Multimodal Prehabilitation for Gynecologic Cancer Surgery
Abstract
1. Introduction
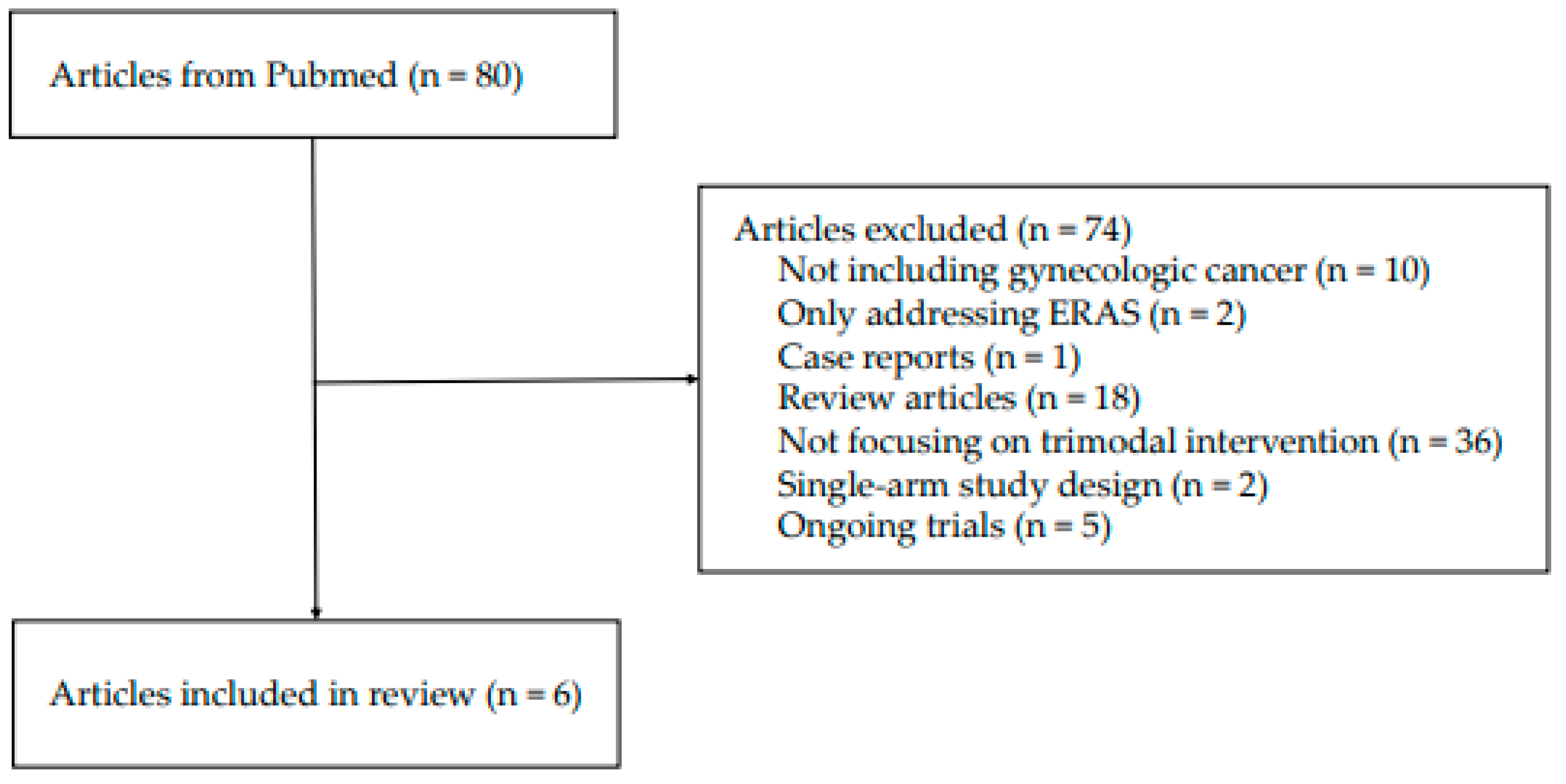
2. Methods
3. Results
4. Prehabilitation in Gynecologic Oncology
4.1. Physical Intervention
4.2. Nutritional Intervention
4.3. Psychological Intervention
4.4. Frailty Assessment and Medical Optimization
4.5. Postoperative Outcomes
4.6. Challenges and Limitations of Prehabilitation in Gynecologic Oncology
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.; Carli, F. Promoting perioperative metabolic and nutritional care. Anesthesiology 2015, 123, 1455–1472. [Google Scholar] [CrossRef] [PubMed]
- Scheede-Bergdahl, C.; Minnella, E.; Carli, F. Multi-modal prehabilitation: Addressing the why, when, what, how, who and where next? Anaesthesia 2019, 74, 20–26. [Google Scholar] [CrossRef]
- Dhanis, J.; Keidan, N.; Blake, D.; Rundle, S.; Strijker, D.; van Ham, M.; Pijnenborg, J.M.; Smits, A. Prehabilitation to improve outcomes of patients with gynaecological cancer: A new window of opportunity? Cancers 2022, 14, 3448. [Google Scholar] [CrossRef]
- Klapheke, A.K.; Keegan, T.H.; Ruskin, R.; Cress, R.D. Depressive symptoms and health-related quality of life in older women with gynecologic Cancers. J. Geriatr. Oncol. 2020, 11, 820–827. [Google Scholar] [CrossRef]
- Silver, J.K.; Baima, J. Cancer prehabilitation: An opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am. J. Phys. Med. Rehabil. 2013, 92, 715–727. [Google Scholar] [CrossRef]
- Mayer, A.; Cibula, D. Optimizing prehabilitation in gynecologic malignancies: Improving acceptance, overcoming barriers, and managing program complexity. Eur. J. Surg. Oncol. 2024, 50, 108739. [Google Scholar] [CrossRef]
- Nelson, G.; Bakkum-Gamez, J.; Kalogera, E.; Glaser, G.; Altman, A.; Meyer, L.A.; Taylor, J.S.; Iniesta, M.; Lasala, J.; Mena, G.; et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations—2019 update. Int. J. Gynecol. Cancer 2019, 29, 651–668. [Google Scholar] [CrossRef]
- Diaz-Feijoo, B.; Agusti-Garcia, N.; Sebio, R.; López-Hernández, A.; Sisó, M.; Glickman, A.; Carreras-Dieguez, N.; Fuste, P.; Marina, T.; Martínez-Egea, J.; et al. Feasibility of a multimodal prehabilitation programme in patients undergoing cytoreductive surgery for advanced ovarian cancer: A pilot study. Cancers 2022, 14, 1635. [Google Scholar] [CrossRef]
- Miralpeix, E.; Mancebo, G.; Gayete, S.; Corcoy, M.; Solé-Sedeño, J.-M. Role and impact of multimodal prehabilitation for gynecologic oncology patients in an Enhanced Recovery After Surgery (ERAS) program. Int. J. Gynecol. Cancer 2019, 29, 1235–1243. [Google Scholar] [CrossRef]
