Canadian Expert Consensus Recommendations for the Diagnosis and Management of Glioblastoma: Results of a Delphi Study
Abstract
1. Introduction
2. Materials and Methods
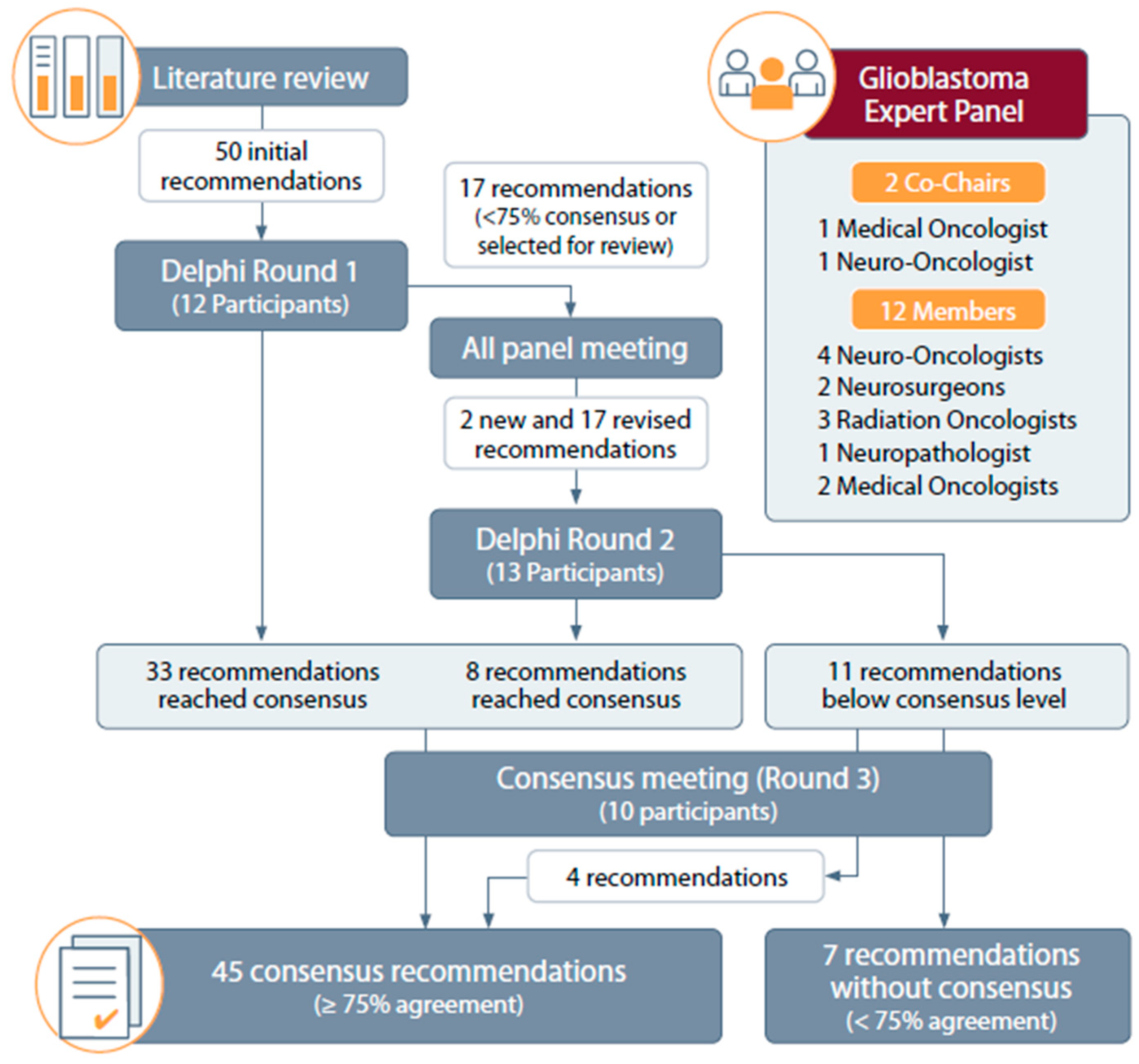
2.1. Expert Panel Selection and Composition
2.2. Literature Review
2.3. Delphi Study Design and Consensus Process
3. Results
3.1. Literature Search Results, Initial Recommendations, and Consensus Process
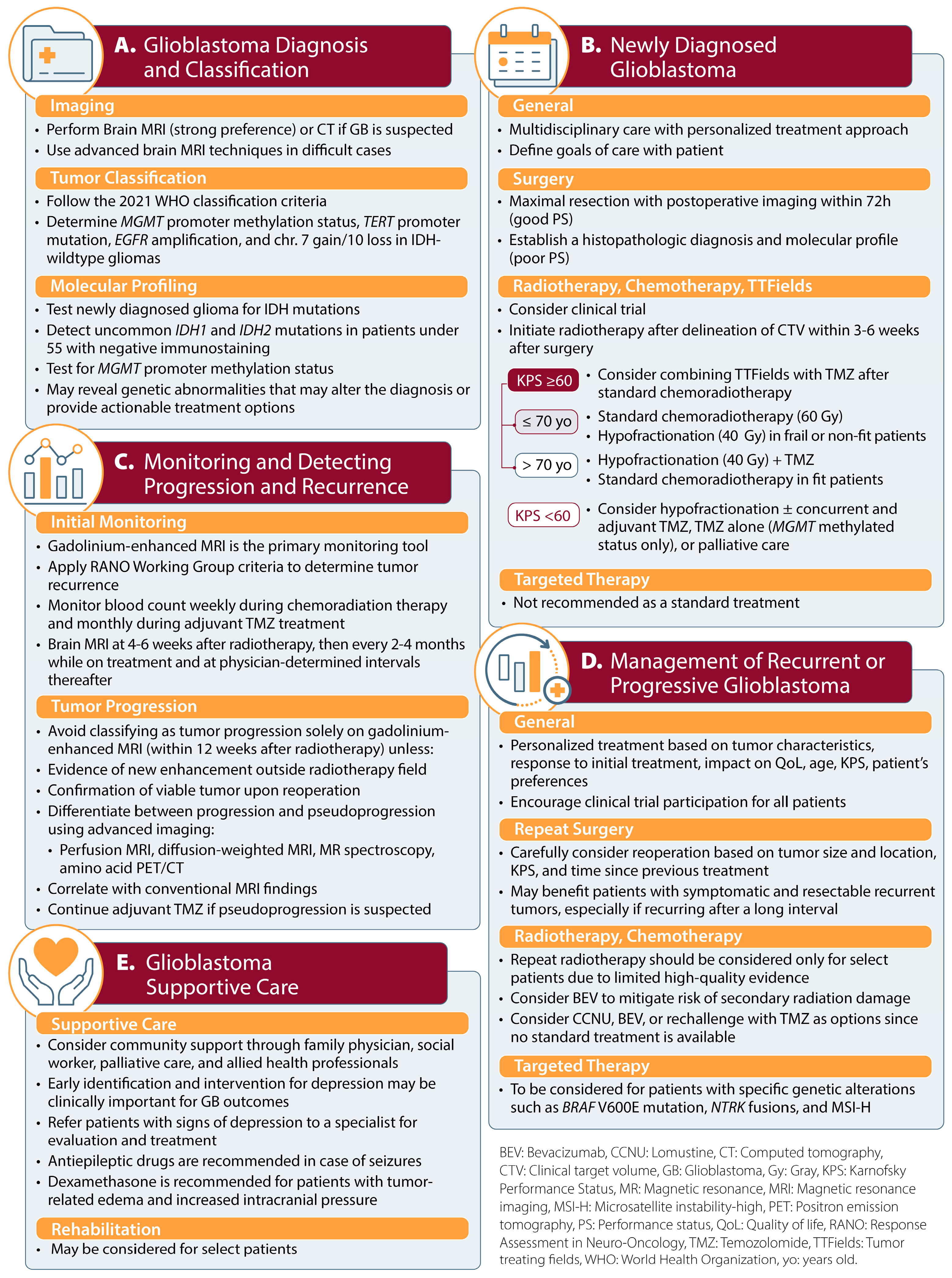
3.2. Recommendations
3.2.1. Glioblastoma Diagnosis and Classification
3.2.2. Management of Newly Diagnosed Glioblastoma
3.2.3. Monitoring Treatment Response and Disease Progression
3.2.4. Management of Recurrent or Progressive Glioblastoma
3.2.5. Supportive Care
3.2.6. Statements That Did Not Reach Consensus
4. Discussion
4.1. Glioblastoma Diagnosis and Classification
4.1.1. Imaging
4.1.2. Tumor Classification
4.1.3. Molecular Profiling
4.2. Management of Newly Diagnosed Glioblastoma
4.2.1. General
4.2.2. Surgery
4.2.3. Radiotherapy and Chemotherapy
4.2.4. New First-Line Treatments
4.3. Monitoring Treatment Response and Disease Progression
4.4. Management of Recurrent or Progressive Glioblastoma
4.4.1. General
4.4.2. Surgery
4.4.3. Radiotherapy and Chemotherapy
4.4.4. New and Emerging Treatments
4.5. Supportive Care
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, E.; Liu, J.; Davis, F.; Climans, S.; Yuan, Y. Brain Tumour Registry of Canada (BTRC): Survival and Prevalence Report 2010–2017; Brain Tumour Registry of Canada (BTRC) A Surveillance Research Collaborative, 2022; Available online: https://braintumourregistry.ca/2022-survival-and-prevalence-report (accessed on 11 November 2024).
- Walker, E.; Zakaria, D.; Yuan, Y.; Yasmin, F.; Shaw, A.; Davis, F. Brain Tumour Registry of Canada (BTRC): Incidence (2013–2017) and Mortality (2014–2018) Report; Brain Tumour Registry of Canada (BTRC) A Surveillance Research Collaborative, 2021; Available online: https://braintumourregistry.ca/incidence-report (accessed on 11 November 2024).
- Hambardzumyan, D.; Bergers, G. Glioblastoma: Defining tumor niches. Trends Cancer 2015, 1, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Taylor, O.G.; Brzozowski, J.S.; Skelding, K.A. Glioblastoma multiforme: An overview of emerging therapeutic targets. Front. Oncol. 2019, 9, 963. [Google Scholar] [CrossRef]
- Konishi, Y.; Muragaki, Y.; Iseki, H.; Mitsuhashi, N.; Okada, Y. Patterns of intracranial glioblastoma recurrence after aggressive surgical resection and adjuvant management: Retrospective analysis of 43 cases. Neurol. Med. Chir. 2012, 52, 577–586. [Google Scholar] [CrossRef]
- Sanghera, P.; Perry, J.; Sahgal, A.; Symons, S.; Aviv, R.; Morrison, M.; Lam, K.; Davey, P.; Tsao, M.N. Pseudoprogression following chemoradiotherapy for glioblastoma multiforme. Can. J. Neurol. Sci. 2010, 37, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Roldan, G.B.; Scott, J.N.; McIntyre, J.B.; Dharmawardene, M.; de Robles, P.A.; Magliocco, A.M.; Yan, E.S.; Parney, I.F.; Forsyth, P.A.; Cairncross, J.G.; et al. Population-based study of pseudoprogression after chemoradiotherapy in GBM. Can. J. Neurol. Sci. 2009, 36, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Kucharczyk, M.J.; Parpia, S.; Whitton, A.; Greenspoon, J.N. Evaluation of pseudoprogression in patients with glioblastoma. Neurooncol. Pract. 2017, 4, 120–134. [Google Scholar] [CrossRef]
- Young, J.S.; Al-Adli, N.; Sibih, Y.E.; Scotford, K.L.; Casey, M.; James, S.; Berger, M.S. Recognizing the psychological impact of a glioma diagnosis on mental and behavioral health: A systematic review of what neurosurgeons need to know. J. Neurosurg. 2023, 139, 11–19. [Google Scholar] [CrossRef]
- Solanki, C.; Sadana, D.; Arimappamagan, A.; Rao, K.; Rajeswaran, J.; Subbakrishna, D.K.; Santosh, V.; Pandey, P. Impairments in quality of life and cognitive functions in long-term survivors of glioblastoma. J. Neurosci. Rural. Pract. 2017, 8, 228–235. [Google Scholar] [CrossRef]
- Easaw, J.C.; Mason, W.P.; Perry, J.; Laperriere, N.; Eisenstat, D.D.; Del Maestro, R.; Belanger, K.; Fulton, D.; Macdonald, D.; Canadian Glioblastoma Recommendations Committee. Canadian recommendations for the treatment of recurrent or progressive glioblastoma multiforme. Curr. Oncol. 2011, 18, e126–e136. [Google Scholar] [CrossRef]
- Mason, W.P.; Maestro, R.D.; Eisenstat, D.; Forsyth, P.; Fulton, D.; Laperriere, N.; Macdonald, D.; Perry, J.; Thiessen, B.; Canadian, G.B.M. Recommendations Committee. Canadian recommendations for the treatment of glioblastoma multiforme. Curr. Oncol. 2007, 14, 110–117. [Google Scholar] [CrossRef]
