The Efficacy and Safety of Hepatic Artery Infusion Chemotherapy Combined with Lenvatinib and Programmed Death (PD)-1 Inhibitors for Unresectable Intrahepatic Cholangiocarcinoma: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
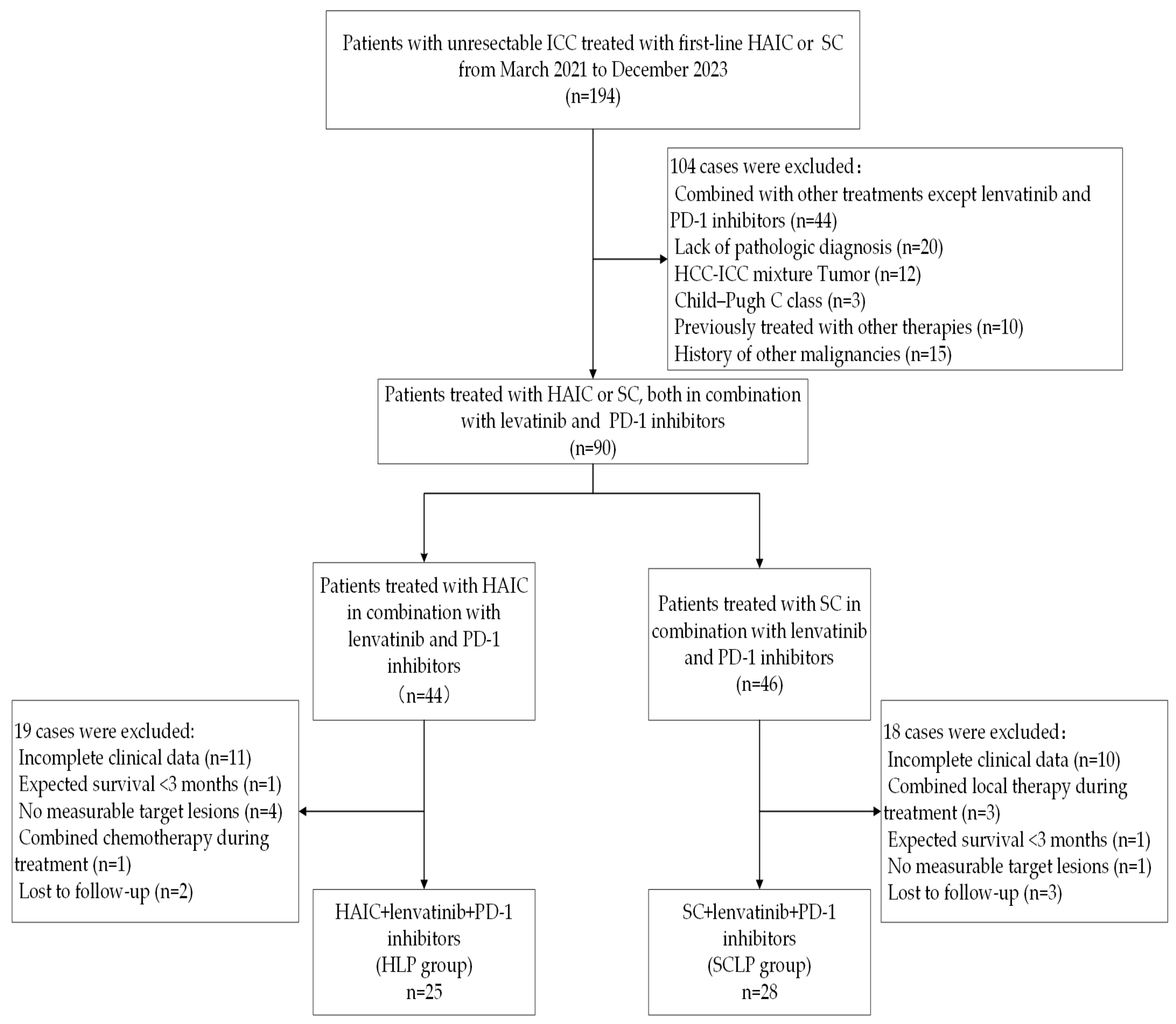
2.1. Study Subject and Selection Criteria
2.2. Treatments
2.3. Data Acquisition and Assessment
2.4. Data Analysis
3. Results
3.1. Patient Characteristics
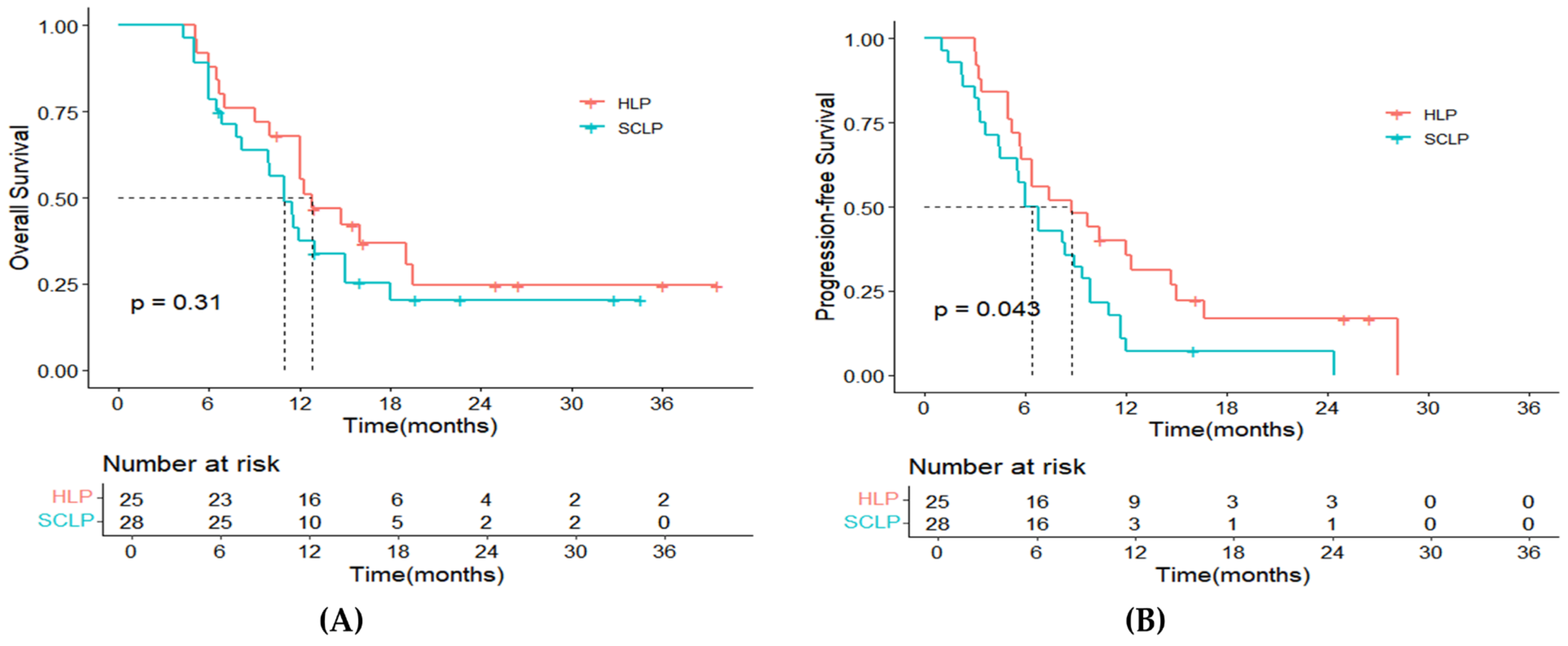
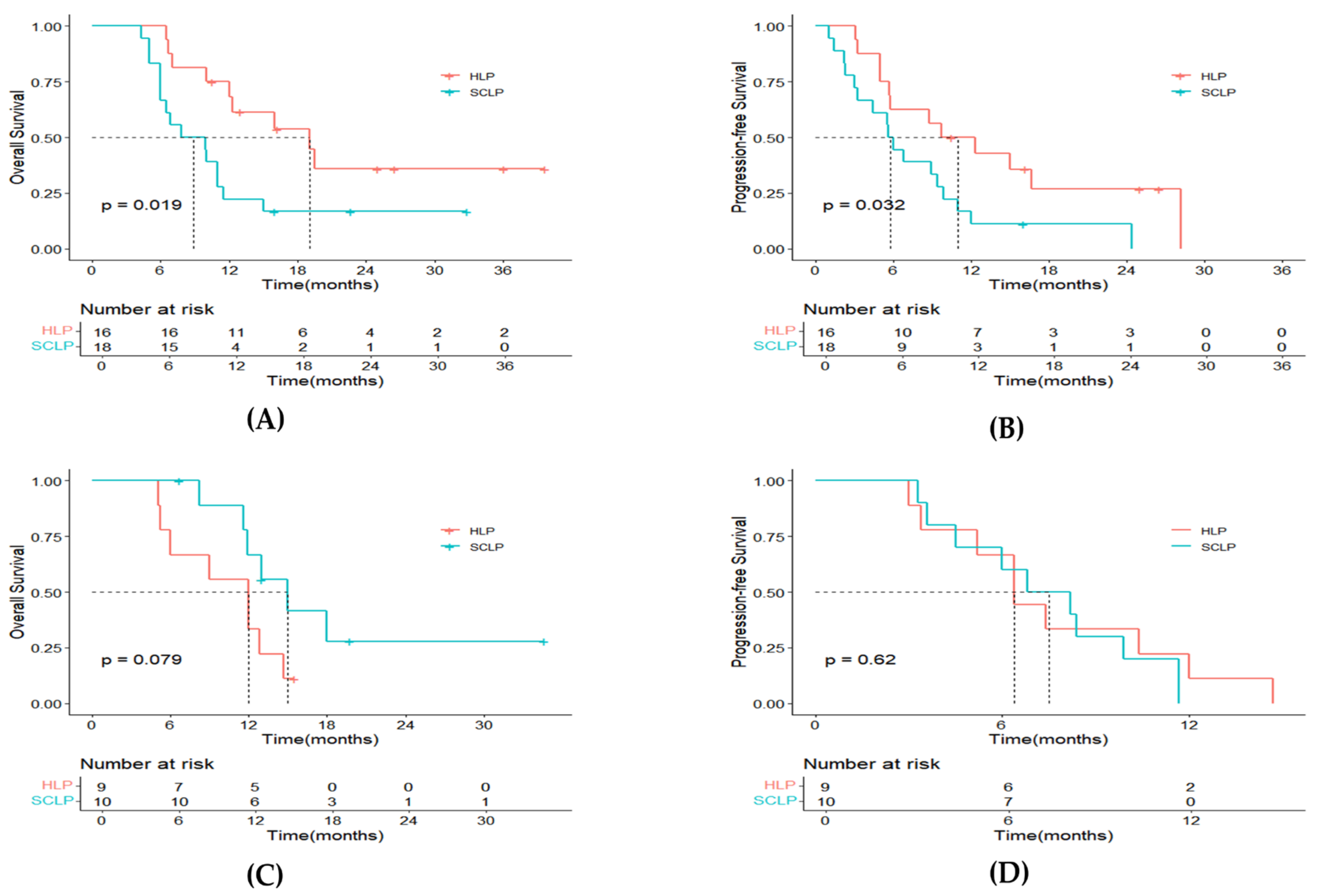
3.2. Survival
3.3. Tumor Response
3.4. Prognostic Factors Analyses
4. Safety
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Valle, J.W.; Borbath, I.; Khan, S.A.; Huguet, F.; Gruenberger, T.; Arnold, D.; Committee, E.G. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v28–v37. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Zhu, A.X.; Fuchs, C.S.; Brooks, G.A. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016, 21, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Esnaola, N.F.; Meyer, J.E.; Karachristos, A.; Maranki, J.L.; Camp, E.R.; Denlinger, C.S. Evaluation and management of intrahepatic and extrahepatic cholangiocarcinoma. Cancer 2016, 122, 1349–1369. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.; Sotiropoulos, G.C.; Sgourakis, G.; Schmitz, K.J.; Paul, A.; Hilgard, P.; Zopf, T.; Trarbach, T.; Malago, M.; Baba, H.A.; et al. Operations for intrahepatic cholangiocarcinoma: Single-institution experience of 158 patients. J. Am. Coll. Surg. 2009, 208, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Nathan, H.; Aloia, T.A.; Vauthey, J.N.; Abdalla, E.K.; Zhu, A.X.; Schulick, R.D.; Choti, M.A.; Pawlik, T.M. A proposed staging system for intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2009, 16, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Endo, I.; Gonen, M.; Yopp, A.C.; Dalal, K.M.; Zhou, Q.; Klimstra, D.; D’Angelica, M.; DeMatteo, R.P.; Fong, Y.; Schwartz, L.; et al. Intrahepatic cholangiocarcinoma: Rising frequency, improved survival, and determinants of outcome after resection. Ann. Surg. 2008, 248, 84–96. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Fiteni, F.; Nguyen, T.; Vernerey, D.; Paillard, M.J.; Kim, S.; Demarchi, M.; Fein, F.; Borg, C.; Bonnetain, F.; Pivot, X. Cisplatin/gemcitabine or oxaliplatin/gemcitabine in the treatment of advanced biliary tract cancer: A systematic review. Cancer Med. 2014, 3, 1502–1511. [Google