- Luther, A.; Gabriel, J.; Watson, R.P.; Francis, N.K. The impact of total body prehabilitation on post-operative outcomes after major abdominal surgery: A systematic review. World J. Surg. 2018, 42, 2781–2791. [Google Scholar] [CrossRef] [PubMed]
- Coderre, D.; Brahmbhatt, P.; Hunter, T.L.; Baima, J. Cancer prehabilitation in practice: The current evidence. Curr. Oncol. Rep. 2022, 24, 1569–1577. [Google Scholar] [CrossRef]
- Ljungqvist, O.; de Boer, H.D.; Balfour, A.; Fawcett, W.J.; Lobo, D.N.; Nelson, G.; Scott, M.J.; Wainwright, T.W.; Demartines, N. Opportunities and challenges for the next phase of enhanced recovery after surgery: A review. JAMA Surg. 2021, 156, 775–784. [Google Scholar] [CrossRef]
- Lario-Perez, S.; Macia, J.-J.; Lillo-Garcia, C.; Caravaca, I.; Lopez-Rodriguez, F.; Calero, A.; Tomas-Rodriguez, M.; Moya-Martinez, A.; Arroyo, A.; Lacueva, F.-J. Home-Based Trimodal Prehabilitation in Patients with Peritoneal Carcinomatosis Undergoing Cytoreductive Surgery: Effect on Functional Walking Capacity and Skeletal Muscle Mass. Ann. Surg. Oncol. 2024, 31, 7133–7141. [Google Scholar] [CrossRef] [PubMed]
- Zębalski, M.A.; Parysek, K.; Krzywon, A.; Nowosielski, K. LUNA EMG as a Marker of Adherence to Prehabilitation Programs and Its Effect on Postoperative Outcomes among Patients Undergoing Cytoreductive Surgery for Ovarian Cancer and Suspected Ovarian Tumors. Cancers 2024, 16, 2493. [Google Scholar] [CrossRef]
- Li, X.; Sha, L.; He, Y.; Yi, J.; Wang, X. The impact of short-term multimodal prehabilitation on functional capacity in patients with gynecologic malignancies during the perioperative period: A prospective study. Eur. J. Oncol. Nurs. 2024, 70, 102577. [Google Scholar] [CrossRef] [PubMed]
- Miralpeix, E.; Sole-Sedeno, J.-M.; Rodriguez-Cosmen, C.; Taus, A.; Muns, M.-D.; Fabregó, B.; Mancebo, G. Impact of prehabilitation during neoadjuvant chemotherapy and interval cytoreductive surgery on ovarian cancer patients: A pilot study. World J. Surg. Oncol. 2022, 20, 46. [Google Scholar] [CrossRef]
- Miralpeix, E.; Fabregó, B.; Rodriguez-Cosmen, C.; Solé-Sedeño, J.-M.; Gayete, S.; Jara-Bogunya, D.; Corcoy, M.; Mancebo, G. Prehabilitation in an ERAS program for endometrial cancer patients: Impact on post-operative recovery. Int. J. Gynecol. Cancer 2023, 33, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Dronkers, J.; Chorus, A.; Van Meeteren, N.; Hopman-Rock, M. The association of pre-operative physical fitness and physical activity with outcome after scheduled major abdominal surgery. Anaesthesia 2013, 68, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Enright, P.L.; McBurnie, M.A.; Bittner, V.; Tracy, R.P.; McNamara, R.; Arnold, A.; Newman, A.B. The 6-min walk test: A quick measure of functional status in elderly adults. Chest 2003, 123, 387–398. [Google Scholar] [CrossRef]