- Berger, T.R.; Wen, P.Y.; Lang-Orsini, M.; Chukwueke, U.N. World Health Organization 2021 classification of central nervous system tumors and implications for therapy for adult-type gliomas: A review. JAMA Oncol. 2022, 8, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Wu, B.; Fu, Z.; Feng, F.; Qiao, E.; Li, Q.; Sun, C.; Ge, M. Prognostic role of IDH mutations in gliomas: A meta-analysis of 55 observational studies. Oncotarget 2015, 6, 17354–17365. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the extent of resection with survival in glioblastoma: A systematic review and meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef]
- Revilla-Pacheco, F.; Rodriguez-Salgado, P.; Barrera-Ramirez, M.; Morales-Ruiz, M.P.; Loyo-Varela, M.; Rubalcava-Ortega, J.; Herrada-Pineda, T. Extent of resection and survival in patients with glioblastoma multiforme: Systematic review and meta-analysis. Medicine 2021, 100, e26432. [Google Scholar] [CrossRef]
- Taylor, C.; Ekert, J.O.; Sefcikova, V.; Fersht, N.; Samandouras, G. Discriminators of pseudoprogression and true progression in high-grade gliomas: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 13258. [Google Scholar] [CrossRef]
- Ballo, M.T.; Conlon, P.; Lavy-Shahaf, G.; Kinzel, A.; Vymazal, J.; Rulseh, A.M. Association of tumor treating fields (TTFields) therapy with survival in newly diagnosed glioblastoma: A systematic review and meta-analysis. J. Neurooncol. 2023, 164, 1–9. [Google Scholar] [CrossRef]
- McKinnon, C.; Nandhabalan, M.; Murray, S.A.; Plaha, P. Glioblastoma: Clinical presentation, diagnosis, and management. BMJ 2021, 374, n1560. [Google Scholar] [CrossRef]
- Gao, H.; Jiang, X. Progress on the diagnosis and evaluation of brain tumors. Cancer Imaging 2013, 13, 466–481. [Google Scholar] [CrossRef]
- Lundy, P.; Domino, J.; Ryken, T.; Fouke, S.; McCracken, D.J.; Ormond, D.R.; Olson, J.J. The role of imaging for the management of newly diagnosed glioblastoma in adults: A systematic review and evidence-based clinical practice guideline update. J. Neurooncol. 2020, 150, 95–120. [Google Scholar] [CrossRef]
- Padelli, F.; Mazzi, F.; Erbetta, A.; Chiapparini, L.; Doniselli, F.M.; Palermo, S.; Aquino, D.; Bruzzone, M.G.; Cuccarini, V. In vivo brain MR spectroscopy in gliomas: Clinical and pre-clinical chances. Clin. Transl. Imaging 2022, 10, 495–515. [Google Scholar]
- Pasquini, L.; Napolitano, A.; Tagliente, E.; Dellepiane, F.; Lucignani, M.; Vidiri, A.; Ranazzi, G.; Stoppacciaro, A.; Moltoni, G.; Nicolai, M.; et al. Deep learning can differentiate IDH-mutant from IDH-wild GBM. J. Pers. Med. 2021, 11, 290. [Google Scholar] [CrossRef]
- Olympios, N.; Gilard, V.; Marguet, F.; Clatot, F.; Di Fiore, F.; Fontanilles, M. TERT promoter alterations in glioblastoma: A systematic review. Cancers 2021, 13, 1147. [Google Scholar] [CrossRef]
- Lim-Fat, M.J.; Macdonald, M.; Lapointe, S.; Climans, S.A.; Cacciotti, C.; Chahal, M.; Perreault, S.; Tsang, D.S.; Gao, A.; Yip, S.; et al. Molecular testing for adolescent and young adult central nervous system tumors: A Canadian guideline. Front. Oncol. 2022, 12, 960509. [Google Scholar] [CrossRef]
- Richardson, T.E.; Yokoda, R.T.; Rashidipour, O.; Vij, M.; Snuderl, M.; Brem, S.; Hatanpaa, K.J.; McBrayer, S.K.; Abdullah, K.G.; Umphlett, M.; et al. Mismatch repair protein mutations in isocitrate dehydrogenase (IDH)-mutant astrocytoma and IDH-wild-type glioblastoma. Neurooncol. Adv. 2023, 5, vdad085. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef]
- Gibson, D.; Ravi, A.; Rodriguez, E.; Chang, S.; Oberheim Bush, N.; Taylor, J.; Clarke, J.; Solomon, D.; Scheffler, A.; Witte, J.; et al. Quantitative analysis of MGMT promoter methylation in glioblastoma suggests nonlinear prognostic effect. Neurooncol. Adv. 2023, 5, vdad115. [Google Scholar] [CrossRef]
- Hegi, M.E.; Liu, L.; Herman, J.G.; Stupp, R.; Wick, W.; Weller, M.; Mehta, M.P.; Gilbert, M.R. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J. Clin. Oncol. 2008, 26, 4189–4199. [Google Scholar] [CrossRef]
- Szylberg, M.; Sokal, P.; Sledzinska, P.; Bebyn, M.; Krajewski, S.; Szylberg, L.; Szylberg, A.; Szylberg, T.; Krystkiewicz, K.; Birski, M.; et al. MGMT promoter methylation as a prognostic factor in primary glioblastoma: A single-institution observational study. Biomedicines 2022, 10, 2030. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Ghosh, H.S.; Patel, R.V.; Woodward, E.; Greenwald, N.F.; Bhave, V.M.; Maury, E.A.; Cello, G.; Hoffman, S.E.; Li, Y.; Gupta, H.; et al. Contemporary prognostic signatures and refined risk stratification of gliomas: An analysis of 4,400 tumors. Neuro Oncol. 2024, 27, 195–208. [Google Scholar] [CrossRef]
- Kiang, K.M.; Sun, S.; Leung, G.K. ADD3 deletion in glioblastoma predicts disease status and survival. Front. Oncol. 2021, 11, 717793. [Google Scholar] [CrossRef]
