The Spatial Proximity of CD8+ FoxP3+PD-1+ Cells to Tumor Cells: A More Accurate Predictor of Immunotherapy Outcomes in Advanced Non-Small-Cell Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
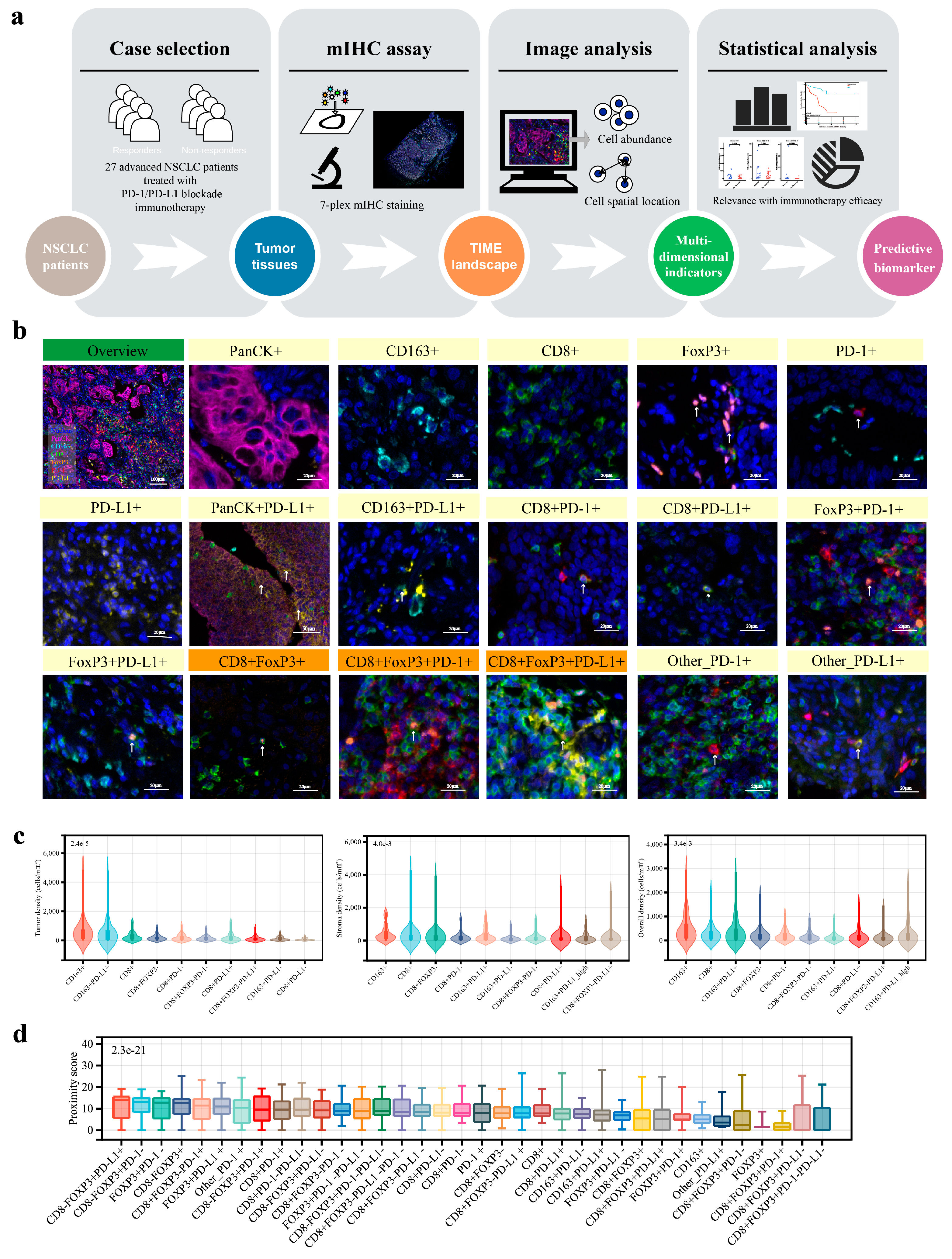
2.2. mIHC Staining and Multispectral Imaging
2.3. Image Data Extraction and Analysis
2.4. Statistics
3. Results
3.1. Clinicopathologic Features of NSCLC Patients
3.2. Analysis Overview of the Advanced NSCLC TIME
3.3. The CD8+FoxP3+ Cells Exhibited Distinctive Correlations with Clinicopathological Parameters and Expression Profiles of PD-1/PD-L1
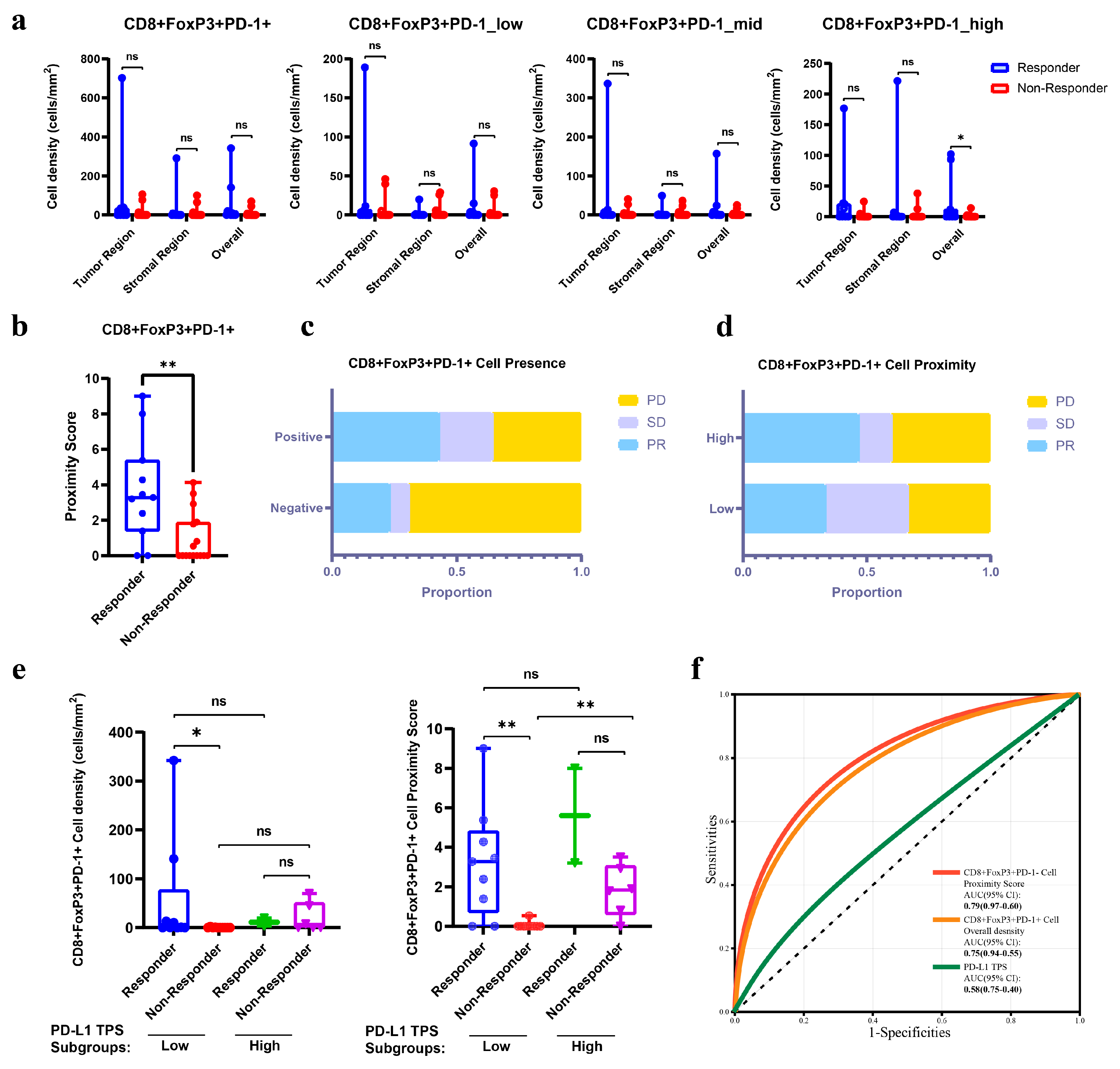
3.4. The Abundance and Spatial Proximity of CD8+FoxP3+PD-1+ Cells Provided Superior Predictive Efficacy for Immunotherapy than PD-L1 TPS
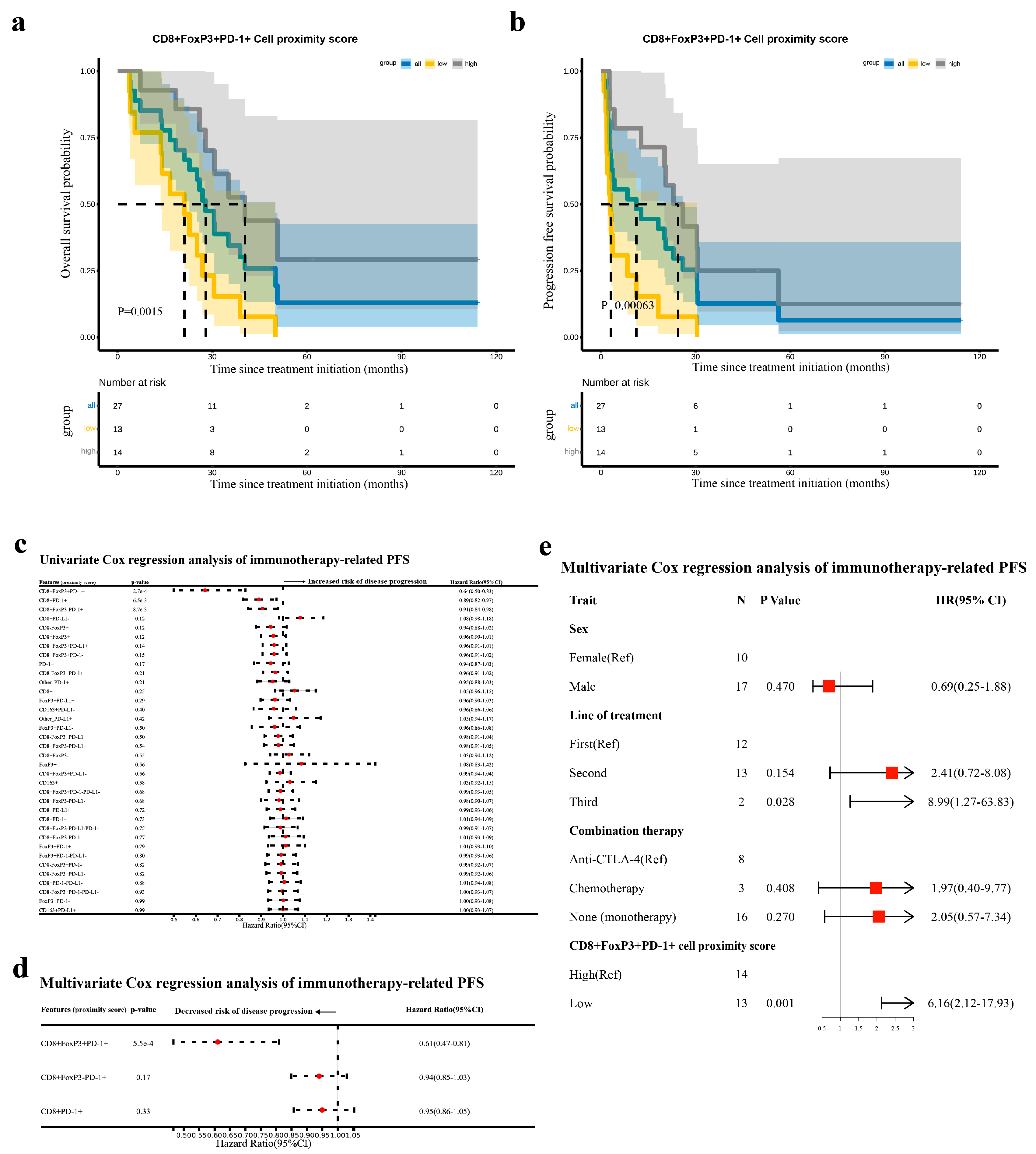
3.5. The Proximity Score of CD8+FoxP3+PD-1+ Cells Was an Independent Prognostic Factor for Immunotherapy-Related PFS in Advanced NSCLC
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N.; et al. Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin. Med. J. 2022, 135, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Hui, L.; Chen, Y. Tumor microenvironment: Sanctuary of the devil. Cancer Lett. 2015, 368, 7–13. [Google Scholar] [CrossRef]
- Taube, J.M.; Galon, J.; Sholl, L.M.; Rodig, S.J.; Cottrell, T.R.; Giraldo, N.A.; Baras, A.S.; Patel, S.S.; Anders, R.A.; Rimm, D.L.; et al. Implications of the tumor immune microenvironment for staging and therapeutics. Mod. Pathol. 