- Symonsi, T.; Sheffield-Moore, M.; Mamerow, M.; Wolfe, R.; Paddon-Jones, D. The anabolic response to resistance exercise and a protein-rich meal is not diminished by age. J. Nutr. Health Aging 2011, 15, 376–381. [Google Scholar] [CrossRef]
- Coca-Martinez, M.; Carli, F. Prehabilitation: Who can benefit? Eur. J. Surg. Oncol. 2024, 50, 106979. [Google Scholar] [CrossRef] [PubMed]
- Obermair, A.; Simunovic, M.; Isenring, L.; Janda, M. Nutrition interventions in patients with gynecological cancers requiring surgery. Gynecol. Oncol. 2017, 145, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Naser, A.Y.; Hameed, A.N.; Mustafa, N.; Alwafi, H.; Dahmash, E.Z.; Alyami, H.S.; Khalil, H. Depression and anxiety in patients with cancer: A cross-sectional study. Front. Psychol. 2021, 12, 585534. [Google Scholar] [CrossRef] [PubMed]
- Villa, G.; Lanini, I.; Amass, T.; Bocciero, V.; Scirè Calabrisotto, C.; Chelazzi, C.; Romagnoli, S.; De Gaudio, A.R.; Lauro Grotto, R. Effects of psychological interventions on anxiety and pain in patients undergoing major elective abdominal surgery: A systematic review. Perioper. Med. 2020, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Volz, S.; Koch, F.; Dayan, D.; Upadhyay, M.; Otto, S.; Schochter, F.; Janni, W.; Ebner, F. Is there evidence behind pre-or perioperative cognitive training in gynaecological patients on the prevention of perioperative cognitive dysfunction? A review. Arch. Gynecol. Obstet. 2022, 306, 937–942. [Google Scholar] [CrossRef]
- Proietti, M.; Cesari, M. Frailty: What is it? In Frailty and Cardiovascular Diseases: Research into an Elderly Population; Springer: Cham, Switzerland, 2020; pp. 1–7. [Google Scholar]
- Bogani, G.; Sarpietro, G.; Ferrandina, G.; Gallotta, V.; Donato, V.D.; Ditto, A.; Pinelli, C.; Casarin, J.; Ghezzi, F.; Scambia, G. Enhanced recovery after surgery (ERAS) in gynecology oncology. Eur. J. Surg. Oncol. 2021, 47, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, Y.; Gondal, U.; Aziz, O. A systematic review of prehabilitation programs in abdominal cancer surgery. Int. J. Surg. 2017, 39, 156–162. [Google Scholar] [CrossRef]
- Dhanis, J.; Strijker, D.; Drager, L.D.; van Ham, M.; van Laarhoven, C.J.; Pijnenborg, J.M.; Smits, A.; van den Heuvel, B. Feasibility of Introducing a Prehabilitation Program into the Care of Gynecological Oncology Patients—A Single Institution Experience. Cancers 2024, 16, 1013. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, E.; Cobián, C.D.; Jørgensen, T.L.; Minet, L.R.; Schnack, T.H.; Vinther, A. Development and feasibility of an exercise therapy intervention for older women with advanced epithelial ovarian cancer referred to neoadjuvant chemotherapy prior to possible interval debulking surgery. Gynecol. Oncol. Rep. 2024, 54, 101441. [Google Scholar] [CrossRef]