Scholar] [CrossRef]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Obi, S.; Sato, S.; Kawai, T. Current Status of Hepatic Arterial Infusion Chemotherapy. Liver Cancer 2015, 4, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Treatment of advanced hepatocellular carcinoma with emphasis on hepatic arterial infusion chemotherapy and molecular targeted therapy. Liver Cancer 2012, 1, 62–70. [Google Scholar] [CrossRef]
- Jarnagin, W.R.; Schwartz, L.H.; Gultekin, D.H.; Gonen, M.; Haviland, D.; Shia, J.; D’Angelica, M.; Fong, Y.; DeMatteo, R.; Tse, A.; et al. Regional chemotherapy for unresectable primary liver cancer: Results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann. Oncol. 2009, 20, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Boehm, L.M.; Jayakrishnan, T.T.; Miura, J.T.; Zacharias, A.J.; Johnston, F.M.; Turaga, K.K.; Gamblin, T.C. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J. Surg. Oncol. 2015, 111, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Piha-Paul, S.A.; Oh, D.Y.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int. J. Cancer 2020, 147, 2190–2198. [Google Scholar] [CrossRef]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients with Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Ikeda, M.; Sasaki, T.; Nagashima, F.; Mizuno, N.; Shimizu, S.; Ikezawa, H.; Hayata, N.; Nakajima, R.; Morizane, C. Phase 2 study of lenvatinib monotherapy as second-line treatment in unresectable biliary tract cancer: Primary analysis results. BMC Cancer 2020, 20, 1105. [Google Scholar] [CrossRef]
- Shi, C.; Li, Y.; Yang, C.; Qiao, L.; Tang, L.; Zheng, Y.; Chen, X.; Qian, Y.; Yang, J.; Wu, D.; et al. Lenvatinib Plus Programmed Cell Death Protein-1 Inhibitor Beyond First-Line Systemic Therapy in Refractory Advanced Biliary Tract Cancer: A Real-World Retrospective Study in China. Front. Immunol. 2022, 13, 946861. [Google Scholar] [CrossRef]
- Guan, R.; Zhang, N.; Deng, M.; Lin, Y.; Huang, G.; Fu, Y.; Zheng, Z.; Wei, W.; Zhong, C.; Zhao, H.; et al. Patients with hepatocellular carcinoma extrahepatic metastases can benefit from hepatic arterial infusion chemotherapy combined with lenvatinib plus programmed death-1 inhibitors. Int. J. Surg. 2024, 110, 4062–4073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, K.; Liu, C.; Gao, W.; Si, T.; Zou, Q.; Guo, Z.; Yang, X.; Li, M.; Liu, D.; et al. Hepatic arterial infusion chemotherapy combined with anti-PD-1/PD-L1 immunotherapy and molecularly targeted agents for advanced hepatocellular carcinoma: A real world study. Front. Immunol. 2023, 14, 1127349. [Google Scholar] [CrossRef] [PubMed]