- Polonara, G.; Aiudi, D.; Iacoangeli, A.; Raggi, A.; Ottaviani, M.M.; Antonini, R.; Iacoangeli, M.; Dobran, M. Glioblastoma: A retrospective analysis of the role of the maximal surgical resection on overall survival and progression-free survival. Biomedicines 2023, 11, 739. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, F.; Ni, W.; Qi, W.; Cao, W.; Xu, C.; Chen, J.; Gao, Y. Survival impact of delaying postoperative chemoradiotherapy in newly-diagnosed glioblastoma patients. Transl. Cancer Res. 2020, 9, 5450–5458. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Oster, C.; Schmidt, T.; Agkatsev, S.; Lazaridis, L.; Kleinschnitz, C.; Sure, U.; Scheffler, B.; Kebir, S.; Glas, M. Are we providing best-available care to newly diagnosed glioblastoma patients? Systematic review of phase III trials in newly diagnosed glioblastoma 2005–2022. Neurooncol. Adv. 2023, 5, vdad105. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Y. The efficacy and safety of radiotherapy with adjuvant temozolomide for glioblastoma: A meta-analysis of randomized controlled studies. Clin. Neurol. Neurosurg. 2020, 196, 105890. [Google Scholar] [CrossRef]
- Gupta, T.; Selvarajan, J.M.P.; Kannan, S.; Menon, N.; Dasgupta, A.; Chatterjee, A. Updated systematic review and meta-analysis of extended adjuvant temozolomide in patients with newly diagnosed glioblastoma. Neurooncol. Adv. 2023, 5, vdad086. [Google Scholar] [CrossRef]
- Attarian, F.; Taghizadeh-Hesary, F.; Fanipakdel, A.; Javadinia, S.A.; Porouhan, P.; PeyroShabany, B.; Fazilat-Panah, D. A systematic review and meta-analysis on the number of adjuvant temozolomide cycles in newly diagnosed glioblastoma. Front. Oncol. 2021, 11, 779491. [Google Scholar] [CrossRef]
- Hegi, M.E.; Oppong, F.B.; Perry, J.R.; Wick, W.; Henriksson, R.; Laperriere, N.J.; Gorlia, T.; Malmstrom, A.; Weller, M. No benefit from TMZ treatment in GB with truly unmethylated MGMT promoter: Reanalysis of the CE.6 and the pooled Nordic/NOA-08 trials in elderly GB patients. Neuro Oncol. 2024, 26, 1867–1875. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology. Central Nervous System Cancers. Version 3.2024. 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf (accessed on 11 November 2024).
- Roa, W.; Kepka, L.; Kumar, N.; Sinaika, V.; Matiello, J.; Lomidze, D.; Hentati, D.; Guedes de Castro, D.; Dyttus-Cebulok, K.; Drodge, S.; et al. International Atomic Energy Agency randomized phase III study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J. Clin. Oncol. 2015, 33, 4145–4150. [Google Scholar] [CrossRef]
- Guedes de Castro, D.; Matiello, J.; Roa, W.; Ghosh, S.; Kepka, L.; Kumar, N.; Sinaika, V.; Lomidze, D.; Hentati, D.; Rosenblatt, E.; et al. Survival outcomes with short-course radiation therapy in elderly patients with glioblastoma: Data from a randomized phase 3 trial. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 931–938. [Google Scholar] [CrossRef]
- Malmström, A.; Grønberg, B.H.; Marosi, C.; Stupp, R.; Frappaz, D.; Schultz, H.; Abacioglu, U.; Tavelin, B.; Lhermitte, B.; Hegi, M.E.; et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol. 2012, 13, 916–926. [Google Scholar] [CrossRef]
- Novocure’s Optune Approved in Canada for the Treatment of Newly Diagnosed and Recurrent Glioblastoma. Press Release. 29 November 2022. Available online: https://www.braintumour.ca/wp-content/uploads/2022/11/Novocure-Press-Release.pdf (accessed on 9 February 2024).
- Rominiyi, O.; Vanderlinden, A.; Clenton, S.J.; Bridgewater, C.; Al-Tamimi, Y.; Collis, S.J. Tumour treating fields therapy for glioblastoma: Current advances and future directions. Br. J. Cancer 2021, 124, 697–709. [Google Scholar] [CrossRef]
- Ram, Z.; Kim, C.Y.; Hottinger, A.F.; Idbaih, A.; Nicholas, G.; Zhu, J.J. Efficacy and safety of tumor treating fields (TTFields) in elderly patients with newly diagnosed glioblastoma: Subgroup analysis of the phase 3 EF-14 clinical trial. Front. Oncol. 2021, 11, 671972. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef]
- Nishikawa, R.; Yamasaki, F.; Arakawa, Y.; Muragaki, Y.; Narita, Y.; Tanaka, S.; Yamaguchi, S.; Mukasa, A.; Kanamori, M. Safety and efficacy of tumour-treating fields (TTFields) therapy for newly diagnosed glioblastoma in Japanese patients using the Novo-TTF System: A prospective post-approval study. Jpn. J. Clin. Oncol. 2023, 53, 371–377. [Google Scholar] [CrossRef]
- Bähr, O.; Tabatabai, G.; Fietkau, R.; Goldbrunner, R.; Glas, M. Tumor treating fields (TTFields) therapy in patients with glioblastoma: Long-term survival results from TTFields in Germany in routine clinical care (TIGER) study. J. Clin. Oncol. 2024, 42, 2036. [Google Scholar] [CrossRef]
- Rapp, M.; Baernreuther, J.; Turowski, B.; Steiger, H.J.; Sabel, M.; Kamp, M.A. Recurrence pattern analysis of primary glioblastoma. World Neurosurg. 2017, 103, 733–740. [Google Scholar] [CrossRef]
- Kim, H.R.; Kim, K.H.; Kong, D.S.; Seol, H.J.; Nam, D.H.; Lim, D.H.; Lee, J.I. Outcome of salvage treatment for recurrent glioblastoma. J. Clin. Neurosci. 2015, 22, 468–473. [Google Scholar] [CrossRef]
