New Therapeutic Scenarios in the Context of Adjuvant Treatment for HR+/HER2−Breast Cancer: The Possible Role of Ribociclib in Treatment Algorithms for Stage II and III
Abstract
1. Introduction
2. Materials and Methods
3. Results
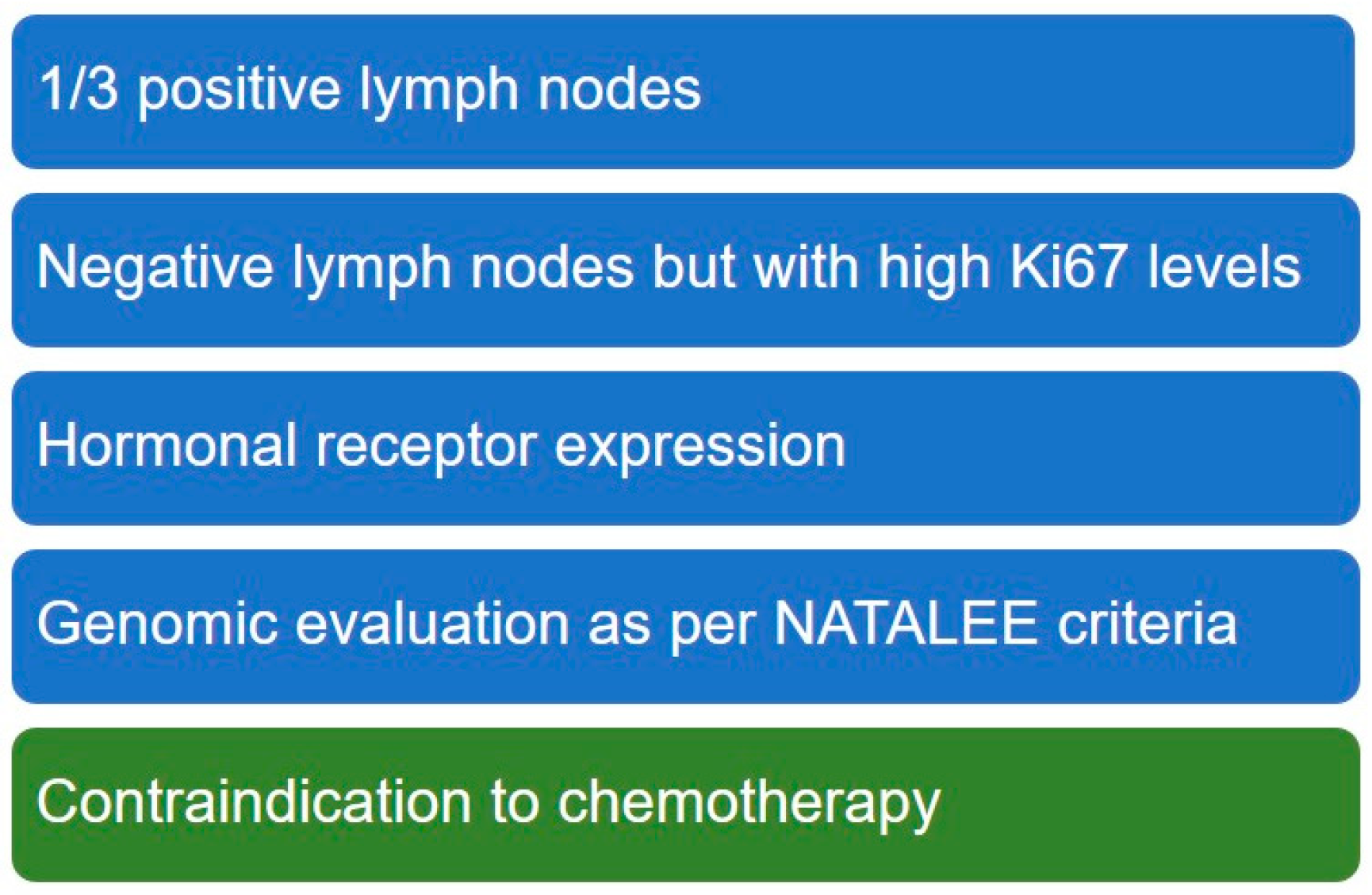
3.1. Drivers for Treatment Choice: Patient Profile and Risk Characterization
3.2. Challenges and Strategies in Managing Adjuvant Therapy with Ribociclib
3.3. Implication of Adherence and QoL in Therapy Management
4. Discussion
| Trial | CDK 4/6 Inhibitor | Patient Population | Treatment Duration | Primary Endpoint | p Value | EMA Approval |
|---|---|---|---|---|---|---|
| monarchE [17] | Abemaciclib | HR+/HER2− EBC: Cohort 1: ≥4 ALN or 1–3 ALN + additional risk factors Cohort 2: 1–3 ALN + Ki-67 ≥ 20% | 2 years | 4-year-iDFS HR (95% CI): 0.66 (0.58–0.76) | p < 0.0001 | Approved [30] |
| NATALEE [19] | Ribociclib | HR+/HER2− EBC (Stage II and III) | 3 years | 3-year-iDFS HR (95% CI): 0.75 (0.62–0.91) | p = 0.003 | Approved [29] |
| PALLAS [24] | Palbociclib | HR+/HER2− EBC (Stage II and III invasive BC) | 2 years | 4-years-iDFS HR (95% CI): 0.96 (0.81–1.14) | p = 0.65 | Not approved |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse Events |
| AI | Aromatase Inhibitor |
| ALN | Axillary Lymph Node |
| CDK | Cyclin-Dependent Kinase |
| EBC | Early breast cancer |
| HER2− | Human Epidermal Growth Factor Receptor 2-Negative |
| HR+ | Hormone Receptor-positive |
| iDFS | invasive Disease-Free Survival |
| PFS | Progression-Free Survival |
| QoL | Quality of Life |
| QTcF | QT interval corrected for heart rate using Fridericia’s formula |
References