- Jolissaint, J.S.; Soares, K.C.; Seier, K.P.; Kundra, R.; Gonen, M.; Shin, P.J.; Boerner, T.; Sigel, C.; Madupuri, R.; Vakiani, E.; et al. Intrahepatic Cholangiocarcinoma with Lymph Node Metastasis: Treatment-Related Outcomes and the Role of Tumor Genomics in Patient Selection. Clin. Cancer Res. 2021, 27, 4101–4108. [Google Scholar] [CrossRef]
- Yang, Z.; Fu, Y.; Wu, W.; Hu, Z.; Pan, Y.; Wang, J.; Chen, J.; Hu, D.; Zhou, Z.; Chen, M.; et al. Comparison of hepatic arterial infusion chemotherapy with mFOLFOX vs. first-line systemic chemotherapy in patients with unresectable intrahepatic cholangiocarcinoma. Front. Pharmacol. 2023, 14, 1234342. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Itano, O.; Morinaga, J.; Shirakawa, H.; Itano, S. Potential efficacy of hepatic arterial infusion chemotherapy using gemcitabine, cisplatin, and 5-fluorouracil for intrahepatic cholangiocarcinoma. PLoS ONE 2022, 17, e0266707. [Google Scholar] [CrossRef]
- Franssen, S.; Holster, J.J.; Jolissaint, J.S.; Nooijen, L.E.; Cercek, A.; D’Angelica, M.I.; Homs, M.Y.V.; Wei, A.C.; Balachandran, V.P.; Drebin, J.A.; et al. Gemcitabine with Cisplatin Versus Hepatic Arterial Infusion Pump Chemotherapy for Liver-Confined Unresectable Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2024, 31, 115–124. [Google Scholar] [CrossRef]
- Tabrizian, P.; Jibara, G.; Hechtman, J.F.; Franssen, B.; Labow, D.M.; Schwartz, M.E.; Thung, S.N.; Sarpel, U. Outcomes following resection of intrahepatic cholangiocarcinoma. HPB 2015, 17, 344–351. [Google Scholar] [CrossRef]
- Burris, H.A., 3rd; Okusaka, T.; Vogel, A.; Lee, M.A.; Takahashi, H.; Breder, V.; Blanc, J.F.; Li, J.; Bachini, M.; Zotkiewicz, M.; et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer (TOPAZ-1): Patient-reported outcomes from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2024, 25, 626–635. [Google Scholar] [CrossRef]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klumpen, H.J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Fan, J.; Shi, G.-M.; Huang, X.-Y.; Wu, D.; Liang, F.; Yang, G.-H.; Lu, J.-C.; Chen, Y.; Ge, N.-L.; et al. Lenvatinib plus toripalimab as first-line treatment for advanced intrahepatic cholangiocarcinoma: A single-arm, phase 2 trial. J. Clin. Oncol. 2021, 39, 4099. [Google Scholar] [CrossRef]
