Recurrent Metastatic Basal Cell Carcinomas of the Face in a Patient with Gorlin–Goltz Syndrome
Abstract
1. Introduction
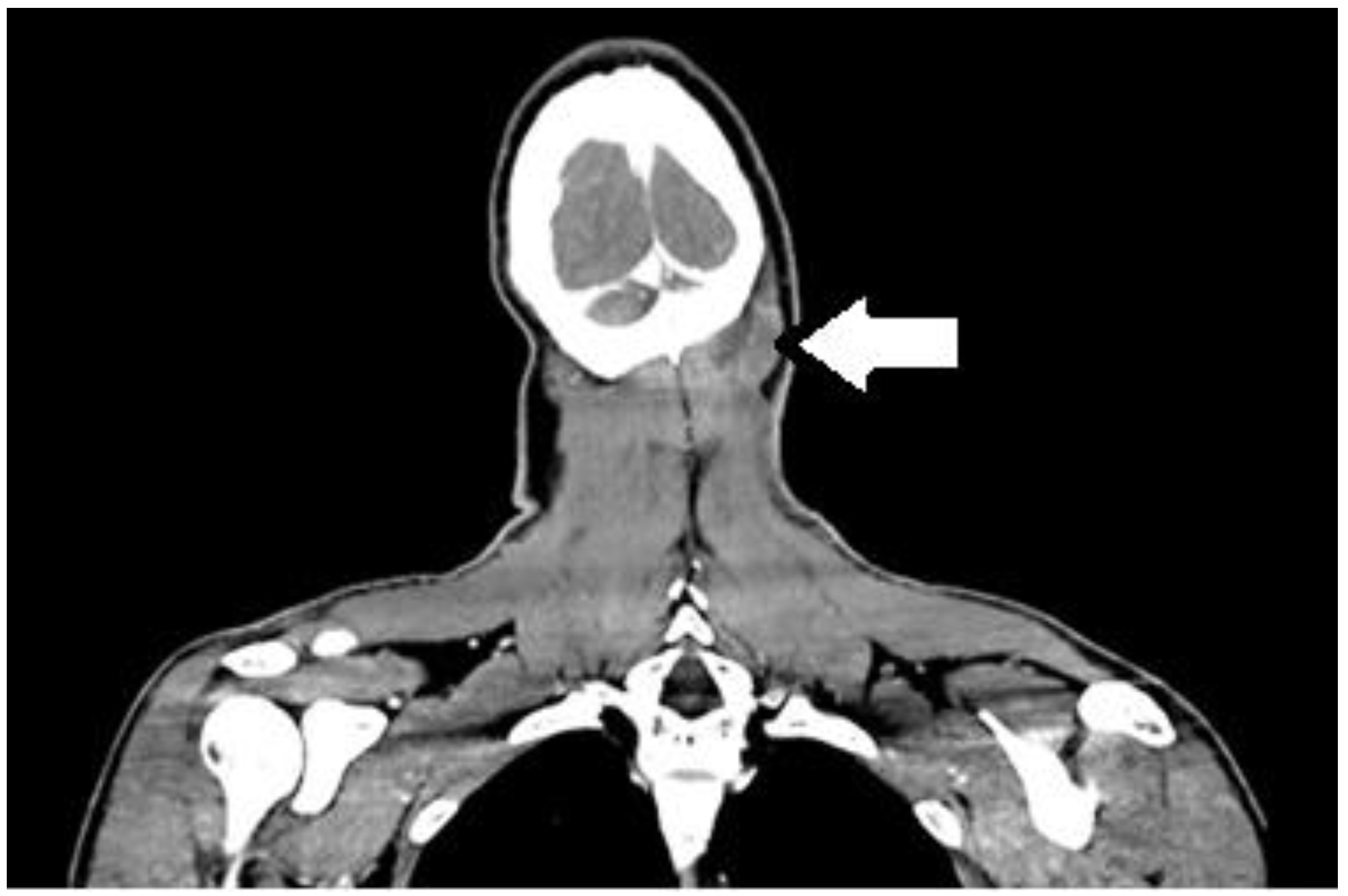
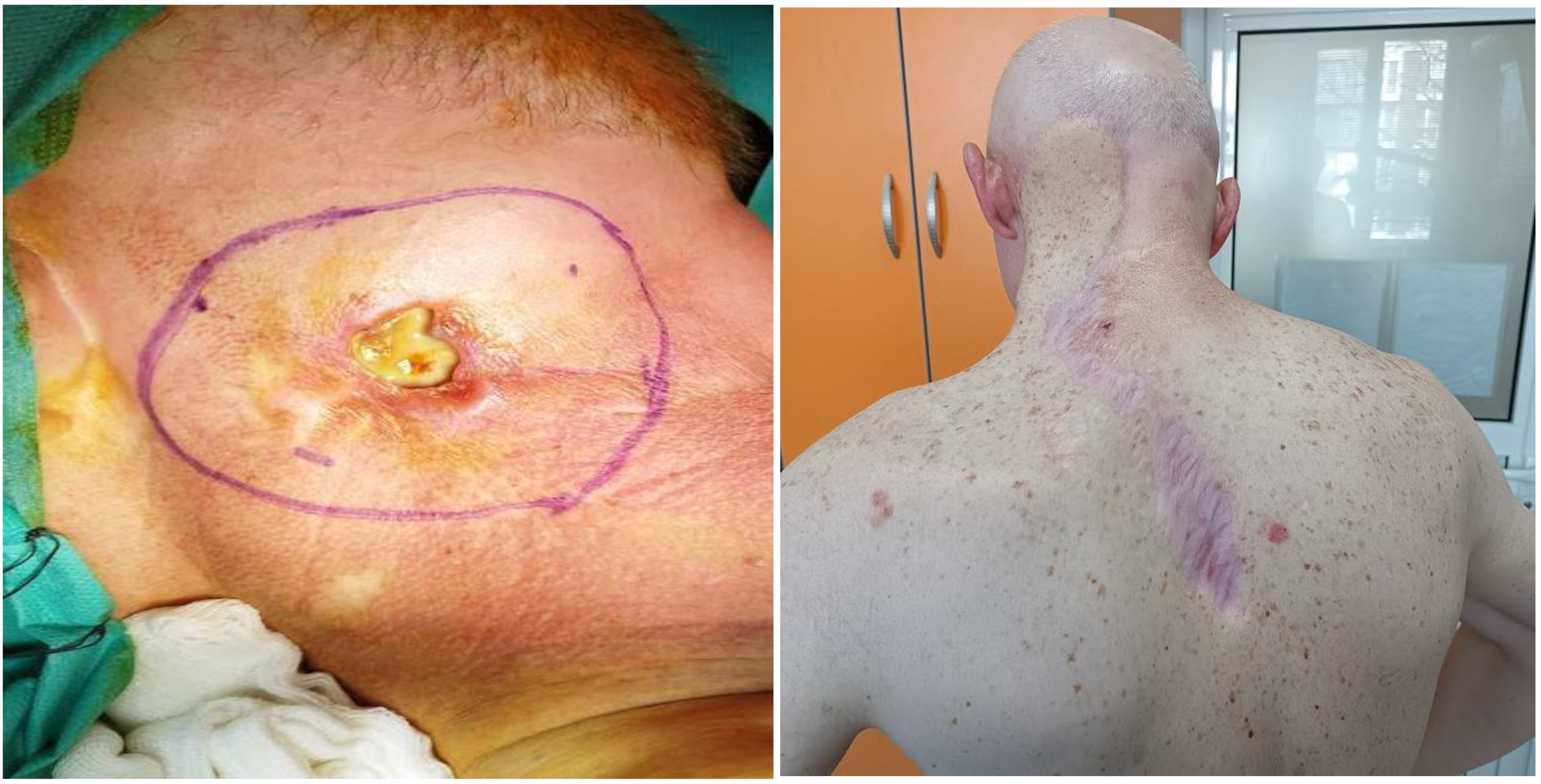
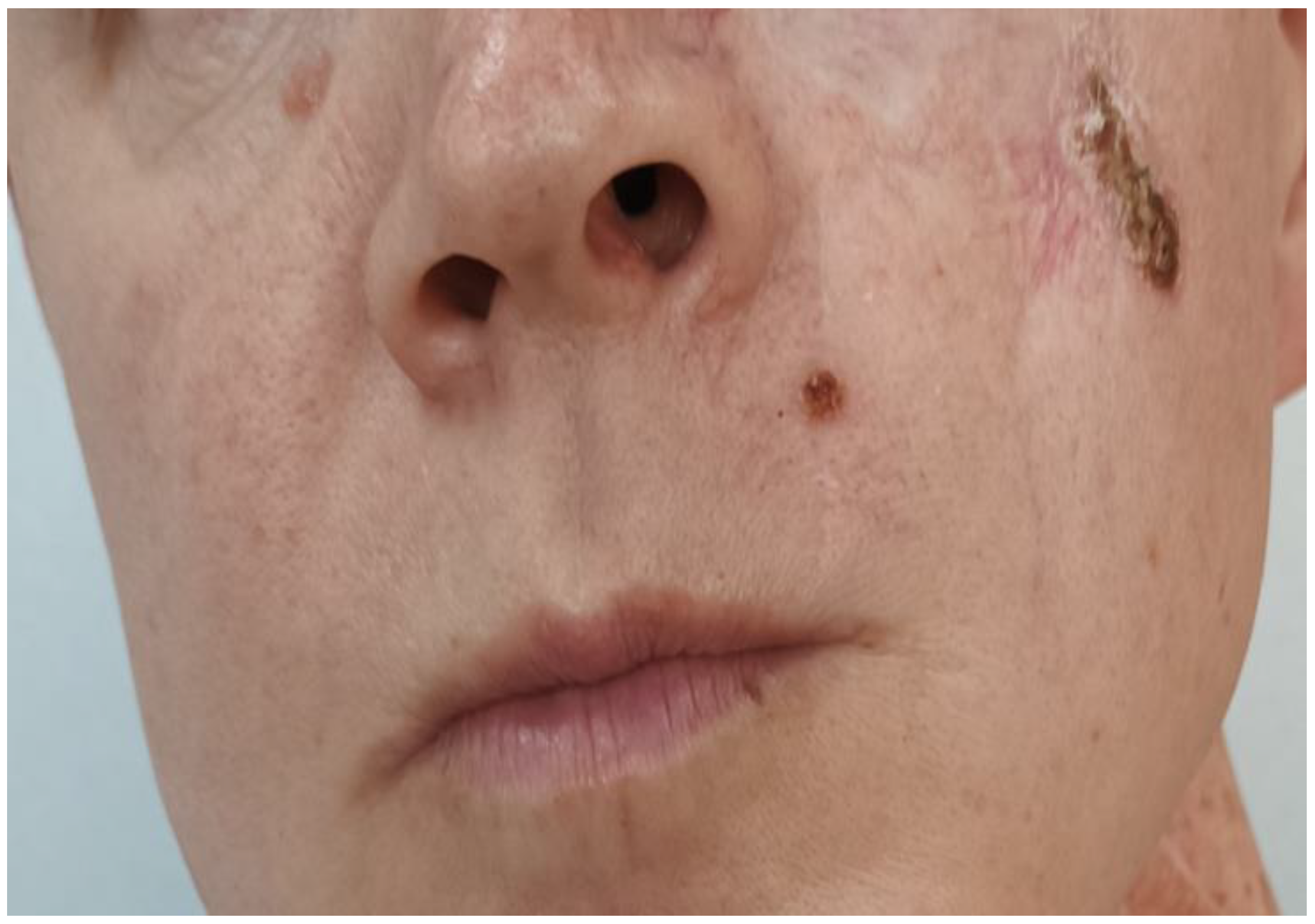
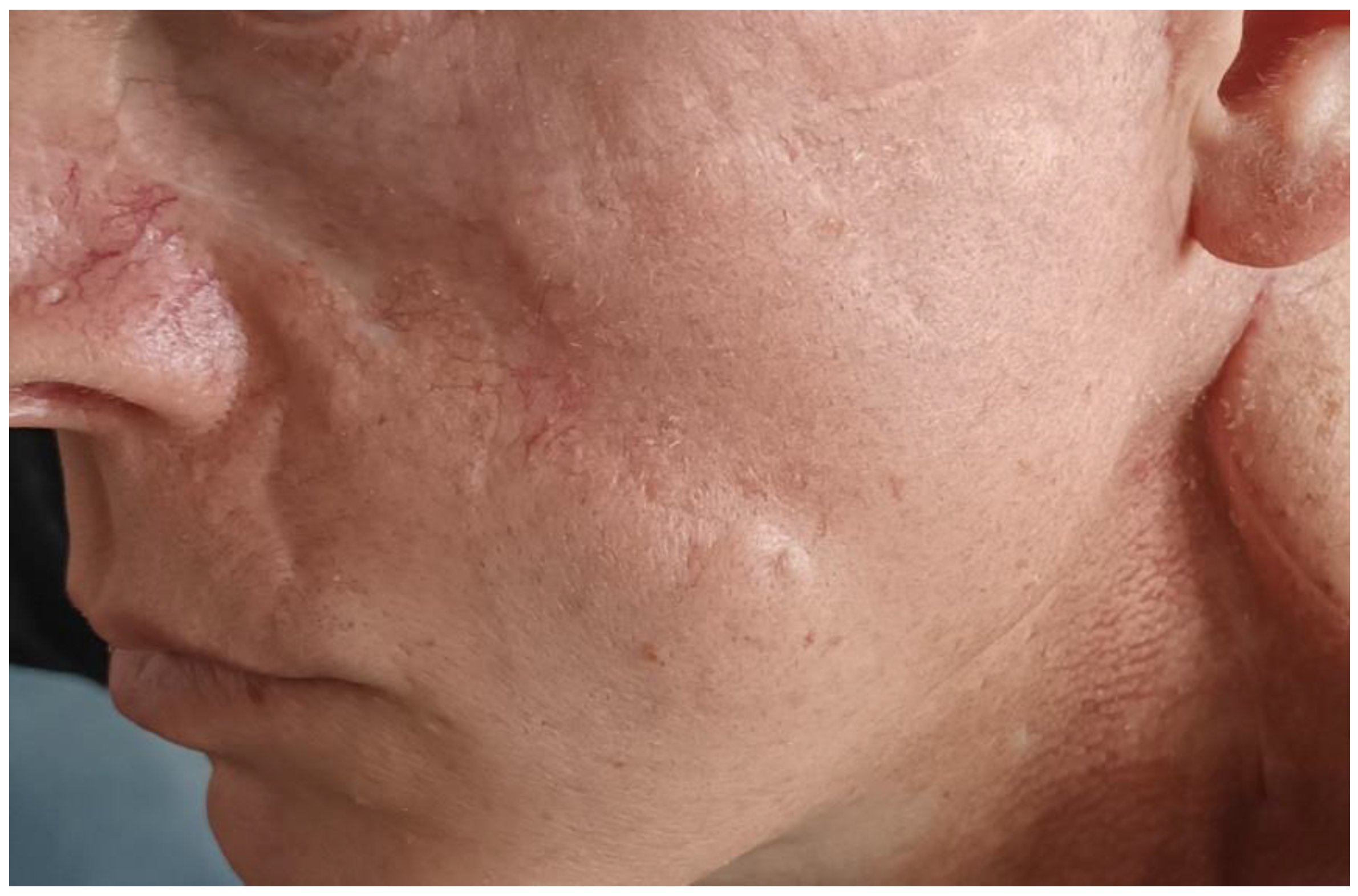
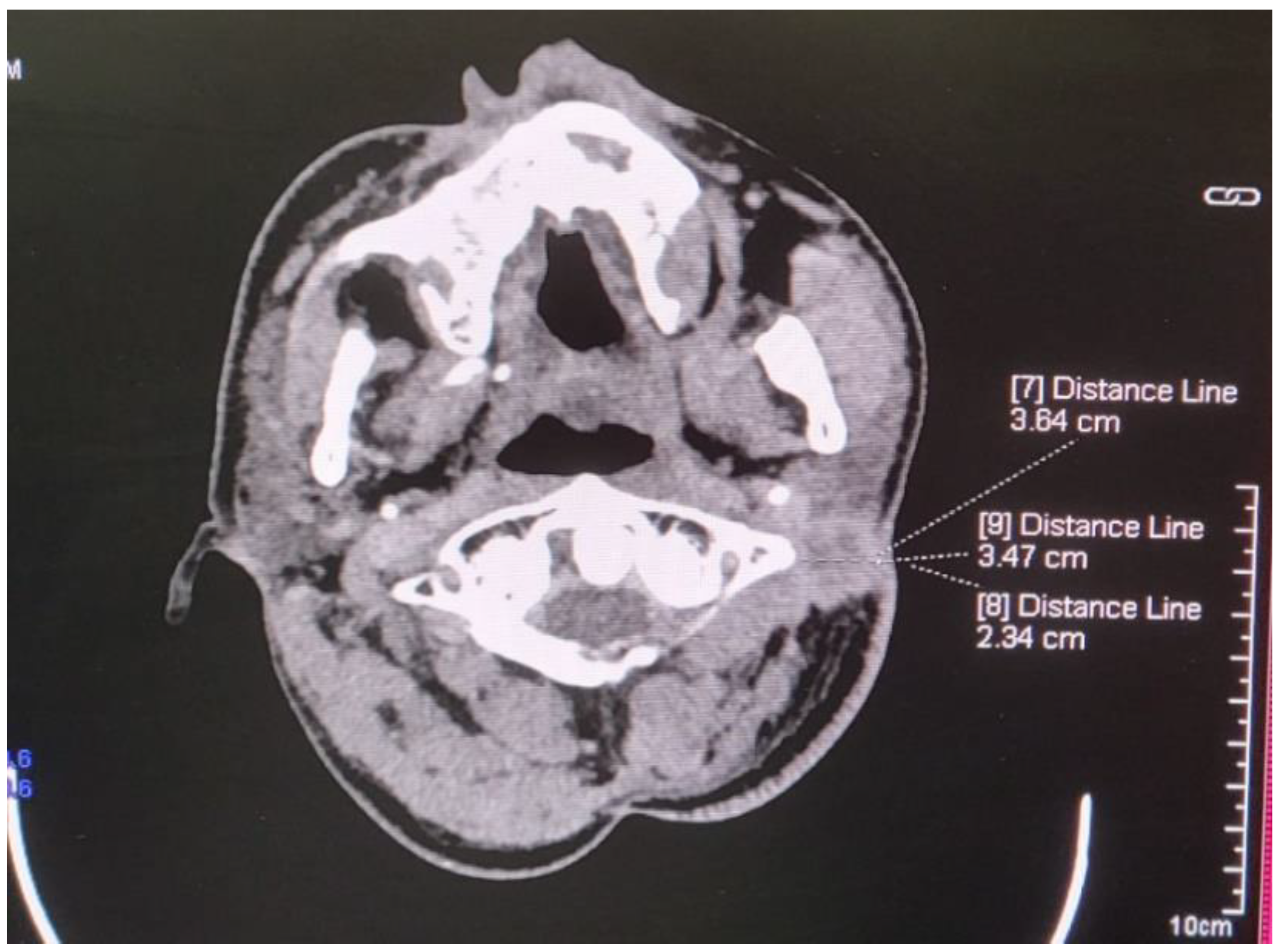
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GGS | Gorlin–Goltz syndrome |
| NBCCS | basal cell carcinoma syndrome |
| BCCs | basal cell carcinomas |
References
- Ljubenovic, M.; Ljubenovic, D.; Binic, I.; Jovanovic, D.; Stanojevic, M. Gorlin-Goltz syndrome. Acta Dermatovenerol. Alp. Panon. Adriat. 2007, 16, 166–169. [Google Scholar]
- Gnanam, A.; Rajkumar, K.; Krishnakumar Raja, V.B.; Preeti, T. Odontogenic keratocyst in nevoid basal cell carcinoma syndrome- A case report. SRM Univ. J. Dent. Sci. 2010, 1, 119–121. [Google Scholar]
- Şereflican, B.; Tuman, B.; Şereflican, M.; Halıcıoğlu, S.; Özyalvaçlı, G.; Bayrak, S. Gorlin-Goltz syndrome. Turk. Pediatri Ars. 2017, 52, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulou, C.; Zampakis, P.; Kazantzi, S.; Kraniotis, P.; Mastronikolis, N.S. Gorlin-Goltz syndrome: Incidental finding on routine CT scan following car accident. Cases J. 2009, 2, 9087. [Google Scholar] [CrossRef]
- Evans, D.G.; Ladusans, E.J.; Rimmer, S.; Burnell, L.D.; Thakker, N.; Farndon, P.A. Complications of the naevoid basal cell carcinoma syndrome: Results of a population-based study. J. Med. Genet. 1993, 30, 460–464. [Google Scholar] [CrossRef]