2018, 31, 214–234. [Google Scholar] [CrossRef]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crino, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Perez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Deng, Y.; Tin, M.S.; Lok, V.; Ngai, C.H.; Zhang, L.; Lucero-Prisno, D.E., 3rd; Xu, W.; Zheng, Z.J.; Elcarte, E.; et al. Distribution, Risk Factors, and Temporal Trends for Lung Cancer Incidence and Mortality: A Global Analysis. Chest 2022, 161, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Fu, Y.; Zeng, Y.; Liu, X.; Peng, Y.; Hu, C.; Deng, C.; Qiu, Z.; Zou, J.; Liu, Y.; et al. The key to immunotherapy: How to choose better therapeutic biomarkers for patients with non-small cell lung cancer. Biomark. Res. 2022, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, X.; Ma, Z.; Ma, Y.; Wang, J.; Cao, B. Research Progress of Biomarkers for Immune Checkpoint Inhibitors on Digestive System Cancers. Front. Immunol. 2022, 13, 810539. [Google Scholar] [CrossRef]
- Galluzzi, L.; Chan, T.A.; Kroemer, G.; Wolchok, J.D.; Lopez-Soto, A. The hallmarks of successful anticancer immunotherapy. Sci. Transl. Med. 2018, 10, eaat7807. [Google Scholar] [CrossRef]
- Grant, M.J.; Herbst, R.S.; Goldberg, S.B. Selecting the optimal immunotherapy regimen in driver-negative metastatic NSCLC. Nat. Rev. Clin. Oncol. 2021, 18, 625–644. [Google Scholar] [CrossRef]
- Wu, F.; Jiang, T.; Chen, G.; Huang, Y.; Zhou, J.; Lin, L.; Feng, J.; Wang, Z.; Shu, Y.; Shi, J.; et al. Multiplexed imaging of tumor immune microenvironmental markers in locally advanced or metastatic non-small-cell lung cancer characterizes the features of response to PD-1 blockade plus chemotherapy. Cancer Commun. 2022, 42, 1331–1346. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, K.; Sun, Y.; Zhang, C.; Li, Y.; Zhang, L.; Chen, Z.; Zhang, J.; Hu, Y.; Yuan, J.; et al. Predicting response to immunotherapy in gastric cancer via multi-dimensional analyses of the tumour immune microenvironment. Nat. Commun. 2022, 13, 4851. [Google Scholar] [CrossRef]
