Simple Summary
Stereotactic radiosurgery (SRS) is a precise, noninvasive medical technique that uses focused radiation to treat small targets inside the brain without opening the skull. It was first imagined by the Swedish neurosurgeon Lars Leksell as an alternative to traditional brain surgery for neurological disorders. With advances in imaging and computing, SRS evolved during the 1990s into an essential tool, mainly for treating brain tumors and vascular malformations, and continued to be an alternative to functional neurosurgery. This literature review traces the historical development of stereotactic methods and the pivotal innovations that enabled precise intracranial targeting for SRS, while exploring the treatment evolution and most recent usage of SRS for various neurological disorders such as intractable pain (e.g., trigeminal neuralgia), movement disorders, epilepsy, and psychiatric conditions (e.g., obsessive–compulsive disorder). This review underscores how technological progress and shifting clinical priorities have transformed SRS from a niche neurosurgical technique into a cornerstone of modern clinical practice, with functional SRS representing its latest clinical field of expansion.
Abstract
Background: Stereotactic radiosurgery (SRS) was originally conceived as a noninvasive alternative to functional neurosurgery by the Swedish neurosurgeon Lars Leksell. This review traces the historical development of stereotactic methods from early mechanical frames to advanced image-guided systems and examines the pivotal innovations that enable precise intracranial targeting for SRS. Methods: Using PubMed and Google Scholar, we reviewed the literature on the general history of functional stereotactic neurosurgery and radiosurgery, its indications, and how their treatment methods evolved, focusing mainly on the early period from the end of the 18th century to the 1990s. Results: The origins of stereotaxy as a principle and technique were traced back to the early 20th century with animal studies by Horsley and Clarke, later adapted for human use by Spiegel and Wycis, and then Talairach in the 1940s. This enabled the precise targeting of deep brain structures for lesion-based interventions in movement, pain, and psychiatric disorders. Deep Brain Stimulation (DBS) emerged in the 1980s as a reversible treatment for tremor. Stereotactic radiosurgery was conceived in 1951 as a noninvasive alternative functional approach and saw limited use until the 1990s due to imaging constraints. With MRI-guided planning, its application has expanded mostly to the management of benign and malignant tumors and vascular disorders, as well as for functional approaches, particularly for trigeminal neuralgia, tremor, epilepsy, and OCD. Conclusions: This review underscores how technological progress and shifting clinical priorities have transformed SRS from a niche neurosurgical technique into a cornerstone of modern clinical practice, with functional SRS representing its latest clinical field of expansion.
1. Introduction
Stereotaxy was the original term coined by Horsley and Clarke in 1908 to describe their method of precisely targeting brain structures using a three-dimensional (3D) coordinate system. The term stereotactic emerged later, especially in human neurosurgery, and gained popularity after a consensus at the 1973 meeting of the World Society for Stereotactic and Functional Neurosurgery [1].
Although contemporary applications of stereotactic radiosurgery (SRS) predominantly focus on oncological treatments, its initial purpose was to be an alternative to invasive functional neurosurgery, for example, intractable pain (e.g., trigeminal neuralgia), movement disorders, epilepsy, and psychiatric disorders [2]. The role of functional neurosurgery in the development and implementation of functional SRS has become critical, particularly through a better understanding of underlying brain disorders and better visualization of brain anatomy with precision and minimal invasiveness. Functional radiosurgery has benefited from technological advancements in imaging and treatment delivery systems. This development underscores the need for less invasive neurosurgical interventions that prioritize patient safety and efficacy [3]. Functional radiosurgery was originally based on Gamma Knife technology; it has evolved since its initial application and significantly influences the management of functional brain disorders [4].
This article aims to provide a comprehensive historical overview of the development and clinical applications of functional stereotaxy, from its origin to its present-day context. This review underscores how technological progress and shifting clinical priorities have transformed SRS into a versatile tool with enduring relevance. We believe that knowledge of the history of functional stereotaxy is important for understanding its present-day context and the expansion of radiation oncology. This paper offers radiation oncologists a comprehensive roadmap for tracing the birth, refinement, and modern-day expansion of SRS. It not only illuminates how Leksell’s early vision evolved into today’s Gamma Knife- and Linac-based platforms but also uncovers emerging non-oncologic applications that could redefine their clinical practice.