- Xie, L.; Huang, J.; Wang, L.; Ren, W.; Tian, H.; Hu, A.; Liang, J.; Jiao, Y.; Li, Y.; Zhou, Q.; et al. Lenvatinib Combined with a PD-1 Inhibitor as Effective Therapy for Advanced Intrahepatic Cholangiocarcinoma. Front. Pharmacol. 2022, 13, 894407. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Wang, S.; Wang, H.; Zhang, N.; Wang, Y.; Yang, X.; Zhu, C.; Ning, C.; Zhang, X.; Xue, J.; et al. Real-world cohort study of PD-1 blockade plus lenvatinib for advanced intrahepatic cholangiocarcinoma: Effectiveness, safety, and biomarker analysis. Cancer Immunol. Immunother. 2023, 72, 3717–3726. [Google Scholar] [CrossRef] [PubMed]
- Auer, T.A.; Collettini, F.; Segger, L.; Pelzer, U.; Mohr, R.; Krenzien, F.; Gebauer, B.; Geisel, D.; Hosse, C.; Schoning, W.; et al. Interventional Treatment Strategies in Intrahepatic Cholangiocarcinoma and Perspectives for Combined Hepatocellular-Cholangiocarcinoma. Cancers 2023, 15, 2655. [Google Scholar] [CrossRef] [PubMed]
- Morawitz, J.; Bruckmann, N.M.; Jannusch, K.; Kirchner, J.; Antoch, G.; Loosen, S.; Luedde, T.; Roderburg, C.; Minko, P. Update on Locoregional Therapies for Cholangiocellular Carcinoma. Cancers 2023, 15, 2368. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.; Makary, M.S.; Beal, E.W. Locoregional Therapy for Intrahepatic Cholangiocarcinoma. Cancers 2023, 15, 2384. [Google Scholar] [CrossRef] [PubMed]
- Amini, N.; Ejaz, A.; Spolverato, G.; Kim, Y.; Herman, J.M.; Pawlik, T.M. Temporal trends in liver-directed therapy of patients with intrahepatic cholangiocarcinoma in the United States: A population-based analysis. J. Surg. Oncol. 2014, 110, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.T.; Paprottka, P.M.; Schon, A.; Bamberg, F.; Haug, A.; Durr, E.M.; Rauch, B.; Trumm, C.T.; Jakobs, T.F.; Helmberger, T.K.; et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: Factors associated with prolonged survival. Cardiovasc. Interv. Radiol. 2012, 35, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Ierardi, A.M.; Angileri, S.A.; Patella, F.; Panella, S.; Lucchina, N.; Petre, E.N.; Pinto, A.; Franceschelli, G.; Carrafiello, G.; Cornalba, G.; et al. The role of interventional radiology in the treatment of intrahepatic cholangiocarcinoma. Med. Oncol. 2017, 34, 11. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.C.G., 2nd; Simo, K.A.; Hansen, P.; Rocha, F.; Philips, P.; McMasters, K.M.; Tatum, C.M.; Kelly, L.R.; Driscoll, M.; Sharma, V.R.; et al. Drug-Eluting Bead, Irinotecan Therapy of Unresectable Intrahepatic Cholangiocarcinoma (DELTIC) with Concomitant Systemic Gemcitabine and Cisplatin. Ann. Surg. Oncol. 2022, 29, 5462–5473. [Google Scholar] [CrossRef]