- Berry, S.; Giraldo, N.A.; Green, B.F.; Cottrell, T.R.; Stein, J.E.; Engle, E.L.; Xu, H.; Ogurtsova, A.; Roberts, C.; Wang, D.; et al. Analysis of multispectral imaging with the AstroPath platform informs efficacy of PD-1 blockade. Science 2021, 372, eaba2609. [Google Scholar] [CrossRef]
- Zheng, X.; Weigert, A.; Reu, S.; Guenther, S.; Mansouri, S.; Bassaly, B.; Gattenlohner, S.; Grimminger, F.; Pullamsetti, S.; Seeger, W.; et al. Spatial Density and Distribution of Tumor-Associated Macrophages Predict Survival in Non-Small Cell Lung Carcinoma. Cancer Res. 2020, 80, 4414–4425. [Google Scholar] [CrossRef]
- Lu, S.; Stein, J.E.; Rimm, D.L.; Wang, D.W.; Bell, J.M.; Johnson, D.B.; Sosman, J.A.; Schalper, K.A.; Anders, R.A.; Wang, H.; et al. Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade. JAMA Oncol. 2019, 5, 1195. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Wang, H.; Song, L.; Li, S.; Che, N.; Zhang, S.; Zhang, H.; Wang, J. Infiltration of CD8(+) FOXP3(+) T cells, CD8(+) T cells, and FOXP3(+) T cells in non-small cell lung cancer microenvironment. Int. J. Clin. Exp. Pathol. 2020, 13, 880–888. [Google Scholar] [PubMed]
- Cohen, E.; Wang, D.; Engle, E.; Green, B.; Caushi, J.; Zhang, J.J.; Sunshine, J.; Uttam, S.; Fotheringham, M.; Fiorante, A.; et al. CD8+FOXP3+cells represent early, effector t-cells and predict outcomes in patients with resectable non-small cell lung carcinoma (nsclc) receiving neoadjuvant anti-pd-1-based therapy. J. Immunother. Cancer 2022, 10, A63. [Google Scholar] [CrossRef]