- Jungles, K.M.; Holcomb, E.A.; Pearson, A.N.; Jungles, K.R.; Bishop, C.R.; Pierce, L.J.; Green, M.D.; Speers, C.W. Updates in combined approaches of radiotherapy and immune checkpoint inhibitors for the treatment of breast cancer. Front. Oncol. 2022, 12, 1022542. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Ginsburg, O.; Rochon, P.A.; Sun, P.; Narod, S.A. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 2015, 313, 165–173. [Google Scholar] [CrossRef]
- B.C Cancer Agency. Breast Cancer Companion Guide. Available online: http://www.bccancer.bc.ca/patient-and-public-info-site/documents/companionguide2014revision.pdf (accessed on 10 June 2024).
- Ito, M.; Amari, M.; Sato, A.; Hikichi, M.; Sakamoto, A.; Yamazaki, A.; Saji, S. Risk factors for late recurrence and postrelapse survival in estrogen receptor (ER)-positive, human epidermal growth factor receptor (HER) 2-negative breast cancer after 5 years of endocrine therapy. Breast 2024, 73, 103604. [Google Scholar] [CrossRef]
- Wangchinda, P.; Ithimakin, S. Factors that predict recurrence later than 5 years after initial treatment in operable breast cancer. World J. Surg. Oncol. 2016, 14, 223. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Gray, R.; Braybrooke, J.; Davies, C.; Taylor, C.; McGale, P.; Peto, R.; Pritchard, K.I.; Bergh, J.; Dowsett, M.; et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N. Engl. J. Med. 2017, 377, 1836–1846. [Google Scholar] [CrossRef]
- Foldi, J.; O’Meara, T.; Marczyk, M.; Sanft, T.; Silber, A.; Pusztai, L. Defining Risk of Late Recurrence in Early-Stage Estrogen Receptor-Positive Breast Cancer: Clinical Versus Molecular Tools. J. Clin. Oncol. 2019, 37, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Associazione Italiana Oncologia Medica. Linee Guida Carcinoma Mammario Avanzato 2023. Available online: https://www.iss.it/documents/20126/8403839/LG_C008_AIOM_Ca-mammario-avanzato-TTT2.pdf (accessed on 14 July 2024).