- Yu, J.; Zheng, J.; Xu, W.; Weng, J.; Gao, L.; Tao, L.; Liang, F.; Zhang, J. Accuracy of (18)F-FDOPA positron emission tomography and (18)F-FET positron emission tomography for differentiating radiation necrosis from brain tumor recurrence. World Neurosurg. 2018, 114, e1211–e1224. [Google Scholar] [CrossRef]
- Azoulay, M.; Santos, F.; Shenouda, G.; Petrecca, K.; Oweida, A.; Guiot, M.C.; Owen, S.; Panet-Raymond, V.; Souhami, L.; Abdulkarim, B.S. Benefit of re-operation and salvage therapies for recurrent glioblastoma multiforme: Results from a single institution. J. Neurooncol. 2017, 132, 419–426. [Google Scholar] [CrossRef]
- Kalita, O.; Kazda, T.; Reguli, S.; Jancalek, R.; Fadrus, P.; Slachta, M.; Pospisil, P.; Krska, L.; Vrbkova, J.; Hrabalek, L.; et al. Effects of reoperation timing on survival among recurrent glioblastoma patients: A retrospective multicentric descriptive study. Cancers 2023, 15, 2530. [Google Scholar] [CrossRef]
- Lu, V.M.; Jue, T.R.; McDonald, K.L.; Rovin, R.A. The survival effect of repeat surgery at glioblastoma recurrence and its trend: A systematic review and meta-analysis. World Neurosurg. 2018, 115, 453–459.e453. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Wang, Z.F.; Pan, Z.Y.; Peus, D.; Delgado-Fernandez, J.; Pallud, J.; Li, Z.Q. A meta-analysis of survival outcomes following reoperation in recurrent glioblastoma: Time to consider the timing of reoperation. Front. Neurol. 2019, 10, 286. [Google Scholar] [CrossRef]
- Kazmi, F.; Soon, Y.Y.; Leong, Y.H.; Koh, W.Y.; Vellayappan, B. Re-irradiation for recurrent glioblastoma (GBM): A systematic review and meta-analysis. J. Neurooncol. 2019, 142, 79–90. [Google Scholar] [CrossRef]
- Vellayappan, B.; Kazmi, F.; Lim, K.H.C.; Yeo, T.T.; Wong, A.; Soon, Y.Y.; Koh, W.Y. Re-irradiation for recurrent glioblastoma multiforme (GBM): Systematic review and meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, E114. [Google Scholar] [CrossRef]
- You, W.C.; Lee, H.D.; Pan, H.C.; Chen, H.C. Re-irradiation combined with bevacizumab for recurrent glioblastoma beyond bevacizumab failure: Survival outcomes and prognostic factors. Sci. Rep. 2023, 13, 9442. [Google Scholar] [CrossRef]
- Marwah, R.; Xing, D.; Squire, T.; Soon, Y.Y.; Gan, H.K.; Ng, S.P. Reirradiation versus systemic therapy versus combination therapy for recurrent high-grade glioma: A systematic review and meta-analysis of survival and toxicity. J. Neurooncol. 2023, 164, 505–524. [Google Scholar] [CrossRef]
- Tsien, C.I.; Pugh, S.L.; Dicker, A.P.; Raizer, J.J.; Matuszak, M.M.; Lallana, E.C.; Huang, J.; Algan, O.; Deb, N.; Portelance, L.; et al. NRG Oncology/RTOG1205: A randomized phase II trial of concurrent bevacizumab and reirradiation versus bevacizumab alone as treatment for recurrent glioblastoma. J. Clin. Oncol. 2023, 41, 1285–1295. [Google Scholar] [CrossRef]
- Villani, V.; Prosperini, L.; Lecce, M.; Tanzilli, A.; Farneti, A.; Benincasa, D.; Telera, S.; Marucci, L.; Piludu, F.; Pace, A. Recurrent glioblastoma: Which treatment? A real-world study from the Neuro-oncology Unit “Regina Elena” National Cancer Institute. Neurol. Sci. 2022, 43, 5533–5541. [Google Scholar] [CrossRef]
- McBain, C.; Lawrie, T.A.; Rogozinska, E.; Kernohan, A.; Robinson, T.; Jefferies, S. Treatment options for progression or recurrence of glioblastoma: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 5, CD013579. [Google Scholar] [CrossRef]
- AVASTIN (bevacizumab). Product Monograph. Hoffmann-La Roche Ltd. 2018. Available online: https://pdf.hres.ca/dpd_pm/00071938.PDF (accessed on 7 February 2024).
- Nagpal, S.; Harsh, G.; Recht, L. Bevacizumab improves quality of life in patients with recurrent glioblastoma. Chemother. Res. Pract. 2011, 2011, 602812. [Google Scholar] [CrossRef]
- Kaka, N.; Hafazalla, K.; Samawi, H.; Simpkin, A.; Perry, J.; Sahgal, A.; Das, S. Progression-free but no overall survival benefit for adult patients with bevacizumab therapy for the treatment of newly diagnosed glioblastoma: A systematic review and meta-analysis. Cancers 2019, 11, 1723. [Google Scholar] [CrossRef]
- Fu, M.; Zhou, Z.; Huang, X.; Chen, Z.; Zhang, L.; Zhang, J.; Hua, W.; Mao, Y. Use of bevacizumab in recurrent glioblastoma: A scoping review and evidence map. BMC Cancer 2023, 23, 544. [Google Scholar] [CrossRef]
- Zhang, T.; Xin, Q.; Kang, J.M. Bevacizumab for recurrent glioblastoma: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6480–6491. [Google Scholar] [CrossRef]
- Schritz, A.; Aouali, N.; Fischer, A.; Dessenne, C.; Adams, R.; Berchem, G.; Huiart, L.; Schmitz, S. Systematic review and network meta-analysis of the efficacy of existing treatments for patients with recurrent glioblastoma. Neurooncol. Adv. 2021, 3, vdab052. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Ai, D.; Li, T.; Xia, L.; Sun, L. Effectiveness of lomustine combined with bevacizumab in glioblastoma: A meta-analysis. Front. Neurol. 2020, 11, 603947. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, L.; Li, X.; Liu, R.; Ren, C.; Du, S. Reduced-dose bevacizumab vs. standard-dose bevacizumab in recurrent high-grade glioma: Which one is better? A meta-analysis. Clin. Neurol. Neurosurg. 2020, 198, 106239. [Google Scholar] [CrossRef] [PubMed]