- Heeren, A.M.; Rotman, J.; Stam, A.G.M.; Pocorni, N.; Gassama, A.A.; Samuels, S.; Bleeker, M.C.G.; Mom, C.H.; Zijlmans, H.; Kenter, G.G.; et al. Efficacy of PD-1 blockade in cervical cancer is related to a CD8(+)FoxP3(+)CD25(+) T-cell subset with operational effector functions despite high immune checkpoint levels. J. Immunother. Cancer 2019, 7, 43. [Google Scholar] [CrossRef]
- Feng, Z.; Bethmann, D.; Kappler, M.; Ballesteros-Merino, C.; Eckert, A.; Bell, R.B.; Cheng, A.; Bui, T.; Leidner, R.; Urba, W.J.; et al. Multiparametric immune profiling in HPV– oral squamous cell cancer. JCI Insight 2017, 2, e93652. [Google Scholar] [CrossRef]
- Shen, W.; Song, Z.; Zhong, X.; Huang, M.; Shen, D.; Gao, P.; Qian, X.; Wang, M.; He, X.; Wang, T.; et al. Sangerbox: A comprehensive, interaction-friendly clinical bioinformatics analysis platform. iMeta 2022, 1, e36. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Shimoda, Y.; Shibaki, R.; Yoshida, T.; Murakami, S.; Shirasawa, M.; Torasawa, M.; Matsumoto, Y.; Masuda, K.; Shinno, Y.; Okuma, Y.; et al. Concurrent High PD-L1 Expression and CD8(+) Immune Cell Infiltration Predict PD-1 Blockade Efficacy in Advanced EGFR-Mutant NSCLC Patients. Clin. Lung Cancer 2022, 23, 477–486. [Google Scholar] [CrossRef]
- Fu, M.; Zhou, H.; Yang, J.; Cao, D.; Yuan, Z. Infiltration of CD8+ cytotoxic T-cells and expression of PD-1 and PD-L1 in ovarian clear cell carcinoma. Sci. Rep. 2025, 15, 4716. [Google Scholar] [CrossRef]
- Ferrata, M.; Schad, A.; Zimmer, S.; Musholt, T.J.; Bahr, K.; Kuenzel, J.; Becker, S.; Springer, E.; Roth, W.; Weber, M.M.; et al. PD-L1 Expression and Immune Cell Infiltration in Gastroenteropancreatic (GEP) and Non-GEP Neuroendocrine Neoplasms With High Proliferative Activity. Front. Oncol. 2019, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Rodak, O.; Peris-Diaz, M.D.; Olbromski, M.; Podhorska-Okolow, M.; Dziegiel, P. Current Landscape of Non-Small Cell Lung Cancer: Epidemiology, Histological Classification, Targeted Therapies, and Immunotherapy. Cancers 2021, 13, 4705. [Google Scholar] [CrossRef] [PubMed]