- Sunil, M.K.; Kumar, R.; Guru, E.N.; Vikash, R. Gorlin-Goltz syndrome. J. Indian Acad. Oral. Med. Radiol. 2010, 22, 18–26. [Google Scholar] [CrossRef]
- Larsen, A.K.; Mikkelsen, D.B.; Hertz, J.M.; Bygum, A. Manifestations of Gorlin-Goltz syndrome. Dan. Med. J. 2014, 61, A4829. [Google Scholar]
- da Silva, L.P.; Rolim, L.S.A.; da Silva, L.A.B.; Pinto, L.P.; de Souza, L.B. The recurrence of odontogenic keratocysts in pediatric patients is associated with clinical findings of Gorlin-Goltz Syndrome. Med. Oral Patol. Oral Y Cir. Bucal 2019, 25, e56. [Google Scholar] [CrossRef]
- Keçeli, O.; Coskun-Benlidayı, İ.; Benlidayı, M.E.; Erdoğan, Ö. An uncommon disorder with multiple skeletal anomalies: Gorlin-Goltz syndrome. Turk. J. Pediatr. 2014, 56, 434–436. [Google Scholar]
- Moramarco, A.; Himmelblau, E.; Miraglia, E.; Mallone, F.; Roberti, V.; Franzone, F.; Iacovino, C.; Giustini, S.; Lambiase, A. Ocular manifestations in Gorlin-Goltz syndrome. Orphanet J. Rare Dis. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Jawa, D.S.; Sircar, K.; Somani, R.; Grover, N.; Jaidka, S.; Singh, S. Gorlin-Goltz syndrome. A case report. J. Oral Maxillofac. Pathol. 2009, 13, 89–92. [Google Scholar] [CrossRef] [PubMed]
- de Matos, J.D.M.; Nakano, L.J.N.; Diamantino, P.J.S.; Lopes, G.D.R.S.; Bottino, M.A.; de Vasconcelos, J.E.L.; de Carvalho Ramos, N.; Andrade, V.C. Gorlin-Goltz syndrome: Systemic and maxillofacial characteristics. Arch. Health Investig. 2021, 10, 49–54. [Google Scholar]