- Melhem, J.M.; Tahir, A.; Calabrese, E.; Granovskaya, I.; Atenafu, E.G.; Sahgal, A.; Lim-Fat, M.J.; Perry, J.R. Dose-dependent efficacy of bevacizumab in recurrent glioblastoma. J. Neurooncol. 2023, 161, 633–641. [Google Scholar] [CrossRef]
- Wick, W.; Gorlia, T.; Bendszus, M.; Taphoorn, M.; Sahm, F.; Harting, I.; Brandes, A.A.; Taal, W.; Domont, J.; Idbaih, A.; et al. Lomustine and bevacizumab in progressive glioblastoma. N. Engl. J. Med. 2017, 377, 1954–1963. [Google Scholar] [CrossRef]
- Stupp, R.; Wong, E.T.; Kanner, A.A.; Steinberg, D.; Engelhard, H.; Heidecke, V.; Kirson, E.D.; Taillibert, S.; Liebermann, F.; Dbaly, V.; et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. Eur. J. Cancer 2012, 48, 2192–2202. [Google Scholar] [CrossRef]
- Kesari, S.; Ram, Z.; Investigators, E.F.T. Tumor-treating fields plus chemotherapy versus chemotherapy alone for glioblastoma at first recurrence: A post hoc analysis of the EF-14 trial. CNS Oncol. 2017, 6, 185–193. [Google Scholar] [CrossRef]
- Wahyuhadi, J.; Immadoel Haq, I.B.; Arifianto, M.R.; Sulistyono, B.; Meizikri, R.; Rosada, A.; Sigit Prakoeswa, C.R.; Susilo, R.I. Active immunotherapy for glioblastoma treatment: A systematic review and meta-analysis. Cancer Control 2022, 29, 10732748221079474. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, S.; Xu, L.; Sun, J.; Chan, W.L.; Zheng, P.; Zhang, J.; Zhang, L. Efficacy and safety of innate and adaptive immunotherapy combined with standard of care in high-grade gliomas: A systematic review and meta-analysis. Front. Immunol. 2023, 14, 966696. [Google Scholar] [CrossRef]
- Zeng, Y.F.; Wei, X.Y.; Guo, Q.H.; Chen, S.Y.; Deng, S.; Liu, Z.Z.; Gong, Z.C.; Zeng, W.J. The efficacy and safety of anti-PD-1/PD-L1 in treatment of glioma: A single-arm meta-analysis. Front. Immunol. 2023, 14, 1168244. [Google Scholar] [CrossRef]
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bahr, O.; et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: The CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020, 6, 1003–1010. [Google Scholar] [CrossRef]
- Das, A.; Fernandez, N.R.; Levine, A.; Bianchi, V.; Stengs, L.K.; Chung, J.; Negm, L.; Dimayacyac, J.R.; Chang, Y.; Nobre, L.; et al. Combined immunotherapy improves outcome for replication-repair-deficient (RRD) high-grade glioma failing anti-PD-1 monotherapy: A report from the International RRD Consortium. Cancer Discov. 2024, 14, 258–273. [Google Scholar] [CrossRef]
- Scherm, A.; Ippen, F.M.; Hau, P.; Baurecht, H.; Wick, W.; Gempt, J.; Knuttel, H.; Leitzmann, M.F.; Seliger, C. Targeted therapies in patients with newly diagnosed glioblastoma—A systematic meta-analysis of randomized clinical trials. Int. J. Cancer 2023, 152, 2373–2382. [Google Scholar] [CrossRef]
- Lombardi, G.; De Salvo, G.L.; Brandes, A.A.; Eoli, M.; Ruda, R.; Faedi, M.; Lolli, I.; Pace, A.; Daniele, B.; Pasqualetti, F.; et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019, 20, 110–119. [Google Scholar] [CrossRef]
- Wen, P.; Alexander, B.; Berry, D.; Buxton, M.; Cavenee, W.; Colman, H.; de Groot, J.; Ellingson, B.; Gordon, G.; Hyddmark, E.; et al. CTNI-85. GBM AGILE platform trial for newly diagnosed and recurrent GBM: Results of first experimental arm, regorafenib. Neuro Oncol. 2023, 25, v97–v98. [Google Scholar] [CrossRef]
- Au, T.H.; Willis, C.; Reblin, M.; Peters, K.B.; Nghiemphu, P.L.; Taylor, J.W.; Colman, H.; Cohen, A.L.; Ormond, D.R.; Chakravarti, A.; et al. Caregiver burden by treatment and clinical characteristics of patients with glioblastoma. Support. Care Cancer 2022, 30, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Lamba, N.; Zheng, L.J.; Cote, D.; Regestein, Q.R.; Liu, C.M.; Tran, Q.; Routh, S.; Smith, T.R.; Mekary, R.A.; et al. Depression and survival of glioma patients: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 2018, 172, 8–19. [Google Scholar] [CrossRef]
- Applebaum, A.J.; Baser, R.E.; Roberts, K.E.; Lynch, K.; Gebert, R.; Breitbart, W.S.; Diamond, E.L. Meaning-centered psychotherapy for cancer caregivers: A pilot trial among caregivers of patients with glioblastoma multiforme. Transl. Behav. Med. 2022, 12, 841–852. [Google Scholar] [CrossRef] [PubMed]
| Statement | Consensus Level | Consensus Strength | Evidence Quality b | Round |
|---|---|---|---|---|
| Imaging | ||||
| All patients with a suspected diagnosis of GB should receive a brain MRI. The minimum assessment should include T2, FLAIR, and pre- and post-contrast T1 sequences. | 100% | Strong | Moderate to high | 1 |
