic Stem Cell Transplant | ||
| Prior transplant | 62 (55) | 16 (48) |
| No prior transplant | 50 (45) | 17 (52) |
| Overall (n = 77) | 100 mg (n = 24) | 80 mg (n = 22) | 60 mg (n = 27) | 40 mg (n = 4) | |
|---|---|---|---|---|---|
| Had change in prescribed dose, n, (%) | 22 (29) | 14 (58) | 7 (32) | 1 (3.7) | 0 (0) |
| Selinexor Regimen n, (%) | |||||
| XVd | 39 (51) | 20 (83) | 12 (55) | 6 (22) | 1 (25) |
| XKd | 18 (23) | 1 (4.2) | 8 (36) | 8 (30) | 1 (25) |
| XDd | 7 (9.1) | 3 (13) | 1 (4.5) | 2 (7.4) | 1 (25) |
| XPd | 13 (17) | 0 (0) | 1 (4.5) | 11 (41) | 1 (25) |
| Characteristic | dPFS Months (95% CI) | rwOS Months (95% CI) |
|---|---|---|
| Overall | 4.7 (3.4, 6.7) | 14.7 (10.6, 20.9) |
| Post-anti-CD38 | 8.7 (5.8, 11.7) | 20.9 (13.4, NE) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whiteley, A.; Ijioma, S.C.; Ray, D.; Langerman, S.S.; Hu, E.; Pierre, A.; Mark, T.; Yimer, H. Real-World Treatment Patterns and Survival Outcomes of Patients with Relapsed/Refractory Multiple Myeloma Treated with a Selinexor-Containing Triplet-Based Regimen. Curr. Oncol. 2025, 32, 268. https://doi.org/10.3390/curroncol32050268
Whiteley A, Ijioma SC, Ray D, Langerman SS, Hu E, Pierre A, Mark T, Yimer H. Real-World Treatment Patterns and Survival Outcomes of Patients with Relapsed/Refractory Multiple Myeloma Treated with a Selinexor-Containing Triplet-Based Regimen. Current Oncology. 2025; 32(5):268. https://doi.org/10.3390/curroncol32050268
Chicago/Turabian StyleWhiteley, Andrew, Stephen C. Ijioma, David Ray, Spencer S. Langerman, Ellen Hu, Amy Pierre, Tomer Mark, and Habte Yimer. 2025. "Real-World Treatment Patterns and Survival Outcomes of Patients with Relapsed/Refractory Multiple Myeloma Treated with a Selinexor-Containing Triplet-Based Regimen" Current Oncology 32, no. 5: 268. https://doi.org/10.3390/curroncol32050268
APA StyleWhiteley, A., Ijioma, S. C., Ray, D., Langerman, S. S., Hu, E., Pierre, A., Mark, T., & Yimer, H. (2025). Real-World Treatment Patterns and Survival Outcomes of Patients with Relapsed/Refractory Multiple Myeloma Treated with a Selinexor-Containing Triplet-Based Regimen. Current Oncology, 32(5), 268. https://doi.org/10.3390/curroncol32050268





