A Dramatic Clinical Response to Trastuzumab-Deruxtecan in a Patient with HER-2 Low Breast Cancer with Untreated Leptomeningeal Metastasis and Hydrocephalus
Abstract
1. Introduction
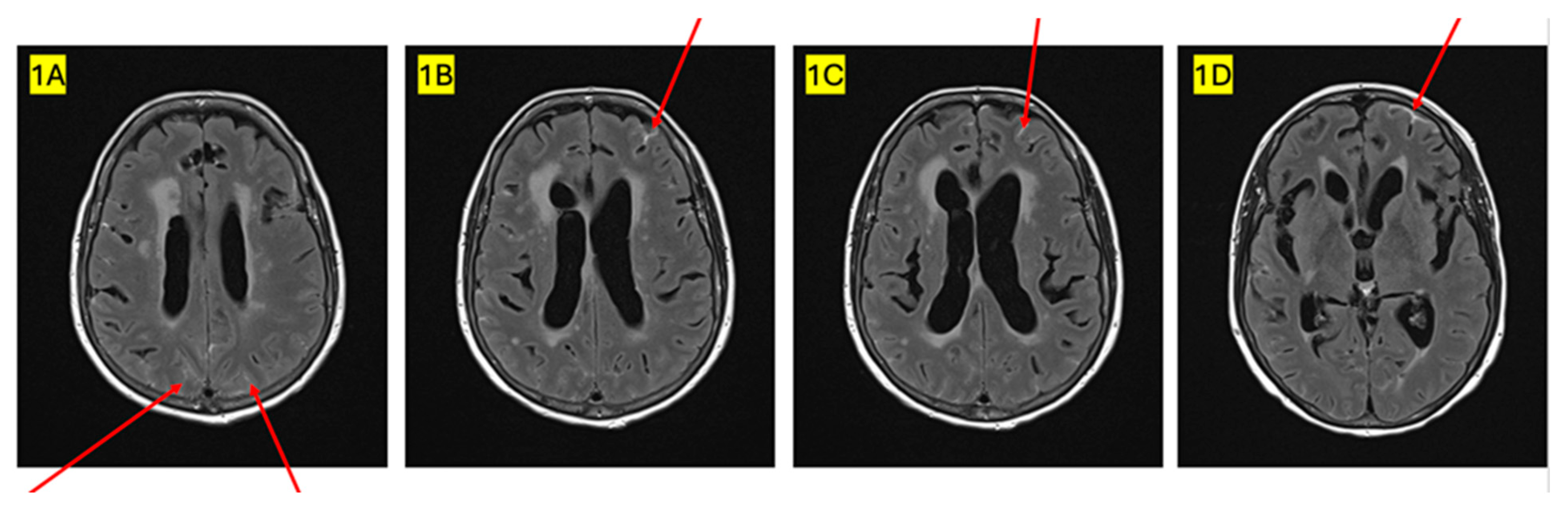
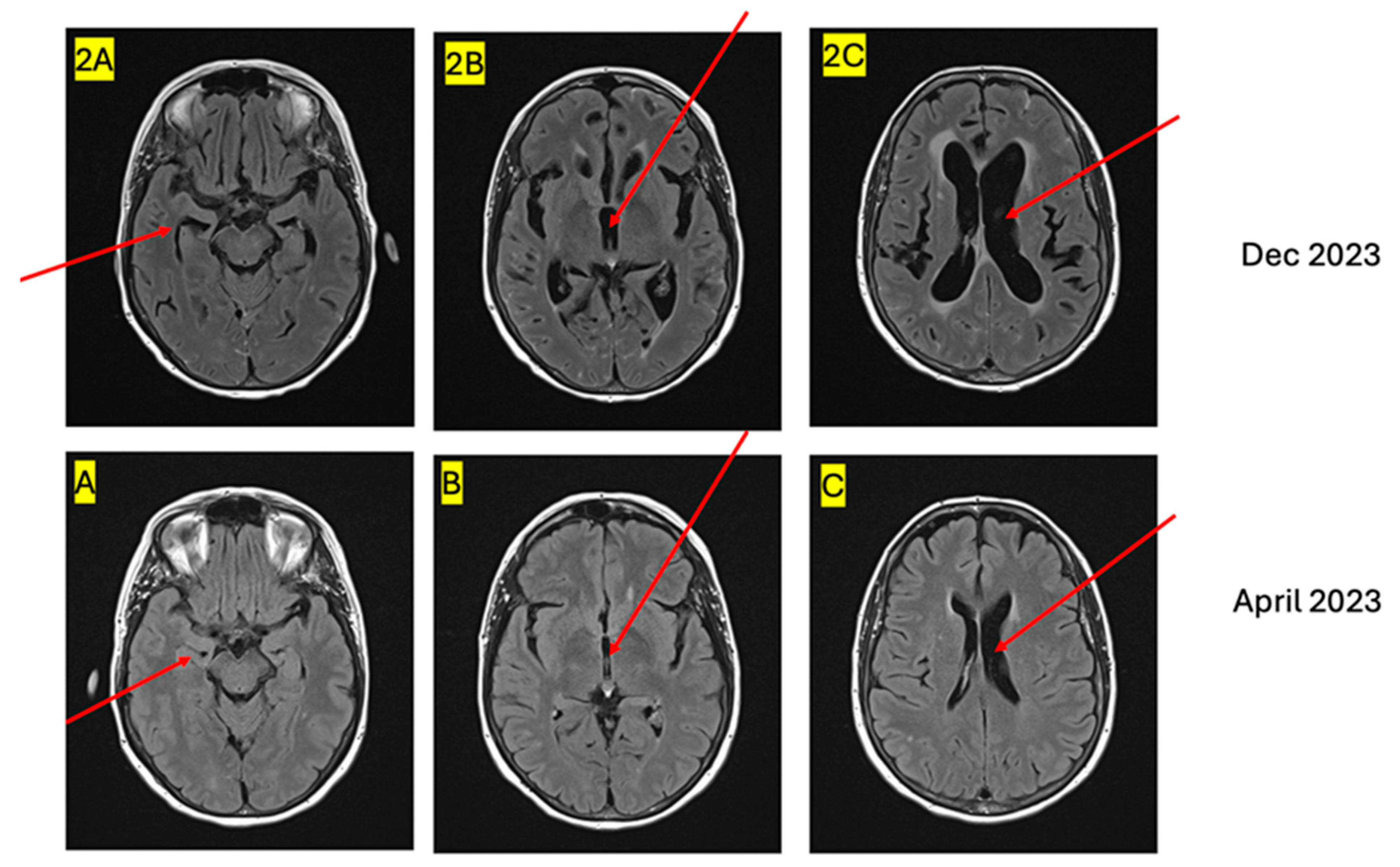
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | Advanced breast cancer |
| ADC | Antibody drug conjugate |
| AI | Aromatase inhibitor |
| ALN | Axillary lymph nodes |
| BM | Brain metastasis |
| CDK 4/6 inhibitor | Cyclin dependent kinase 4/6 inhibitor |
| CNS | Central nervous system |
| CR | Complete response |
| CSF | Cerebrospinal fluid |
| CSI | Craniospinal irradiation |
| ER | Estrogen receptor |
| HER2 | Human epidermal growth factor receptor 2 |
| HR | Hormone receptor |
| HRCT | High resolution computed tomography |
| IC | Intracranial |
| ICR | Intracranial response rate |
| IHC | Immunohistochemistry |
| IV | Intravenous |
| IT | Intrathecal |
| LM | Leptomeningeal metastasis |
| MRI | Magnetic resonance imaging |
| ORR | Overall response rate |
| pCSI | Proton craniospinal irradiation |
| PFS | Progression free survival |
| PR | Progesterone receptor |
| SISH | Silver in situ hybridization |
| SRS | Stereotactic radiosurgery |
| SRT | Stereotactic radiotherapy |
| T-DXd | Trastuzumab-deruxtecan |
| WBRT | Whole brain radiotherapy |
References
- Mollica, L.; Leli, C.; Puglisi, S.; Sardi, S.; Sottotetti, F. Leptomeningeal carcinomatosis and breast cancer: A systematic review of current evidence on diagnosis, treatment and prognosis. Drugs Context 2021, 10, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Sacco, K.; Muhammad, A.; Saleem, W.; Alshaker, H.; Monzon, L.; Islam, M.R.; Pchejetski, D. Leptomeningeal carcinomatosis as the primary presentation of relapse in breast cancer. Oncol. Lett. 2016, 12, 779–782. [Google Scholar] [CrossRef][Green Version]
- Lamovec, J.; Bracko, M. Metastatic pattern of infiltrating lobular carcinoma of the breast: An autopsy study. J. Surg. Oncol. 1991, 48, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Jin, Q.; Tayob, N.; Jeselsohn, R.M.; Schnitt, S.J.; Vincuilla, J.; Parker, T.; Tyekucheva, S.; Li, T.; Lin, N.U.; et al. Prognostic and biologic significance of ERBB2-low expression in early-stage breast cancer. JAMA Oncol. 2022, 8, 1177–1183. [Google Scholar] [CrossRef]