- Konstantinidis, I.T.; Groot Koerkamp, B.; Do, R.K.; Gonen, M.; Fong, Y.; Allen, P.J.; D’Angelica, M.I.; Kingham, T.P.; DeMatteo, R.P.; Klimstra, D.S.; et al. Unresectable intrahepatic cholangiocarcinoma: Systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer 2016, 122, 758–765. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, X.; Yang, X.; Zheng, K.; Wang, Y.; Wang, Y.; Sang, X.; Lu, X.; Xu, Y.; Wang, X.; et al. Different interventional time of hepatic arterial infusion with PD-1 inhibitor for advanced biliary tract cancer: A multicenter retrospective study. Am. J. Cancer Res. 2022, 12, 3455–3463. [Google Scholar]
- Wang, Y.; Xun, Z.; Yang, X.; Wang, Y.; Wang, S.; Xue, J.; Zhang, N.; Yang, X.; Lu, Z.; Zhou, J.; et al. Local-regional therapy combined with toripalimab and lenvatinib in patients with advanced biliary tract cancer. Am. J. Cancer Res. 2023, 13, 1026–1037. [Google Scholar] [PubMed]
- Ni, J.Y.; Sun, H.L.; Guo, G.F.; Zhou, X.; Wei, J.X.; Xu, L.F. Hepatic arterial infusion of GEMOX plus systemic gemcitabine chemotherapy combined with lenvatinib and PD-1 inhibitor in large unresectable intrahepatic cholangiocarcinoma. Int. Immunopharmacol. 2024, 140, 112872. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; Kroemer, G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 2008, 8, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kato, Y.; Ozawa, Y.; Kodama, K.; Ito, J.; Ichikawa, K.; Yamada, K.; Hori, Y.; Tabata, K.; Takase, K.; et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018, 109, 3993–4002. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Z.; Song, M.; Pan, Q.; Zhao, J.; Huang, Y.; Han, Y.; Ouyang, D.; Yang, C.; Chen, H.; et al. Lenvatinib improves anti-PD-1 therapeutic efficacy by promoting vascular normalization via the NRP-1-PDGFRbeta complex in hepatocellular carcinoma. Front. Immunol. 2023, 14, 1212577. [Google Scholar] [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | HLP Group n = 25 | SCLP Group n = 28 | p-Value |
|---|---|---|---|
| Gender, n (%) | 0.610 | ||
| Male | 16 (64%) | 16 (57%) | |
| Female | 9 (16%) | 12 (43%) | |
| Age, median (IQR), years | 63 (53.5–66) | 58.5 (52.75–65) | 0.574 |
| HBV, n (%) | 0.365 | ||
| postive | 12 (48.0%) | 10 (35.7%) | |
| negative | 13 (52.0%) | 18 (64.3%) | |