- Carron, M.; Safaee Fakhr, B.; Ieppariello, G.; Foletto, M. Perioperative care of the obese patient. J. Br. Surg. 2020, 107, e39–e55. [Google Scholar] [CrossRef]
- Saravana-Bawan, B.; Goplen, M.; Alghamdi, M.; Khadaroo, R.G. The relationship between visceral obesity and post-operative complications: A meta-analysis. J. Surg. Res. 2021, 267, 71–81. [Google Scholar] [CrossRef]
- Arora, L.; Sharma, S.; Carillo, J.F. Obesity and anesthesia. Curr. Opin. Anesthesiol. 2024, 37, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Polen-De, C.; Langstraat, C.; Asiedu, G.B.; Jatoi, A.; Kumar, A. Advanced ovarian cancer patients identify opportunities for prehabilitation: A qualitative study. Gynecol. Oncol. Rep. 2021, 36, 100731. [Google Scholar] [CrossRef] [PubMed]
- Dholakia, J.; Cohn, D.E.; Straughn, J.M., Jr.; Dilley, S.E. Prehabilitation for medically frail patients undergoing surgery for epithelial ovarian cancer: A cost-effectiveness analysis. J. Gynecol. Oncol. 2021, 32, e92. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.-L.L.; Cass, G.; Collins, A.; Adishesh, M.; Addley, S.; Baker-Rand, H.; Bharathan, R.; Blake, D.; Beirne, J.; Canavan, L.; et al. FARGO-360: A multi-disciplinary survey of practice and perspectives on provision of care for patients with frailty presenting with gynecological cancers in the UK and Ireland. Int. J. Gynecol. Cancer 2022, 32, 924–930. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, L.; Lukez, A.; Ma, Y.; Baima, J.; Moni, J. Prehabilitation for patient positioning: Pelvic exercises assist in minimizing inter-fraction sacral slope variability during radiation therapy. Med. Oncol. 2020, 37, 3. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Yamada, A.M.T.D.; de Campos Cardenas, T.; de Carvalho, J.N.; de Azevedo Oliveira, E.; da Silva, M.E.R.; Andrade, J.F.M.; de Souza Neto, E.; do Rêgo Barros, L.A.; Costa, R.L.R. PROPER—PRehabilitatiOn Plus Enhanced Recovery after surgery versus enhanced recovery after surgery in gynecologic oncology: A randomized clinical trial. Int. J. Gynecol. Cancer 2022, 32, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Prehabilitation in Gynaecological Cancer Patients (PHOCUS). Available online: https://ctv.veeva.com/study/prehabilitation-in-gynaecological-cancer-patients (accessed on 25 December 2024).