| CT should be used to evaluate patients with suspected GB only if MRI is unavailable or contraindicated. | 92% | Strong | High | 1 |
| Advanced brain MRI techniques, such as diffusion-weighted MRI, perfusion-weighted MRI, PET, and MR spectroscopy, may be considered to assess the presence of GB in difficult cases (e.g., in individuals with intracranial hemorrhage) or to help distinguish GB from other tumor types and progression from radionecrosis. | 92% | Moderate | Moderate | 1 |
| Tumor Classification | ||||
| IDH-wildtype grade 4 gliomas should be classified as GB in accordance with the 2021 WHO classification criteria. | 77% | Strong | High | 1 |
| IDH-wildtype astrocytic gliomas in adults should be considered as GB if any of the following criteria are met: microvascular proliferation, necrosis, TERT promoter mutation, EGFR gene amplification, and +7/−10 chromosome copy number changes. | 77% | Strong | High | 1 |
| MGMT promoter methylation and TERT promoter mutations should be determined in patients with IDH-wildtype diffuse gliomas. | 85% | Moderate | Moderate | 1 |
| Molecular Profiling | ||||
| All patients with newly diagnosed gliomas should be tested for IDH mutations. | 100% | Strong | High | 1 |
| Sequencing of IDH1 and IDH2 is recommended to detect uncommon IDH1 and IDH2 mutations in patients under 55 years of age with negative immunostaining, without postponing treatment. | 100% | Strong | Moderate | 2 |
| In patients with confirmed GB (IDH-wildtype) or grade 4 IDH-mutated tumors, MGMT promoter methylation status should be tested to inform prognosis and predict response to temozolomide chemotherapy. | 77% | Strong | Moderate | 1 |
| Analysis of H3K27 mutations should be considered in patients with high-grade midline, IDH-wildtype gliomas. | 100% | Strong | High | 2 |
| Key molecular markers of patients with GB include TERT promoter mutation, EGFR amplification, and chromosome 7 gain and 10 loss, without postponing treatment. Molecular profiling may reveal other genetic abnormalities that may alter the diagnosis or provide actionable treatment options. | 75% | Moderate | Moderate | 3 |
| Statement | Consensus Level | Consensus Strength | Evidence Quality b | Round |
|---|---|---|---|---|
| General | ||||
| A multidisciplinary approach is required for GB management. Multidisciplinary care teams would optimally include neurosurgeons, medical oncologists or neuro-oncologists, radiation oncologists, neuropathologists, radiologists, and neurologists. | 92% | Strong | Moderate | 1 |
| Follow a personalized treatment approach for the management of newly diagnosed GB patients, considering the tumor characteristics (size, location, and molecular profile) and the patient’s age, functional ability (measured as KPS), symptoms, clinical needs, and preferences. | 100% | Strong | High | 1 |
| Given that the intent of management is palliative from the outset, part of the discussion on further management should involve a conversation with the patient and their family about goals of care and whether they wish to proceed with active therapies. For some patients with a very poor performance status, palliative care alone is a reasonable option. | 92% | Moderate | High | 1 |
| Surgery | ||||
| Maximal safe resection is recommended for all patients with newly diagnosed GB when the tumors are operable and for all patients who have good performance status. In cases not amenable to resection (i.e., poor performance status and/or those with unfavorable tumor location), stereotactic biopsy is recommended to establish a histopathologic diagnosis and molecular profile (including cytogenetic alterations). Whenever feasible, tissue should be saved for tumor banking. | 83% | Moderate-to-strong | Moderate | 1 |
| Advanced techniques, such as fluorescence-guided surgery, may be used to optimize tumor removal and preserve normal brain tissue. | 75% | Moderate | Low | 3 |
| Postoperative imaging within 72 h is recommended for patients undergoing surgery for newly diagnosed GB. | 100% | Strong | Moderate | 1 |
| Radiotherapy, Chemotherapy, and TTFields | ||||
| Where possible, patients should be considered for a clinical trial. | 100% | Strong | High | 1 |
| Radiation therapy should be initiated as soon as it is safely permissible, ideally within 3–6 weeks after surgery. | 92% | Moderate | Moderate | 1 |
| Use gadolinium-enhanced T1-weighted MRI and FLAIR to determine the CTV. | 92% | Strong-to-moderate | High-to-moderate | 2 |
| Consider combining TTFields with adjuvant temozolomide after treatment with standard chemoradiotherapy for patients who have good performance status (KPS ≥ 60), regardless of MGMT promoter methylation status. | 85% | Strong | High-to-moderate | 2 |