| ECOG performance status, n (%) | 0.963 | ||
| 0 | 18 (72.0%) | 20 (71.4%) | |
| 1 | 7 (28.0%) | 8 (28.6%) | |
| Child–Pugh class, n (%) | 0.355 | ||
| A | 24 (96.0%) | 24 (85.7%) | |
| B | 1 (4.0%) | 4 (14.3%) | |
| Liver cirrhosis, n (%) | 0.243 | ||
| Yes | 10 (40.0%) | 7 (25%) | |
| No | 15 (60.0%) | 21 (75%) | |
| Cycle times, median (IQR) | 3 (2–4) | 3 (2–5) | 0.270 |
| Extrahepatic metastasis, n (%) | 0.983 | ||
| Yes | 9 (36.0%) | 10 (35.7%) | |
| No | 16 (64.0%) | 18 (64.3%) | |
| Lymph node metastasis, n (%) | 0.706 | ||
| Yes | 19 (76.0%) | 20 (71.4%) | |
| No | 6 (24.0%) | 8 (28.6%) | |
| Vascular invasion, n (%) | 0.135 | ||
| Yes | 9 (36.0%) | 5 (17.9%) | |
| No | 16 (64.0%) | 23 (82.1%) | |
| Tumor numbers, n (%) | 0.239 | ||
| Single | 12 (48.0%) | 9 (32.1%) | |
| Multiple | 13 (52.0%) | 19 (67.9%) | |
| TNM stage, n (%) | 1.000 | ||
| I–II | 4 (16.0%) | 4 (14.3%) | |
| III–IV | 21 (84.0%) | 24 (85.7%) | |
| Largest tumor dimension, mean ± SD, cm | 8.13 ± 3.59 | 7.10 ± 3.52 | 0.323 |
| CA19-9, median (IQR), U/ml | 165 (30.975–901) | 417.65 (23.275–2554.65) | 0.715 |
| CEA, median (IQR), U/m | 4.45 (2.335–15.455) | 3.56 (1.703–8.01) | 0.340 |
| AST, median (IQR), U/L | 38.4 (28.15–50.25) | 35.5 (22.83–44.15) | 0.190 |
| ALT, median (IQR), U/L | 25.7 (17.2–45.5) | 23.8 (20.0–37.14) | 0.831 |
| ALB, mean ± SD, g/L | 38.42 ± 3.494 | 38.812 ± 4.097 | 0.712 |
| GGT, median (IQR), U/L | 147 (70.50–259.55) | 78.67 (53.95–183.75) | 0.113 |
| ALP, median (IQR), U/L | 164.1 (93.10–240.20) | 132.77 (97.80–174.91) | 0.412 |
| Tumor Response | RECIST1.1 | mRECIST | ||||
|---|---|---|---|---|---|---|
| HLP Group n, (%) | SCLP Group n, (%) | p-Value | HLP Group n, (%) | SCLP Group n, (%) | p-Value | |
| CR | 0 (0) | 0 (0) | - | 1 (4.0%) | 0 (0) | - |
| PR | 13 (52.0%) | 7 (25.0%) | - | 16 (64.0%) | 9 (32.1%) | - |
| SD | 11 (48.0%) | 15 (53.6%) | - | 9 (36.0%) | 13 (46.4%) | - |
| PD | 1 (4.0%) | 6 (21.4%) | - | 1 (4.0%) | 6 (21.4%) | - |
| ORR | 13 (44.0%) | 7 (25.0%) | 0.043 | 17 (68.0%) | 9 (32.1%) | 0.009 |
| DCR | 24 (96.0%) | 22 (78.6%) | 0.143 | 24 (96.0%) | 22 (78.6%) | 0.143 |
| surgery after treatment | 2 (8.0%) | 1 (3.6%) | 0.919 | 2 (8.0%) | 1 (3.6%) | 0.919 |
| Variables | Overall Survival | Progression-Free Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR95%CI | p-Vaule | HR95%CI | p-Vaule | HR95%CI | p-Vaule | HR95%CI | p-Vaule | |