2. Methods
To construct a robust historical and clinical narrative of functional stereotactic neurosurgery and SRS, we conducted an extensive literature review using PubMed and Google Scholar.
For the first portion of our paper regarding the history of stereotaxy and its clinical beginnings, searches were conducted from the beginning of stereotaxy, at the end of the 18th century, up to the development of SRS by Leksell in the 1950s. The term “history” was first combined with “stereotaxy”, and we subsequently added “epilepsy”, “pain”, “movement disorders”, and “psychosurgery”.
For the section on Deep Brain Stimulation (DBS), we used the terms “history” and “deep brain stimulation”, and we combined them with “epilepsy”, “pain”, “movement disorders”, and “psychosurgery”.
Finally, for the section focused on SRS, searches were conducted without date restrictions using the terms “stereotactic radiosurgery” and “functional neurosurgery”, combined with “epilepsy”, “pain”, “movement disorders”, and “psychosurgery” or “psychiatric disorders”.
The authors are fluent in English, French, and German, ensuring a comprehensive assessment of the literature published in these languages.
Peer-reviewed journal articles were studied, such as historical monographs and archival documents, conference proceedings, case reports, and clinical trials. Articles were screened based on title and abstract, and relevant articles were then fully read to assess their inclusion in this study.
Inclusion Criteria: Studies describing the development or clinical application of stereotactic techniques; articles detailing instrumentation, targeting methods, or imaging advancements; reports on outcomes of SRS or DBS in functional indications; and historical accounts of key figures and technological milestones. Exclusion Criteria: Studies focusing on cavernous malformations or cancer treatment, including vestibular schwannomas, as well as gray literature.
Data extraction and organization: The extracted data were organized into a timeline to trace the evolution of stereotactic principles, devices, and clinical applications. The findings were grouped into four major domains of functional neurosurgery. Historical techniques were compared to modern interventions to highlight shifts in clinical practice and ethical considerations. Studies have evaluated the impact of SRS on its present-day context, especially in cases where SRS has emerged as a viable alternative to open surgery. Our analysis combines historical narratives with a comparative evaluation. Limitations Considered: Recognized gaps in the literature account for the underreporting of early radiosurgical outcomes and limited long-term data for functional SRS applications.
3. Brief History of Stereotaxy
The history of stereotaxy can be traced back to the 19th century. It is compelling how many different specialists from multiple countries shaped the beginnings of stereotaxy. The idea that different parts of the brain are associated with a specific function was presented by Paul Broca in a meeting in April 1861 [5]. This theory led Wilhelm Dittmar, a German physiologist, to develop a device in 1873, one of the earliest devices used to stabilize and localize intracranial brain structures in animals. His device was created to guide a cutting knife and form lesions in the vasomotor center of the medulla oblongata of rabbits [6]. Dittmar’s work was built on earlier studies initiated by his predecessor Carl Ludwig (1816–1895). Ludwig was one of the founders of modern experimental physiology, and he hypothesized that the contraction of arterial vessels was controlled by a specific region of the brain. Initial surgical approaches to the medulla were performed freehand and lacked precision, often resulting in significant damage. In response, Dittmar engineered a mechanical apparatus to enable more accurate targeting while minimizing harm to the surrounding tissues. This was an early step towards stereotactic principles that would later define the field [6].