- Chen, M.; Chen, W.; Liu, D.; Chen, W.; Shen, K.; Wu, J.; Zhu, L. Prognostic values of clinical and molecular features in HER2 low-breast cancer with hormonal receptor overexpression: Features of HER2-low breast cancer. Breast Cancer 2022, 29, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.G.; DeSouza, T.G.; Farkash, A.; Shafran, B.; Pack, D.; Rehman, F.; Fuks, J.; Portenoy, R. Leptomeningeal metastases: Comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J. Neurooncol. 1990, 9, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Le Rhun, E.; Weller, M.; Brandsma, D.; Van den Bent, M.; de Azambuja, E.; Henriksson, R.; Boulanger, T.; Peters, S.; Watts, C.; Wick, W.; et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann. Oncol. 2017, 28, iv84–iv99. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.D.; Fomchenko, E.I.; Guerrieri, R.A.; Glitza Oliva, I.C. Challenges and advances in diagnosis and treatment of leptomeningeal disease (LMD). Front. Oncol. 2022, 11, 800053. [Google Scholar] [CrossRef] [PubMed]
- Vogelbaum, M.A.; Brown, P.D.; Messersmith, H.; Brastianos, P.K.; Burri, S.; Cahill, D.; Dunn, I.F.; Gaspar, L.E.; Gatson NT, N.; Gondi, V.; et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J. Clin. Oncol. 2021, 40, 492–516. [Google Scholar] [CrossRef]
- Volkov, A.A.; Filis, A.K.; Vrionis, F.D. Surgical treatment for leptomeningeal disease. Cancer Control. 2017, 24, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Bazan, F.; Dobi, E.; Royer, B.; Curtit, E.; Mansi, L.; Menneveau, N.; Paillard, M.J.; Meynard, G.; Villanueva, C.; Pivot, X.; et al. Systemic high-dose intravenous methotrexate in patients with central nervous system metastatic breast cancer. BMC Cancer 2019, 19, 1–6. [Google Scholar] [CrossRef]
- Rogers, L.R.; Remer, S.E.; Tejwani, S. Durable response of breast cancer leptomeningeal metastasis to capecitabine monotherapy. Neuro. Oncol. 2004, 6, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Kabraji, S.; Ni, J.; Sammons, S.; Li, T.; Van Swearingen AE, D.; Wang, Y.; Pereslete, A.; Hsu, L.; DiPiro, P.J.; Lascola, C.; et al. Preclinical and clinical efficacy of trastuzumab deruxtecan in breast cancer brain metastases. Clin. Cancer Res. 2023, 29, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Kubeczko, M.; Jarząb, M.; Krzywon, A.; Gräupner, D.; Polakiewicz-Gilowska, A.; Gabryś, D. Efficacy of CDK 4/6 inhibitors and radiotherapy in breast cancer patients with brain metastases. J. Clin. Med. 2023, 12, 2044. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; de Matos, L.V.; Cardoso, D.; Saraiva, M.; Medeiros-Mirra, R.; Coelho, A.; Miranda, H.; Martins, A. Endocrine therapy for the treatment of leptomeningeal carcinomatosis in luminal breast cancer: A comprehensive review. CNS Oncol. 2020, 9, CNS65. [Google Scholar] [CrossRef]