- Cabo, H.; Kolm, I.; Puig, S.; Malvehy, J. Palmar basal cell carcinoma in a patient with Gorlin-Goltz syndrome. Arch. Dermatol. 2007, 143, 799–816. [Google Scholar]
- Ponti, G.; Pellacani, G.; Tomasi, A.; Sammaria, G.; Manfredini, M. Skeletal stigmata as keys to access to the composite and ancient Gorlin–Goltz syndrome history: The Egypt, Pompeii and Herculaneum lessons. Gene 2016, 589, 104–111. [Google Scholar] [CrossRef]
- Ortega García de Amezaga, A.; García Arregui, O.; Zepeda Nuño, S.; Acha Sagredo, A.; Aguirre Urizar, J.M. Gorlin-Goltz syndrome: Clinicopathologic aspects. Med. Oral. Patol. Oral. Cir. Bucal. 2008, 13, E338–E343. [Google Scholar] [PubMed]
- Nabih, O.; Rachdy, Z.; Alaoui, O.; Yahya, I. Syndrome de Gorlin-Goltz: Du diagnostic au traitement: À propos d’une observation. EDP Sci. Numéro Spécial Pathol. 2017, 2, 285. [Google Scholar] [CrossRef][Green Version]
- Voorsmit, R.A.; Stollinga, P.T.; van Hallst, V.J. The management of keratocyst. J. Maxillofac. Surg. 1981, 9, 228–236. [Google Scholar] [CrossRef]
- Potenza, C.; Bernardini, N.; Balduzzi, V.; Losco, L.; Mambrin, A.; Marchesiello, A.; Tolino, E.; Zuber, S.; Skroza, N.; Proietti, I. A review of the literature of surgical and nonsurgical treatments of invasive squamous cells carcinoma. BioMed. Res. Int. 2018, 2018, 9489163. [Google Scholar] [CrossRef]