| Treatment regimen (HLP) | 0.721 (0.379–1.369) | 0.317 | 0.551 (0.305–0.994) | 0.048 | 0.434 (0.228–0.828) | 0.011 | ||
| Gender (Male) | 1.253 (0.647–2.428) | 0.504 | 1.506 (0.831–2.730) | 0.177 | ||||
| Age (≥60) | 1.043 (0.549–1.983) | 0.898 | 1.233 (0.682–2.227) | 0.488 | ||||
| HBV (Postive) | 1.154 (0.607–2.192) | 0.663 | 0.782 (0.432–1.415) | 0.416 | ||||
| ECOG performance status (1) | 1.561 (0.785–3.107) | 0.204 | 1.810 (0.965–3.396) | 0.065 | 2.212 (1.1.108–4.057) | 0.023 | ||
| Child–Pugh (A) | 0.488 (0.210–1.185) | 0.113 | 0.755 (0.318–1.793) | 0.525 | ||||
| Liver cirrhosis (Yes) | 0.998 (0.509–1.955) | 0.994 | 0.860 (0.464–1.595) | 0.632 | ||||
| Lymph node metastasis (Yes) | 1.817 (0.823–4.009) | 0.139 | 1.513 (0.776–2.953) | 0.225 | ||||
| Extrahepatic metastasis (Yes) | 1.009 (0.519–1.960) | 0.979 | 1.540 (0.840–2.824) | 0.163 | ||||
| Vascular invasion (Yes) | 1.109 (0.537–2.288) | 0.780 | 0.937 (0.482–1.818) | 0.847 | ||||
| Tumor numbers (Multiple) | 1.485 (0.776–2.878) | 0.242 | 2.001 (1.085–3.690) | 0.026 | 1.841 (0.985–3.442) | 0.056 | ||
| Largest tumor dimension (≥10) | 1.555 (0.779–3.105) | 0.210 | 1.198 (0.617–2.326) | 0.593 | ||||
| TNM stage (III-IV) | 2.448 (0.870–7.115) | 0.089 | 2.257 (0.546–0.935) | 0.014 | 2.395 (0.985–5.824) | 0.054 | 1.841 (0.985–3.442) | 0.266 |
| Cycle times (≥4) | 0.393 (0.199–0.778) | 0.007 | 0.434 (0.216–0.873) | 0.019 | 0.463 (0.257–0.832) | 0.010 | 0.383 (0.201–0.732) | 0.004 |
| CA19-9 (≥100) | 1.338 (0.723–2.664) | 0.324 | 0.914 (0.510–1.636) | 0.761 | ||||
| GGT (≥60) | 3.176 (1.236–8.161) | 0.016 | 2.305 (0.868–6.120) | 0.094 | 1.217 (0.630–2.349) | 0.558 | ||
| ALB (<35) | 2.694 (1.162–6.245) | 0.021 | 2.415 (1.018–5.728) | 0.045 | 1.959 (0.847–4.530) | 0.116 | ||
| Adverse Events | Any Grade | Grade 3–4 | ||||
|---|---|---|---|---|---|---|
| HLP Group | SCLP Group | p-Value | HLP Group | SCLP Group | p-Value | |
| n = 25 | n = 28 | n = 25 | n = 28 | |||
| Treatment-related AEs, n (%) | ||||||
| Fatigue | 3 (12.0%) | 11 (39.3%) | 0.025 | 0 | 0 | - |
| Fever | 4 (16.0%) | 4 (14.3%) | 1.000 | 1 (4.0%) | 0 | 0.954 |
| Vomiting | 9 (36.0%) | 20 (71.4%) | 0.010 | 1 (4.0%) | 8 (28.6%) | 0.044 |
| Abdominal pain | 10 (40.0%) | 5 (18.9%) | 0.074 | 2 (8.0%) | 0 | 0.422 |
| Rash | 5 (20.0%) | 7 (25.0%) | 0.664 | 0 | 0 | - |
| Hand-foot syndrome | 7 (28.0%) | 8 (28.6%) | 0.963 | 1 (4.0%) | 1 (3.6%) | 1.000 |
| Diarrhea or constipation | 1 (4.0%) | 2 (7.1%) | 1.000 | 0 | 0 | - |
| Loss of appetite | 2 (8.0%) | 7 (25.0%) | 0.148 | 0 | 0 | - |