- Lambaudie, E.; Bannier, C.; Villaron, C.; Zemmour, C.; Boher, J.-M.; Soussan, P.B.; Pakradouni, J.; Brun, C.; Almeida, L.L.; Marino, P. TRAINING-Ovary 01 (connecTed pRehabiliAtIoN pelvIc caNcer surGery): Multicenter randomized study comparing neoadjuvant chemotherapy for patients managed for ovarian cancer with or without a connected pre-habilitation program. Int. J. Gynecol. Cancer 2021, 31, 920–924. [Google Scholar] [CrossRef]
- Díaz-Feijoo, B.; Agusti, N.; Sebio, R.; Sisó, M.; Carreras-Dieguez, N.; Domingo, S.; Díaz-Cambronero, O.; Torne, A.; Martinez-Palli, G.; Arguís, M.J. A multimodal prehabilitation program for the reduction of post-operative complications after surgery in advanced ovarian cancer under an ERAS pathway: A randomized multicenter trial (SOPHIE). Int. J. Gynecol. Cancer 2022, 32, 1463–1468. [Google Scholar] [CrossRef]
- Beck, A.; Thaysen, H.V.; Soegaard, C.H.; Blaakaer, J.; Seibaek, L. Investigating the experiences, thoughts, and feelings underlying and influencing prehabilitation among cancer patients: A qualitative perspective on the what, when, where, who, and why. Disabil. Rehabil. 2022, 44, 202–209. [Google Scholar] [CrossRef]
- van der Zanden, V.; Van der Zaag-Loonen, H.J.; Paarlberg, K.M.; Meijer, W.J.; Mourits, M.J.; van Munster, B.C. PREsurgery thoughts–thoughts on prehabilitation in oncologic gynecologic surgery, a qualitative template analysis in older adults and their healthcare professionals. Disabil. Rehabil. 2022, 44, 5930–5940. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Rotimi, O.; Laza-Cagigas, R.; Rampal, T. The feasibility and effects of a telehealth-delivered home-based prehabilitation program for cancer patients during the pandemic. Curr. Oncol. 2021, 28, 2248–2259. [Google Scholar] [CrossRef] [PubMed]
- Dorgo, S.; King, G.A.; Brickey, G.D. The application of peer mentoring to improve fitness in older adults. J. Aging Phys. Act. 2009, 17, 344–361. [Google Scholar] [CrossRef] [PubMed]
| Author (Year) Cancer Type | Study Design Population | Intervention and Duration | Outcome Measures | Results |
|---|---|---|---|---|
| Diaz-Feijoo (2022) [9] Ovary | Mixed cohort of before-and-after intervention Pilot study N = 34 Prehabilitation: 15, Control: 19 | Supervised exercise Nutritional optimization by dietitian (protein supplementation: 1.6–2 g/kg of body weight/day, whey protein, immunomodulatory formula) Psychological preparation ERAS program Duration: 2 (2–3) weeks | Quality of life (QLQ-C30) Physical activity (6MWT, Yale Physical Activity Survey) Nutritional status (Global Leadership Initiative on Malnutrition criteria) HADS | Prehabilitation group: No results available |
| Hospital stay | Shorter | |||
| Postoperative complications | NS | |||
| Time to start of chemotherapy | Earlier | |||
| Miralpeix (2022) [17] Ovary | Retrospective cohort of consecutive group Pilot study N = 29 NACT/ICS Prehabilitation: 14, Control: 15 | Physical exercise recommendations Nutritional counseling (homemade recipes for protein supplementation) Psychological support Preoperative carbohydrate loading Inspiratory threshold-loading device ERAS program Duration: 13.5 ± 2.0 weeks | Mean total protein levels (g/dL): Preoperative Postoperative | Prehabilitation group: Higher Higher |
| Intraoperative complications (Clavien–Dindo) | Lower | |||
| Intraoperative blood transfusion | Lower | |||
| First ambulation day, postoperative complications rate, and hospital stay | NS | |||
| Lario-Perez (2024) [14] Peritoneum | Prospective cohort N = 62 Ovarian: 30 (48.4%) | Home-based Physical (aerobic and muscular resistance) and relaxation exercises Protein-rich diet and hyperproteic nutritional supplementation (≥1.2 to 1.5 g of protein/kg of body weight/day, immunonutrition supplements) Duration: 27 (19–34) days | Functional walking capacity (T6MWT) | Improved |