| For patients aged >70 years with good performance status (KPS ≥ 60), consider 40 Gy hypofractionated radiotherapy plus concurrent temozolomide 75 mg/m2 for 21 days, followed by adjuvant temozolomide 150–200 mg/m2 in a 5/28-day schedule for 6–12 cycles. Standard radiotherapy may also be considered in place of hypofractionated radiotherapy in fit patients. | 85% | Moderate | Moderate | 2 |
| For patients aged ≤ 70 years with a good performance status (KPS ≥ 60), consider 60 Gy radiotherapy plus concurrent temozolomide 75 mg/m2 for 42 days, followed by adjuvant temozolomide 150–200 mg/m2 in a 5/28-day schedule for six cycles. Hypofractionated radiotherapy may also be considered in place of standard radiotherapy if patients are ineligible for chemotherapy, frail, or present significant comorbidities. | 75% | Strong | High | 3 |
| In patients with poor performance status (KPS < 60), consider hypofractionated radiotherapy with or without concurrent or adjuvant temozolomide, temozolomide alone (only in the presence of MGMT methylation), or palliative care alone. | 100% | Moderate | Moderate | 2 |
| Targeted Therapy and Immunotherapy | ||||
| Targeted therapy and immunotherapy have not shown clear survival benefits in patients with newly diagnosed GB and are not recommended as the standard of care for this population. | 92% | Moderate | Moderate | 1 |
| Statement | Consensus Level | Consensus Strength | Evidence Quality b | Round |
|---|---|---|---|---|
| Tumor progression or recurrence should be monitored using gadolinium-enhanced MRI, and tumor recurrence should be determined according to the RANO Working Group criteria. | 75% | Moderate | Moderate | 1 |
| Blood counts should be monitored weekly during chemoradiation therapy and monthly during adjuvant temozolomide treatment. | 83% | Strong | Moderate | 1 |
| Brain MRI is recommended 4–6 weeks after radiotherapy and then every 2–4 months while on treatment and at physician-determined intervals thereafter. | 75% | Strong | Moderate | 3 |
| Patients receiving chemoradiation should not be classified as having tumor progression based on gadolinium-enhanced MRI within the first 12 weeks after the end of radiotherapy unless new enhancement is evident outside the radiotherapy field or the presence of a viable tumor is confirmed by a pathologist at the time of reoperation. | 100% | Moderate-to-strong | Moderate | 1 |
| Adjuvant temozolomide should be continued in patients with suspected pseudoprogression. | 92% | Strong | Moderate | 1 |
| In patients with suspected progressive GB, pseudoprogression may be difficult to distinguish from true progression based on clinical and radiological criteria. Therefore, advanced imaging techniques, such as perfusion MRI, diffusion-weighted MRI, MR spectroscopy, and amino acid PET/CT, can be used in correlation with conventional MRI findings to distinguish pseudoprogression from true progression. | 83% | Moderate | Low-to-moderate | 1 |
| Statement | Consensus Level | Consensus Strength | Evidence Quality b | Round |
|---|---|---|---|---|
| General | ||||
| Follow a personalized treatment approach for the management of recurrent or progressive GB, considering the tumor characteristics (tumor size, location, and molecular profile), the response to initial treatment, the impact of treatment on the patient’s quality of life, and the patient’s age, functional ability (measured as KPS), symptoms, needs, and preferences. | 100% | Strong | Moderate | 1 |
| Clinical Trials | ||||
| Clinical trial participation should be encouraged for all patients with recurrent GB. | 100% | Strong | Moderate | 1 |
| Surgery | ||||
| In consultation with the multidisciplinary team and the patient, reoperation should be considered for patients with recurrent GB, depending on factors such as tumor size, location, performance status, and time since previous treatment. | 100% | Moderate-to-strong | Moderate | 1 |
| Reoperation may benefit select patients with large, symptomatic, and resectable recurrent tumors, especially if the tumor has recurred after a long interval. | 100% | Strong | Moderate | 1 |
| Reoperation may provide some survival benefits for select patients, but it also carries risks of complications. Repeat surgery should be considered only for patients with a high-performance status score (KPS > 70) and those with a tumor in a favorable location until high-quality evidence on the benefit of repeat surgery and the optimal timing of reoperation becomes available. | 83% | Moderate | Moderate | 1 |
| Radiotherapy, Chemotherapy, and TTFields | ||||
| There is no high-quality evidence to support the use of repeat radiotherapy in patients with recurrent GB, and re-irradiation should be considered only for select patients with recurrent GB, such as those with a small tumor size, a favorable location, and a good performance status, as well as those for whom a long time has passed since previous treatment. | 83% | Moderate | Moderate | 1 |