| Elevated blood pressure | 7 (28.0%) | 5 (18.9%) | 0.378 | 1 (4.0%) | 0 | 0.954 |
| Canker sore | 3 (12.0%) | 5 (18.9%) | 0.833 | 0 | 0 | - |
| Dysuria | 7 (28.0%) | 2 (7.1%) | 0.098 | 0 | 0 | - |
| Laboratory-related AEs, n (%) | ||||||
| Elevated AST | 12 (48.0%) | 10 (35.7%) | 0.365 | 1 (4.0%) | 0 | 0.954 |
| Elevated ALT | 10 (40.0%) | 8 (28.6%) | 0.380 | 0 | 1 (3.6%) | 0.954 |
| Elevated total bilirubin | 5 (20.0%) | 4 (14.3%) | 0.719 | 0 | 0 | - |
| Hypothyroidism | 4 (16.0%) | 3 (10.7%) | 0.694 | 0 | 0 | - |
| Hypokalemia | 1 (4.0%) | 0 | 0.954 | 0 | 0 | - |
| Elevated creatinine | 0 | 1 (3.6%) | 1.000 | 0 | 0 | - |
| Hypoproteinemia | 7 (28.0%) | 5 (18.9%) | 0.378 | 1 (4.0%) | 0 | 0.954 |
| Hyperalgesia | 3 (12.0%) | 0 | 0.196 | 0 | 0 | - |
| Anemic | 3 (12.0%) | 13 (46.4%) | 0.006 | 0 | 6 (21.4%) | 0.024 |
| Thrombocytopenia | 2 (8.0%) | 7 (25%) | 0.148 | 0 | 1 (3.6%) | 0.954 |
| Leukopenia | 5 (20.0%) | 15 (53.6%) | 0.012 | 0 | 6 (21.4%) | 0.024 |
| Neutropenia | 3 (12.0%) | 8 (28.6%) | 0.138 | 0 | 2 (7.1%) | 0.492 |
| Proteinuria | 1 (4.0%) | 0 | 0.954 | 0 | 0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Wen, W.; Xia, Y.; Wan, R. The Efficacy and Safety of Hepatic Artery Infusion Chemotherapy Combined with Lenvatinib and Programmed Death (PD)-1 Inhibitors for Unresectable Intrahepatic Cholangiocarcinoma: A Retrospective Study. Curr. Oncol. 2025, 32, 87. https://doi.org/10.3390/curroncol32020087
Cai Y, Wen W, Xia Y, Wan R. The Efficacy and Safety of Hepatic Artery Infusion Chemotherapy Combined with Lenvatinib and Programmed Death (PD)-1 Inhibitors for Unresectable Intrahepatic Cholangiocarcinoma: A Retrospective Study. Current Oncology. 2025; 32(2):87. https://doi.org/10.3390/curroncol32020087
Chicago/Turabian StyleCai, Yingxiao, Wu Wen, Yangshuo Xia, and Renhua Wan. 2025. "The Efficacy and Safety of Hepatic Artery Infusion Chemotherapy Combined with Lenvatinib and Programmed Death (PD)-1 Inhibitors for Unresectable Intrahepatic Cholangiocarcinoma: A Retrospective Study" Current Oncology 32, no. 2: 87. https://doi.org/10.3390/curroncol32020087
APA StyleCai, Y., Wen, W., Xia, Y., & Wan, R. (2025). The Efficacy and Safety of Hepatic Artery Infusion Chemotherapy Combined with Lenvatinib and Programmed Death (PD)-1 Inhibitors for Unresectable Intrahepatic Cholangiocarcinoma: A Retrospective Study. Current Oncology, 32(2), 87. https://doi.org/10.3390/curroncol32020087