- Carstens, J.L.; Correa de Sampaio, P.; Yang, D.; Barua, S.; Wang, H.; Rao, A.; Allison, J.P.; LeBleu, V.S.; Kalluri, R. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat. Commun. 2017, 8, 15095. [Google Scholar] [CrossRef] [PubMed]
- Keren, L.; Bosse, M.; Marquez, D.; Angoshtari, R.; Jain, S.; Varma, S.; Yang, S.R.; Kurian, A.; Van Valen, D.; West, R.; et al. A Structured Tumor-Immune Microenvironment in Triple Negative Breast Cancer Revealed by Multiplexed Ion Beam Imaging. Cell 2018, 174, 1373–1387.e1319. [Google Scholar] [CrossRef]
- Yang, S.-R.; Gedvilaite, E.; Ptashkin, R.; Chang, J.; Ziegler, J.; Mata, D.A.; Villafania, L.B.; Nafa, K.; Hechtman, J.F.; Benayed, R.; et al. Microsatellite Instability and Mismatch Repair Deficiency Define a Distinct Subset of Lung Cancers Characterized by Smoking Exposure, High Tumor Mutational Burden, and Recurrent Somatic MLH1 Inactivation. J. Thorac. Oncol. 2024, 19, 409–424. [Google Scholar] [CrossRef]

| Items | Total N = 27 |
|---|---|
| Age | |
| Median, IQR | 58 (50,65) |
| Sex | |
| Male | 17 (63.0%) |
| Female | 10 (37.0%) |
| Smoking history | |
| ≥400 cigarette-years | 11 (40.7%) |
| <400 cigarette-years | 16 (59.3%) |
| ECOG PS | |
| 1 | 27 (100.0%) |
| Disease stage | |
| IIIB/IIIC | 3 (11.1%) |
| IV | 24 (88.9%) |
| Histological type | |
| Adenocarcinoma | 13 (48.1%) |
| Non-adenocarcinoma a | 14 (51.9%) |
| PD-L1 TPS | |
| <1% | 8 (29.6%) |
| ≥1% | 19 (73.4%) |
| 1–49% | 11 (40.7%) |
| ≥50% | 8 (29.6%) |
| Line of treatment | |