- Osiecka, B.J.; Nockowski, P.; Szepietowski, J.C. The use of the photodynamic method in the treatment of recurrent basal cell carcinoma on the example of Gorlin-Goltz syndrome-management algorithm. Dermatol. Ther. 2020, 33, e14499. [Google Scholar]
- Agrawal, A.; Murari, A.; Vutukuri, S.; Singh, A. Gorlin-Goltz Syndrome: Case Report of a Rare Hereditary Disorder. Case Rep. Dent. 2012, 2012, 475439. [Google Scholar] [CrossRef][Green Version]
- Kamil, A.H.; Tarakji, B. Odontogenic keratocyst in children: A review. Open Dent. J. 2016, 10, 117. [Google Scholar] [PubMed]
- Witmanowski, H.; Szychta, P.; Błochowiak, K.; Jundziłł, A.; Czajkowski, R. Basal cell nevus syndrome (Gorlin-Goltz syndrome): Genetic predisposition, clinical picture and treatment. Adv. Dermatol. Allergol./Postępy Dermatol. I Alergol. 2017, 34, 381–387. [Google Scholar]
- Nagy, K.; Kiss, E.; Erdei, C.; Oberna, F.; Fejérdy, P.; Márton, K.; Vajo, Z. Complex care by multiple medical and dental specialists of a patient with aggressive Gorlin–Goltz syndrome. Postgrad. Med. J. 2008, 84, 330–332. [Google Scholar] [PubMed]
- Tiodorovic-Zivkovic, D.; Zalaudek, I.; Ferrara, G.; Giorgio, C.M.; Di Nola, K.; Procaccini, E.M.; Argenziano, G. Clinical and dermatoscopic findings in Bazex-Dupré-Christol and Gorlin–Goltz syndromes. J. Am. Acad. Dermatol. 2010, 63, 722–724. [Google Scholar]