- Troussier, I.; Canova, C.; Klausner, G. Complete response of leptomeningeal carcinomatosis secondary to breast cancer. Breast 2020, 54, 328–330. [Google Scholar] [CrossRef]
- Yamashita, T.; Sohn, J.H.; Tokunaga, E.; Niikura, N.; Park, Y.H.; Lee, K.S.; Chae, Y.S.; Xu, B.; Wang, X.; Im, S.A.; et al. Trastuzumab deruxtecan versus treatment of physician’s choice in previously treated Asian patients with HER2-low unresectable/metastatic breast cancer: Subgroup analysis of the DESTINY-Breast04 study. Breast Cancer 2024, 31, 858–868. [Google Scholar] [CrossRef]
- Saura, C.; Modi, S.; Krop, I.; Park, Y.H.; Kim, S.B.; Tamura, K.; Iwata, H.; Tsurutani, J.; Sohn, J.; Mathias, E.; et al. Trastuzumab deruxtecan in previously treated patients with HER2-positive metastatic breast cancer: Updated survival results from a phase II trial (DESTINY-Breast01). Ann. Oncol. 2024, 35, 302–307. [Google Scholar] [CrossRef]
- André, F.; Hee Park, Y.; Kim, S.B.; Takano, T.; Im, S.A.; Borges, G.; Lima, J.P.; Aksoy, S.; Gavila Gregori, J.; De Laurentiis, M.; et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 401, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.; Hurvitz, S.A.; Im, S.A.; Iwata, H.; Curigliano, G.; Kim, S.B.; Chiu JW, Y.; Pedrini, J.L.; Li, W.; Yonemori, K.; et al. Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer: Long-term survival analysis of the DESTINY-Breast03 trial. Nat. Med. 2024, 30, 2208–2215. [Google Scholar] [CrossRef]
- André, F.; Hamilton, E.P.; Loi, S.; Schmid, P.; Anders, C.K.; Yu, T.; Boston, S.; D’Cruz, C.M.; Herbolsheimer, P.; Jhaveri, K.L. Trastuzumab deruxtecan (T-DXd) combinations in patients with HER2-positive advanced or metastatic breast cancer: A phase 1b/2, open-label, multicenter, dose-finding and dose-expansion study (DESTINY-Breast07). J. Clin. Oncol. 2021, 39, TPS1096. [Google Scholar] [CrossRef]
- André, F.; Cortés, J.; Curigliano, G.; Modi SLi, W.; Park, Y.H.; Chung, W.P.; Kim, S.B.; Yamashita, T.; Pedrini, J.L.; Im, S.A. A pooled analysis of trastuzumab deruxtecan in patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer with brain metastases. Ann. Oncol. 2024, 35, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- ESMO Daily Reporter. Antibody-Drug Conjugates Offer Hope for Breast Cancer Patients. 2024. Available online: https://dailyreporter.esmo.org/esmo-congress-2024/breast-cancer/antibody-drug-conjugates-offer-hope-for-patients-with-breast-cancer-including-patients-with-brain-metastases (accessed on 14 October 2024).