| Skeletal muscle mass (ASMI) | No improvement | |||
| HADS 90-day postoperative morbidity (Clavien–Dindo II–V) | No results available Only independent risk factor: T6MWT (<360 m) | |||
| Hospital stay | NS between results of T6MWT after prehabilitation | |||
| Zębalski (2024) [15] Ovary | Prospective randomized interventional study N = 70 Prehabilitation: 36, Control: 34 | Physical activity: resistance and cardio-type aerobic exercises Proper diet and protein supplementation: 1.5–2.0 g/kg of body weight/day, immunomodulators Eliminating risky behaviors and reducing stimulant use: alcohol and smoking cessation Psychological support: refer to psychologist Optimization of laboratory test results ERAS program Duration: 24.5 ± 26.9 days | Physical capacity - 6MWT | Prehabilitation group: Increase |
| - VO2 max | No differences | |||
| Maximum muscle tension (LUNA EMG device) | Increase | |||
| Quality of life (standardized EORTC QLQ-C30) HADS Malnutrition (MNA, MUST) Frailty score (G8) | No major impact | |||
| Postoperative complications (Clavien–Dindo) | Fewer | |||
| Hospital stay | Shorter Negative correlation with maximum muscle tension prior to surgery | |||
| Miralpeix (2023) [18] Endometrium | Retrospective cohort of consecutive group N = 128 undergoing laparoscopic surgery Prehabilitation: 68, Control: 60 | Physical exercise Nutritional counseling Psychological support ERAS program Duration: 25 (18–35) days | Hospital stay | Prehabilitation group: Shorter |
| Normal oral diet restart | Earlier | |||
| Postoperative complications (Clavien–Dindo > II) | NS | |||
| Li (2024) [16] Gynecology (cervix, endometrium, and ovary) | Prospective cohort of consecutive group N = 97 Prehabilitation: 49, Control: 48 | Short-term, hospital-based Exercise intervention (stretching, resistance, aerobic, respiratory function, and Kegel) Nutrition intervention (carbohydrate:protein:fat ratio of 6:3:1, 1.2 g of protein/kg of body weight/day, whey protein, dietitian) Coping strategies to reduce anxiety (yoga relaxation and psychological consultant) ERAS program Duration: 7.2 ± 1.6 days | 6MWT Psychological status (HAS) Overall health (RAND 12-Item Health Survey v2 | Prehabilitation group: Better Better Better on 1 day before surgery and 30th day after surgery |
| Short-term postoperative recovery quality (QoR-9) | Higher quality of recovery for three consecutive days | |||
| Postoperative first ambulation time | Earlier | |||
| First gastrointestinal exhaust time | Earlier | |||
| Hospital stay | NS |
| Study Cancer Type | Objective | N | Intervention and Duration | Outcomes |
|---|---|---|---|---|
| PROPER—PRehabilitatiOn Plus Enhanced Recovery after surgery versus enhanced recovery after surgery in gynecologic oncology: a randomized clinical trial (NCT04596800) [39] Gynecologic cancer | To evaluate the impact of a prehabilitation program on postoperative recovery time for patients who will undergo gynecologic surgery, following the ERAS guidelines | 194 (1:1) | Physiotherapy: aerobic, inspiratory, and stretching exercises, muscle strengthening, 135 min per week in three sessions Nutrition: hypercaloric and hyperproteic nutritional supplement, or whey protein 1 or 2 times/day if indicated; counseling Psychology: counseling, image-based exercises and visualization for relaxation, breathing exercises Duration: 2–3 weeks | Primary: time between surgery and day patient is ready for discharge Secondary: compliance to ERAS guidelines, postoperative complication rates, rates of ICU admissions, health-related QOL, and changes in functional capacity, muscle strength, body mass index, and patient anxiety and depression |