- Gennari, A.; Andre, F.; Barrios, C.H.; Cortes, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef]
- Iwase, H. Treatment strategy for metastatic breast cancer with estrogen receptor-positive tumor. Int. J. Clin. Oncol. 2015, 20, 249–252. [Google Scholar] [CrossRef]
- Yardley, D.A. MONALEESA clinical program: A review of ribociclib use in different clinical settings. Future Oncol. 2019, 15, 2673–2686. [Google Scholar] [CrossRef]
- Johnston, S.; Martin, M.; Di Leo, A.; Im, S.A.; Awada, A.; Forrester, T.; Frenzel, M.; Hardebeck, M.C.; Cox, J.; Barriga, S.; et al. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019, 5, 5. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Nardin, S.; Ruelle, T.; Giannubilo, I.; Del Mastro, L. Adjuvant treatment in hormone receptor-positive early breast cancer: New approaches of endocrine therapy. Tumori 2024, 110, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Rastogi, P.; Martin, M.; Tolaney, S.M.; Shao, Z.M.; Fasching, P.A.; Huang, C.S.; Jaliffe, G.G.; Tryakin, A.; Goetz, M.P.; et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Updated efficacy and Ki-67 analysis from the monarchE study. Ann. Oncol. 2021, 32, 1571–1581. [Google Scholar] [CrossRef]
- Johnston, S.R.D.; Harbeck, N.; Hegg, R.; Toi, M.; Martin, M.; Shao, Z.M.; Zhang, Q.Y.; Martinez Rodriguez, J.L.; Campone, M.; Hamilton, E.; et al. Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2−, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J. Clin. Oncol. 2020, 38, 3987–3998. [Google Scholar] [CrossRef]
- Johnston, S.R.D.; Toi, M.; O’Shaughnessy, J.; Rastogi, P.; Campone, M.; Neven, P.; Huang, C.S.; Huober, J.; Jaliffe, G.G.; Cicin, I.; et al. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): Results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023, 24, 77–90. [Google Scholar] [CrossRef]
- Slamon, D.J.; Fasching, P.A.; Hurvitz, S.; Chia, S.; Crown, J.; Martin, M.; Barrios, C.H.; Bardia, A.; Im, S.A.; Yardley, D.A.; et al. Rationale and trial design of NATALEE: A Phase III trial of adjuvant ribociclib + endocrine therapy versus endocrine therapy alone in patients with HR+/HER2− early breast cancer. Ther. Adv. Med. Oncol. 2023, 15, 17588359231178125. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.; Lipatov, O.; Nowecki, Z.; McAndrew, N.; Kukielka-Budny, B.; Stroyakovskiy, D.; Yardley, D.A.; Huang, C.S.; Fasching, P.A.; Crown, J.; et al. Ribociclib plus Endocrine Therapy in Early Breast Cancer. N. Engl. J. Med. 2024, 390, 1080–1091. [Google Scholar] [CrossRef]
- Paranjpe, R.; John, G.; Trivedi, M.; Abughosh, S. Identifying adherence barriers to oral endocrine therapy among breast cancer survivors. Breast Cancer Res. Treat. 2019, 174, 297–305. [Google Scholar] [CrossRef]
- Murphy, C.C.; Bartholomew, L.K.; Carpentier, M.Y.; Bluethmann, S.M.; Vernon, S.W. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: A systematic review. Breast Cancer Res. Treat. 2012, 134, 459–478. [Google Scholar] [CrossRef]
- Moon, Z.; Moss-Morris, R.; Hunter, M.S.; Carlisle, S.; Hughes, L.D. Barriers and facilitators of adjuvant hormone therapy adherence and persistence in women with breast cancer: A systematic review. Patient Prefer. Adherence 2017, 11, 305–322. [Google Scholar] [CrossRef]