- Mosele, F.; Deluche, E.; Lusque, A.; Le Bescond, L.; Filleron, T.; Pradat, Y.; Ducoulombier, A.; Pistilli, B.; Bachelot, T.; Viret, F.; et al. Trastuzumab deruxtecan in metastatic breast cancer with variable HER2 expression: The phase 2 DAISY trial. Nat. Med. 2023, 29, 2110–2120. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, R.; Berghoff, A.S.; Furtner, J.; Marhold, M.; Bergen, E.S.; Roider-Schur, S.; Mair, M.J.; Starzer, A.M.; Forstner, H.; Rottenmanner, B.; et al. Final outcome analysis from the phase II TUXEDO-1 trial of trastuzumab-deruxtecan in HER2-positive breast cancer patients with active brain metastases. Neuro Oncol. 2024, 26, 2305–2315. [Google Scholar] [CrossRef]
- Alder, L.; Trapani, D.; Bradbury, C.; Van Swearingen AE, D.; Tolaney, S.M.; Khasraw, M.; Anders, C.K.; Lascola, C.D.; Hsu, L.; Lin, N.U.; et al. Durable responses in patients with HER2+ breast cancer and leptomeningeal metastases treated with trastuzumab deruxtecan. NPJ Breast Cancer 2023, 9, 19. [Google Scholar] [CrossRef]
- Pérez-García, J.M.; Vaz Batista, M.; Cortez, P.; Ruiz-Borrego, M.; Cejalvo, J.M.; de la Haba-Rodriguez, J.; Garrigós, L.; Racca, F.; Servitja, S.; Blanch, S.; et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: The DEBBRAH trial. Neuro Oncol. 2023, 25, 157–166. [Google Scholar] [CrossRef]
- Niikura, N.; Yamanaka, T.; Nomura, H.; Shiraishi, K.; Kusama, H.; Yamamoto, M.; Matsuura, K.; Inoue, K.; Takahara, S.; Kita, S.; et al. Treatment with trastuzumab deruxtecan in patients with HER2-positive breast cancer and brain metastases and/or leptomeningeal disease (ROSET-BM). NPJ Breast Cancer 2023, 9, 82. [Google Scholar] [CrossRef]
- Rugo, H.S.; Crossno, C.L.; Gesthalter, Y.B.; Kelley, K.; Moore, H.B.; Rimawi, M.F.; Westbrook, K.E.; Buys, S.S. Real-world perspectives and practices for pneumonitis/interstitial lung disease associated with trastuzumab deruxtecan use in human epidermal growth factor receptor 2-expressing metastatic breast cancer. JCO Oncol. Pract. 2023, 19, 539–546. [Google Scholar] [CrossRef]
- Wolf, B.; Dunet, V.; Dubruc, E.; Dolcan, A.; Nicod Lalonde, M.; Schiappacasse, L.; Zaman, K. Response of brain and meningeal metastases to trastuzumab-deruxtecan in a patient with her2-low breast cancer: A case report. Case Rep. Oncol. 2023, 16, 1425–1435. [Google Scholar] [CrossRef]