- Katase, N.; Nagatsuka, H.; Tsujigiwa, H.; Gunduz, M.; Tamamura, R.; Pwint, H.P.; Rivera, R.S.; Nakajima, M.; Naomoto, Y.; Nagai, N. Analysis of the neoplastic nature and biological potential of sporadic and nevoid basal cell carcinoma syndrome-associated keratocystic odontogenic tumor. J. Oral. Pathol. Med. 2007, 36, 550–554. [Google Scholar] [CrossRef]
- Woolgar, J.A.; Rippen, J.W.; Browne, R.M. The odontogenic keratocyst and its occurrence in the nevoid basal cell carcinoma syndrome. Oral. Surg. Oral. Med. Oral. Pathol. 1987, 64, 727–730. [Google Scholar] [CrossRef]
- Acocella, A.; Sacco, R.; Bertolai, R.; Sacco, N. Genetic and clinicopathologic aspects of Gorlin-Goltz syndrome (NBCCS): Presentation of two case reports. Minerva Stomatol. 2009, 58, 43–53. [Google Scholar]
- De Amezaga, A.O.G.; Arregui, O.G.; Nuño, S.Z.; Sagredo, A.A.; Urizar, J.A. Gorlin-Goltz syndrome: Clinicopathologic aspects. Carcinogenesis 2008, 26, 28. [Google Scholar]
- Bahadure, R.N.; Jain, E.S.; Badole, G.P. Gorlin and Goltz syndrome: A case report with surgical review. Int. J. Clin. Pediatr. Dent. 2013, 6, 104. [Google Scholar]
- Gundlach, K.K.; Kiehn, M. Multiple basal cell carcinomas and keratocysts—The Gorlin and Goltz syndrome. J. Maxillofac. Surg. 1979, 7, 299–307. [Google Scholar]
- Casaroto, A.R.; Rocha Loures, D.C.; Moreschi, E.; Veltrini, V.C.; Trento, C.L.; Gottardo, V.D.; Lara, V.S. Early diagnosis of Gorlin-Goltz syndrome: Case report. Head Face Med. 2011, 7, 1–5. [Google Scholar]