- Yussof, I.; Mohd Tahir, N.A.; Hatah, E.; Mohamed Shah, N. Factors influencing five-year adherence to adjuvant endocrine therapy in breast cancer patients: A systematic review. Breast 2022, 62, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Gnant, M.; Dueck, A.C.; Frantal, S.; Martin, M.; Burstein, H.J.; Greil, R.; Fox, P.; Wolff, A.C.; Chan, A.; Winer, E.P.; et al. Adjuvant Palbociclib for Early Breast Cancer: The PALLAS Trial Results (ABCSG-42/AFT-05/BIG-14-03). J. Clin. Oncol. 2022, 40, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Fasching, P.A.; Slamon, D.; Nowecki, Z.; Kukielka-Budny, B.; Stroyakovskiy, D.; Yardley, D.A.; Huang, C.S.; Chan, A.; Chia, S.; Martin, M.; et al. Health-related quality of life in patients with HR+/HER2− early breast cancer treated with ribociclib plus a nonsteroidal aromatase inhibitor: Results from the NATALEE trial. Clin. Cancer Res. 2025. [Google Scholar] [CrossRef]
- Rugo, H.S.; O’Shaughnessy, J.; Boyle, F.; Toi, M.; Broom, R.; Blancas, I.; Gumus, M.; Yamashita, T.; Im, Y.H.; Rastogi, P.; et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Safety and patient-reported outcomes from the monarchE study. Ann. Oncol. 2022, 33, 616–627. [Google Scholar] [CrossRef]
- O’Shaughnessy, J.; Yardley, D.; Hart, L.; Razavi, P.; Graff, S.L.; Wogen, J.; McDermott, C.; Dionne, P.-A.; Haftchenary, S.; Pathak, P.; et al. Abstract P3-03-12: Risk of recurrence with adjuvant endocrine therapy in real world patients with hormone receptor positive/human epidermal growth factor receptor-negative early breast cancer: A US database analysis. Cancer Res. 2023, 83, P3-03-12. [Google Scholar] [CrossRef]
- FDA. FDA Approves Ribociclib with an Aromatase Inhibitor and Ribociclib and Letrozole Co-Pack for Early High-Risk Breast Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ribociclib-aromatase-inhibitor-and-ribociclib-and-letrozole-co-pack-early-high-risk-0 (accessed on 7 February 2025).
- EMA. Ribociclib. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kisqali (accessed on 12 February 2025).
- EMA. Abemaciclib. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/verzenios (accessed on 12 February 2025).
- Habel, L.A.; Shak, S.; Jacobs, M.K.; Capra, A.; Alexander, C.; Pho, M.; Baker, J.; Walker, M.; Watson, D.; Hackett, J.; et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006, 8, R25. [Google Scholar] [CrossRef]
- Harbeck, N.; Thomssen, C. A new look at node-negative breast cancer. Oncologist 2011, 16 (Suppl. S1), 51–60. [Google Scholar] [CrossRef]
- Fasching, P.A.; Stroyakovskiy, D.Y.D.; Huang, C.; Crown, J.P.; Bardia, A.; Chia, S.; Im, S.; Martin Jimenez, M.; Xu, B.; Loi, S.; et al. LBA13—Adjuvant ribociclib (RIB) plus nonsteroidal aromatase inhibitor (NSAI) in patients (Pts) with HR+/HER2− early breast cancer (EBC): 4-year outcomes from the NATALEE trial. Ann. Oncol. 2024, 35, S1207. [Google Scholar] [CrossRef]
- Natarajan, A.; Tolaney, S.M. Is adjuvant ribociclib ready for prime time? Ann. Oncol. 2024, 35, 1200–1201. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Lacko, A.; Sohn, J.; Cruz, F.; Ruiz Borrego, M.; Manikhas, A.; Hee Park, Y.; Stroyakovskiy, D.; Yardley, D.A.; Huang, C.S.; et al. A phase III trial of adjuvant ribociclib plus endocrine therapy versus endocrine therapy alone in patients with HR-positive/HER2-negative early breast cancer: Final invasive disease-free survival results from the NATALEE trial. Ann. Oncol. 2025, 36, 149–157. [Google Scholar] [CrossRef]