| Radiotherapy with bevacizumab to mitigate the increased risk of radiation damage during secondary radiation may be considered for the treatment of select patients with second recurrence. | 75% | Moderate | Low-to-moderate | 1 |
| There is no standard treatment for recurrent GB. Options include lomustine and rechallenge with temozolomide. Bevacizumab may be added to chemotherapy with select patients. | 75% | Moderate | Moderate | 1 |
| Targeted Therapy and Immunotherapy | ||||
| The use of targeted therapy and immunotherapy could be considered for the management of recurrence in patients with specific genetic alterations such as BRAF V600E mutation, NTRK fusions, and microsatellite instability—high (MSI-H). | 92% | Moderate | Moderate | 2 |
| Statement | Consensus Level | Consensus Strength | Evidence Quality b | Round |
|---|---|---|---|---|
| Multidisciplinary care for GB may include community support through the family physician, a social worker, palliative care, and allied health professionals. | 100% | Strong | Moderate | 2 |
| Early identification and intervention for depression in patients with GB may be clinically important in terms of GB outcomes, and patients with signs of depression should be referred to a specialist for evaluation and treatment. | 83% | Moderate | Moderate | 1 |
| Treatment with antiepileptic drugs (e.g., levetiracetam, lacosamide, and perampanel) is recommended for patients with seizures. | 100% | Strong | High | 1 |
| Treatment with dexamethasone is recommended for patients with tumor-related edema and increased intracranial pressure. | 83% | Strong | Moderate | 1 |
| Rehabilitation may be considered for select patients with GB. | 83% | Moderate | Moderate | 1 |
| Statement | Consensus Status |
|---|---|
| Management of Newly Diagnosed GB | |
| The use of laser-interstitial thermal therapy, awake craniotomy, and functional MRI may be an option for patients with deep-seated inoperable tumors. | Did not reach consensus (consensus level: 46%) |
| For patients aged ≤ 70 years with good performance status (KPS ≥ 60), maintenance therapy could be continued for up to 12 months among patients who show continued benefit, improvement, or a partial response. | Did not reach consensus (consensus level: 46%) |
| Management of Recurrent or Progressive GB | |
| Consider monotherapy with TTFields for patients with recurrent or progressive GB. | Did not reach consensus (consensus level: 67%) |
| Supportive Care | |
| VTE prophylaxis with low-molecular-weight heparin is recommended within 24 h of surgical tumor resection for a minimum of seven days. | Did not reach consensus (consensus level: 46%) |
| Patients who develop VTE in the course of their illness should be treated with either low-molecular-weight heparin or direct oral anticoagulants for a duration determined on an individualized basis. | Did not reach consensus (consensus level: 57%) |
| Prophylaxis for Pneumocystis pneumonia is recommended for patients receiving chemoradiotherapy or adjuvant chemotherapy with temozolomide. | Did not reach consensus (consensus level: 23%) |
| TTFields prescriptions for patients with GB should be provided under the guidance of a supervising oncologist. | Did not reach consensus (consensus level: 62%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mason, W.P.; Harrison, R.A.; Lapointe, S.; Lim-Fat, M.J.; MacNeil, M.V.; Mathieu, D.; Perry, J.R.; Pitz, M.W.; Roberge, D.; Tsang, D.S.; et al. Canadian Expert Consensus Recommendations for the Diagnosis and Management of Glioblastoma: Results of a Delphi Study. Curr. Oncol. 2025, 32, 207. https://doi.org/10.3390/curroncol32040207
Mason WP, Harrison RA, Lapointe S, Lim-Fat MJ, MacNeil MV, Mathieu D, Perry JR, Pitz MW, Roberge D, Tsang DS, et al. Canadian Expert Consensus Recommendations for the Diagnosis and Management of Glioblastoma: Results of a Delphi Study. Current Oncology. 2025; 32(4):207. https://doi.org/10.3390/curroncol32040207
Chicago/Turabian StyleMason, Warren P., Rebecca A. Harrison, Sarah Lapointe, Mary Jane Lim-Fat, Mary V. MacNeil, David Mathieu, James R. Perry, Marshall W. Pitz, David Roberge, Derek S. Tsang, and et al. 2025. "Canadian Expert Consensus Recommendations for the Diagnosis and Management of Glioblastoma: Results of a Delphi Study" Current Oncology 32, no. 4: 207. https://doi.org/10.3390/curroncol32040207
APA StyleMason, W. P., Harrison, R. A., Lapointe, S., Lim-Fat, M. J., MacNeil, M. V., Mathieu, D., Perry, J. R., Pitz, M. W., Roberge, D., Tsang, D. S., Tsien, C., van Landeghem, F. K. H., Zadeh, G., & Easaw, J. (2025). Canadian Expert Consensus Recommendations for the Diagnosis and Management of Glioblastoma: Results of a Delphi Study. Current Oncology, 32(4), 207. https://doi.org/10.3390/curroncol32040207