- Valenti, M.; Di Giulio, S.; Carugno, A.; Frascione, P.; Marzano, A.V.; Mercuri, S.R.; Nazzaro, G.; Spallone, G.; Paolino, G.; Ardigò, M. Real-world experience with vismodegib and sonidegib in advanced basal cell carcinoma: A multicenter Italian study. Dermatol. Rep. 2025. [Google Scholar] [CrossRef]
- Spadari, F.; Pulicari, F.; Pellegrini, M.; Scribante, A.; Garagiola, U. Multidisciplinary approach to Gorlin-Goltz syndrome: From diagnosis to surgical treatment of jawbones. Maxillofac. Plast. Reconstr. Surg. 2022, 44, 25. [Google Scholar]
- Nilius, M.; Nilius, M.; Leonhardt, H.; Weißflog, A.; Lauer, G. Backwards Screening for Gorlin-Goltz syndrome–Does It Make Sense?—A Family Case Report. Arch. Dent. 2020, 2, 28–34. [Google Scholar]
- Shear, M. The aggressive nature of odontogenic keratocyst: Is it a benign cystic neoplasm? Part 3 Immunocytochemistry of cytokeratin and other epithelial marker. Oral Oncol. 2002, 38, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Santander, P.; Schwaibold, E.M.C.; Bremmer, F.; Batschkus, S.; Kauffmann, P. Multiple, Multiloculated, and Recurrent Keratocysts of the Mandible and Maxilla in Association with Gorlin-Goltz (Nevoid Basal-Cell Carcinoma) Syndrome: A Pediatric Case Report and Follow-up over 5 Years. Case Rep. Dent. 2018, 2018, 7594840. [Google Scholar] [PubMed]
- Reaz, S.; Sammi, S.; Gholkar, G. A Rare Case of Cardiac Fibroma Diagnosis in Gorlin–Goltz Syndrome with Information on Management. Future Cardiol. 2022, 18, 561–567. [Google Scholar] [CrossRef]