| First-line | 12 (44.4%) |
| Second-line | 13 (48.1%) |
| Third-line | 2 (7.4%) |
| Combination therapy | |
| None (monotherapy) | 16 (59.3%) |
| Anti-CTLA-4 | 3 (11.1%) |
| Chemotherapy | 8 (29.6%) |
| Best Response | |
| CR | 0 (0.0%) |
| PR | 11 (40.7%) |
| SD | 4 (14.8%) |
| PD | 12 (44.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Hu, Z.; Chen, K.; Huang, H.; Zhong, X.; Wang, Y.; Chen, J.; He, X.; Shi, D.; Zeng, Y.; et al. The Spatial Proximity of CD8+ FoxP3+PD-1+ Cells to Tumor Cells: A More Accurate Predictor of Immunotherapy Outcomes in Advanced Non-Small-Cell Lung Cancer. Curr. Oncol. 2025, 32, 262. https://doi.org/10.3390/curroncol32050262
Hu Z, Hu Z, Chen K, Huang H, Zhong X, Wang Y, Chen J, He X, Shi D, Zeng Y, et al. The Spatial Proximity of CD8+ FoxP3+PD-1+ Cells to Tumor Cells: A More Accurate Predictor of Immunotherapy Outcomes in Advanced Non-Small-Cell Lung Cancer. Current Oncology. 2025; 32(5):262. https://doi.org/10.3390/curroncol32050262
Chicago/Turabian StyleHu, Zijuan, Zhihuang Hu, Keji Chen, Huixia Huang, Xinyang Zhong, Yaxian Wang, Jiayu Chen, Xuefeng He, Di Shi, Yupeng Zeng, and et al. 2025. "The Spatial Proximity of CD8+ FoxP3+PD-1+ Cells to Tumor Cells: A More Accurate Predictor of Immunotherapy Outcomes in Advanced Non-Small-Cell Lung Cancer" Current Oncology 32, no. 5: 262. https://doi.org/10.3390/curroncol32050262
APA StyleHu, Z., Hu, Z., Chen, K., Huang, H., Zhong, X., Wang, Y., Chen, J., He, X., Shi, D., Zeng, Y., Li, J., Zhou, X., & Wei, P. (2025). The Spatial Proximity of CD8+ FoxP3+PD-1+ Cells to Tumor Cells: A More Accurate Predictor of Immunotherapy Outcomes in Advanced Non-Small-Cell Lung Cancer. Current Oncology, 32(5), 262. https://doi.org/10.3390/curroncol32050262