- Farias, A.J.; Ornelas, I.J.; Hohl, S.D.; Zeliadt, S.B.; Hansen, R.N.; Li, C.I.; Thompson, B. Exploring the role of physician communication about adjuvant endocrine therapy among breast cancer patients on active treatment: A qualitative analysis. Support. Care Cancer 2017, 25, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Janz, N.K.; Li, Y.; Zikmund-Fisher, B.J.; Jagsi, R.; Kurian, A.W.; An, L.C.; McLeod, M.C.; Lee, K.L.; Katz, S.J.; Hawley, S.T. The impact of doctor-patient communication on patients’ perceptions of their risk of breast cancer recurrence. Breast Cancer Res. Treat. 2017, 161, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, C.H.; Hershman, D.L.; Gomez, S.L.; Adams, S.R.; Eldridge, E.H.; Kwan, M.L.; Ergas, I.J.; Kubo, A.; Kushi, L.H. Personal and clinical social support and adherence to adjuvant endocrine therapy among hormone receptor-positive breast cancer patients in an integrated health care system. Breast Cancer Res. Treat. 2018, 170, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Shim, E.J.; Park, J.E.; Yi, M.; Jung, D.; Lee, K.M.; Hahm, B.J. Tailoring communications to the evolving needs of patients throughout the cancer care trajectory: A qualitative exploration with breast cancer patients. BMC Womens Health 2016, 16, 65. [Google Scholar] [CrossRef]
- Isakoff, S.J.; Said, M.R.; Kwak, A.H.; Glieberman, E.; O’Rourke, E.A.; Stroiney, A.; Spring, L.M.; Moy, B.; Bardia, A.; Horick, N.; et al. Feasibility of introducing a smartphone navigation application into the care of breast cancer patients (The FIONA Study). Breast Cancer Res. Treat. 2023, 199, 501–509. [Google Scholar] [CrossRef]
- Pistelli, M.; Mora, A.D.; Ballatore, Z.; Berardi, R. Aromatase inhibitors in premenopausal women with breast cancer: The state of the art and future prospects. Curr. Oncol. 2018, 25, e168–e175. [Google Scholar] [CrossRef]
- Cardoso, F.; Rihani, J.; Aubel, D.; de Courcy, J.; Harmer, V.; Harbeck, N.; Casas, A.; Rugo, H.S.; Fasching, P.A.; Haftchenary, S.; et al. 178P Assessment of side effects (SEs) impacting quality of life (QoL) in patients (pts) undergoing treatment (tx) for advanced breast cancer (ABC) in clinical practice: A real-world (RW) multi-country survey. Ann. Oncol. 2022, 33, S208–S209. [Google Scholar] [CrossRef]
- Geyer, C.E., Jr.; Garber, J.E.; Gelber, R.D.; Yothers, G.; Taboada, M.; Ross, L.; Rastogi, P.; Cui, K.; Arahmani, A.; Aktan, G.; et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann. Oncol. 2022, 33, 1250–1268. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battelli, N.; Mocerino, C.; Montedoro, M.; Pistelli, M.; Portarena, I.; Rosanova, M.; Sidoni, T.; Vici, P. New Therapeutic Scenarios in the Context of Adjuvant Treatment for HR+/HER2−Breast Cancer: The Possible Role of Ribociclib in Treatment Algorithms for Stage II and III. Curr. Oncol. 2025, 32, 192. https://doi.org/10.3390/curroncol32040192
Battelli N, Mocerino C, Montedoro M, Pistelli M, Portarena I, Rosanova M, Sidoni T, Vici P. New Therapeutic Scenarios in the Context of Adjuvant Treatment for HR+/HER2−Breast Cancer: The Possible Role of Ribociclib in Treatment Algorithms for Stage II and III. Current Oncology. 2025; 32(4):192. https://doi.org/10.3390/curroncol32040192
Chicago/Turabian StyleBattelli, Nicola, Carmela Mocerino, Michele Montedoro, Mirco Pistelli, Ilaria Portarena, Mario Rosanova, Tina Sidoni, and Patrizia Vici. 2025. "New Therapeutic Scenarios in the Context of Adjuvant Treatment for HR+/HER2−Breast Cancer: The Possible Role of Ribociclib in Treatment Algorithms for Stage II and III" Current Oncology 32, no. 4: 192. https://doi.org/10.3390/curroncol32040192
APA StyleBattelli, N., Mocerino, C., Montedoro, M., Pistelli, M., Portarena, I., Rosanova, M., Sidoni, T., & Vici, P. (2025). New Therapeutic Scenarios in the Context of Adjuvant Treatment for HR+/HER2−Breast Cancer: The Possible Role of Ribociclib in Treatment Algorithms for Stage II and III. Current Oncology, 32(4), 192. https://doi.org/10.3390/curroncol32040192