- Puttaraju, M.K.; Theenathayalan, M. Nevoid basal cell carcinoma (Gorlin-Goltz) syndrome: An incidental finding. BMJ Case Reports CP 2024, 17, e259937. [Google Scholar]
- Kofla-Dlubacz, A.; Stawarski, A.; Pytrus, T.; Gil, J. Phacomatoses, genetic testing for personalisation of clinical management (part 1). Nowotwory. J. Oncol. 2021, 71, 420–426. [Google Scholar] [CrossRef]
- Queirolo, P.; Cinquini, M.; Argenziano, G.; Bassetto, F.; Bossi, P.; Boutros, A.; Clemente, C.; de Giorgi, V.; Del Vecchio, M.; Patuzzo, R.; et al. Guidelines for the diagnosis and treatment of cutaneous squamous cell carcinoma: A GRADE approach for evidence evaluation and recommendations by the Italian Association of Medical Oncology. ESMO Open 2024, 9, 103005. [Google Scholar] [CrossRef]
- Dessinioti, C.; Stratigos, A.J. Immunotherapy and its timing in advanced basal cell carcinoma treatment. Dermatol. Pract. Concept. 2023, 13, e2023252. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Gurizzan, C.; Ottini, A.; Caspani, F.; Bergamini, C.; Locati, L.D.; Marchiselli, C.; Alberti, A.; Lorini, L.; Licitra, L.F.; et al. The association of cemiplimab plus sonidegib for synchronous cutaneous squamous cell carcinoma and basal cell carcinoma of the head and neck: Two case reports. Front. Oncol. 2023, 13, 1111146. [Google Scholar]