- Yang, J.T.; Wijetunga, N.A.; Pentsova, E.; Wolden, S.; Young, R.J.; Correa, D.; Zhang, Z.; Zheng, J.; Steckler, A.; Bucwinska, W.; et al. Randomized phase II trial of proton craniospinal irradiation versus photon involved-field radiotherapy for patients with solid tumor leptomeningeal metastasis. J. Clin. Oncol. 2022, 40, 3858–3867. [Google Scholar] [CrossRef]
- Perlow, H.K.; Matsui, J.K.; Ewing, A.; Cadieux, C.; Blakaj, D.M.; Beyer, S.; Thomas, E.M.; Grecula, J.C.; Raval, R.; Palmer, J.D. Volumetric modulated arc therapy craniospinal irradiation utilizing a vertebral body sparing approach: A toxicity analysis. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, S174. [Google Scholar] [CrossRef]
| 2006 | 2018 | 2022 |
|---|---|---|
| Stage IIIB, ER+/PR+/HER-2(-ve) | Peritoneal metastasis ER+/PR+/HER-2(-ve) | Multiple new bone metastasis |
| Up front surgery Adjuvant CT (EC-T) Adjuvant Tamoxifen | Surgical debulking CDK 4/6 inhibitor (Palbociclib)+AI | Capecitabine chemotherapy |
| 2023 (July) | 2023 (December) | 2024 (March) |
| New liver metastasis and significant increase in retroperitoneal disease | Leptomeningeal metastasis causing hydrocephalus | Disease progression and worsening Neurologic symptoms |
| Weekly paclitaxel | T-DXd | No further therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Nordal, R.; Ng, D.; Willson, M.; Feng, X. A Dramatic Clinical Response to Trastuzumab-Deruxtecan in a Patient with HER-2 Low Breast Cancer with Untreated Leptomeningeal Metastasis and Hydrocephalus. Curr. Oncol. 2025, 32, 81. https://doi.org/10.3390/curroncol32020081
Hussain S, Nordal R, Ng D, Willson M, Feng X. A Dramatic Clinical Response to Trastuzumab-Deruxtecan in a Patient with HER-2 Low Breast Cancer with Untreated Leptomeningeal Metastasis and Hydrocephalus. Current Oncology. 2025; 32(2):81. https://doi.org/10.3390/curroncol32020081
Chicago/Turabian StyleHussain, Sarah, Robert Nordal, Danny Ng, Morgan Willson, and Xiaolan Feng. 2025. "A Dramatic Clinical Response to Trastuzumab-Deruxtecan in a Patient with HER-2 Low Breast Cancer with Untreated Leptomeningeal Metastasis and Hydrocephalus" Current Oncology 32, no. 2: 81. https://doi.org/10.3390/curroncol32020081
APA StyleHussain, S., Nordal, R., Ng, D., Willson, M., & Feng, X. (2025). A Dramatic Clinical Response to Trastuzumab-Deruxtecan in a Patient with HER-2 Low Breast Cancer with Untreated Leptomeningeal Metastasis and Hydrocephalus. Current Oncology, 32(2), 81. https://doi.org/10.3390/curroncol32020081