| Prehabilitation in Gynaecological Cancer Patients (PHOCUS) (NCT04789694) [40] Gynecologic cancer | To evaluate the role of multimodal prehabilitation in patients with gynecological cancer | 64 (1:1) | Physical activity: individualized home-based resistance exercises, step count increase by 20% by time of surgery Psychological and nutritional support Duration: 9–12 weeks Recruitment: 36 months | Primary: 6MWT shortly before surgery Secondary: postoperative morbidity, length of postoperative hospital stay, adherence to training program, effects of nutritional support (analyzed from blood), QOL, and psychological health |
| TRAINING-Ovary 01 (connecTed pRehabiliAtIoN pelvIc caNcer surGery): a multicenter randomized study comparing neoadjuvant chemotherapy for patients managed for ovarian cancer with or without a connected prehabilitation program (NCT04451369) [41] Ovary | To determine if a connected prehabilitation program during NACT for patients treated for an advanced ovarian cancer will improve physical capacity before major abdomino-pelvic surgery | 136 (1:1) | Physical activity training program at home: short videos available on smartphone app, daily physical activity sessions and weekly supervision with connected device Nutritional care (ESPEN guidelines) adapted according to information transmitted weekly Psychological support with coping strategies Recruitment: 24 months Follow up: 5 years | Primary: variation in VO2 max between baseline and surgery Secondary: compliance, physical and nutritional status, QOL, morbidity, postoperative outcomes, return to intended oncologic treatments, oncologic outcomes, and cost-effectiveness |
| A multimodal prehabilitation program for the reduction of postoperative complications after surgery in advanced ovarian cancer under an ERAS pathway: a randomized multicenter trial (SOPHIE) (NCT04862325) [42] Ovary | To compare the postoperative complications of a multimodal prehabilitation program in patients undergoing cytoreductive surgery for advanced ovarian cancer with standard preoperative care | 146 (1:1) | Physical interventions: supervised high-intensity training program (endurance and resistance training), inspiratory muscle training program using threshold resistance device, promotion of physical activity through health mobile application Psychological and cognitive behavioral therapy: weekly group-based sessions Nutritional intervention: protein goals (1.6–2 g/kg/day), whey protein isolate, immunomodulatory formula Exclusion: patients with <75% adherence Duration: >2 weeks Recruitment: 4 years | Primary: overall postoperative complication rate (CCI) Secondary: length of hospital stay and days until initiation of chemotherapy; baseline, preoperative, and 1-month postoperative QOL; physical, nutritional, and cognitive test assessments; and prehabilitation and ERAS program compliance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, C.H.; Yim, G.W. Multimodal Prehabilitation for Gynecologic Cancer Surgery. Curr. Oncol. 2025, 32, 109. https://doi.org/10.3390/curroncol32020109
Kim J, Lee CH, Yim GW. Multimodal Prehabilitation for Gynecologic Cancer Surgery. Current Oncology. 2025; 32(2):109. https://doi.org/10.3390/curroncol32020109
Chicago/Turabian StyleKim, Jeongyun, Chae Hyeong Lee, and Ga Won Yim. 2025. "Multimodal Prehabilitation for Gynecologic Cancer Surgery" Current Oncology 32, no. 2: 109. https://doi.org/10.3390/curroncol32020109
APA StyleKim, J., Lee, C. H., & Yim, G. W. (2025). Multimodal Prehabilitation for Gynecologic Cancer Surgery. Current Oncology, 32(2), 109. https://doi.org/10.3390/curroncol32020109