- Lewis, K.D.; Peris, K.; Sekulic, A.; Stratigos, A.J.; Dunn, L.; Eroglu, Z.; Chang, A.L.S.; Migden, M.R.; Yoo, S.Y.; Mohan, K.; et al. Final analysis of phase II results with cemiplimab in metastatic basal cell carcinoma after hedgehog pathway inhibitors. Ann. Oncol. 2024, 35, 221–228. [Google Scholar] [PubMed]
- Li, X.; Wang, H.; Lu, Q. Immunotherapy for locally advanced and metastatic basal cell carcinoma: A narrative review. Transl. Cancer Res. 2024, 13, 6565. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrov, P.; Shopova, D.; Goranov, G.; Dinkova, A.; Stoyanova, N.; Yanev, N. Recurrent Metastatic Basal Cell Carcinomas of the Face in a Patient with Gorlin–Goltz Syndrome. Curr. Oncol. 2025, 32, 193. https://doi.org/10.3390/curroncol32040193
Petrov P, Shopova D, Goranov G, Dinkova A, Stoyanova N, Yanev N. Recurrent Metastatic Basal Cell Carcinomas of the Face in a Patient with Gorlin–Goltz Syndrome. Current Oncology. 2025; 32(4):193. https://doi.org/10.3390/curroncol32040193
Chicago/Turabian StylePetrov, Petko, Dobromira Shopova, Georgi Goranov, Atanaska Dinkova, Nina Stoyanova, and Nikolay Yanev. 2025. "Recurrent Metastatic Basal Cell Carcinomas of the Face in a Patient with Gorlin–Goltz Syndrome" Current Oncology 32, no. 4: 193. https://doi.org/10.3390/curroncol32040193
APA StylePetrov, P., Shopova, D., Goranov, G., Dinkova, A., Stoyanova, N., & Yanev, N. (2025). Recurrent Metastatic Basal Cell Carcinomas of the Face in a Patient with Gorlin–Goltz Syndrome. Current Oncology, 32(4), 193. https://doi.org/10.3390/curroncol32040193