Towards the end of the 1800s, numerous cranial localization devices were developed worldwide, mainly to observe and localize cranial and cortical regions in relation to bone landmarks. These included Emil Theodor Kocher’s craniometer in 1892 in Bern, Switzerland, which was the most precise method at the time, compared to similar instruments, such as Wilson’s cyrtometer or the cephalometer of Kroenlein and Köhler [5]. All these devices shared the same goal: to accurately relate the surface structures of the cranium to brain structures in the hopes of realizing the least invasive procedure possible, guiding the creation of an opening through a small trephine hole. Kocher aimed to account for variations in human cranial shapes by developing a more universal apparatus to relate surface topography to brain structure [5]. Around the same period, in 1889, Dmitry Nikolaevich Zernov (1843–1917), a Russian anatomist, presented the encephalometer, a precursor of modern stereotactic apparatuses. This device is based on polar coordinates and can precisely locate any point on the skull or brain surface. It has been used clinically in humans to create burr holes after precisely locating various gyri and fissures in the brain. In 1907, another Russian neurologist and psychologist, Grigory Ivanovich Rossolimo, tried to improve this device, which he called the brain topography [7]. Regrettably, their device did not achieve a significant breakthrough in the English medical literature during this period.
The first stereotactic device was developed in 1905 by Sir Victor Horsley and Robert Henry Clarke, a British surgeon and a physiologist, respectively. In 1908, they introduced their stereotactic apparatus to the public, which utilized Cartesian coordinates to investigate deep brain structures in animals, such as the gray nuclei and thalamus, by referencing bone landmarks [7]. Their goal was not immediately clinical; it was to enable precise, reproducible targeting of deep brain structures for experimental purposes. Their initial experiment, published in 1908, provided evidence that the cerebellum functions as a recipient structure rather than as an efferent one. Although this was the prevailing hypothesis at the time, the use of rudimentary lesion techniques often resulted in extensive damage or complications involving injuries to other parts of the brain, rendering accurate interpretation challenging. Utilizing their precise stereotactic apparatus to accurately locate and conduct targeted electrolysis while preserving the integrity of the cerebellar nuclei, they effectively dispelled any potential doubts [8]. Owing to the precision of their apparatus, they subsequently advanced to the development of brain atlases in cats and monkeys [7]. Their invention was a turning point: it transformed brain surgery from a largely exploratory endeavor into a targeted, science-driven discipline. This has allowed researchers to study the function of specific brain regions by creating lesions or stimulating them with remarkable accuracy. While their initial work focused on basic neuroscience, the long-term clinical aim was to translate this precision into human neurosurgery by mapping the brain in three dimensions and creating atlases.
Prior to this point, although devices for guiding procedures on the human brain were available, no stereotactic devices had been developed. However, in 1918, Aubrey Mussen, a Canadian neuropathologist who worked with Horsley and Clarke, created the first stereotactic device for human use. Unfortunately, he never succeeded in convincing a neurosurgeon to use his apparatus, likely due to the absence of a human brain atlas at the time [9]. Many years passed, and both Mussen’s and the Horsley–Clarke apparatus were forgotten.
In the mid-1940s, key breakthroughs occurred on both sides of the Atlantic. Spiegel (1895–1985) and Wycis (1911–1972) transformed neurosurgery together by bringing precision and structure to brain interventions. Spiegel and Wycis, working in the United States, introduced their modified version of the Horsley–Clarke apparatus for human use in 1947 at Temple University in Philadelphia. In 1946, Swiss physiologist Marcel Monnier established a crucial foundation by linking animal experiments to stereotactic neurosurgery, aspiring to develop a neurosurgical approach that utilizes intracerebral electrodes instead of traditional surgical tools [10]. This led to the first human stereotactic surgery performed in 1947 by Spiegel and Wycis, based on intracranial landmarks to account for extracranial brain variability between humans and with Cartesian coordinates. Their approach used ventriculography for image guidance.
Spiegel and Wycis deployed their new human stereotactic frame across a spectrum of indications (psychiatric illness, pain, cyst aspiration, movement disorders) almost immediately after their 1947 publication in Science [11]. Subsequently, they published their own human brain atlas in 1952 [12].
In France, Jean Talairach (1911–2007), a psychiatrist who transitioned to neurosurgery, made significant advancements in the field. In 1947, he developed a specialized stereotactic methodology that utilized a stereotactic frame equipped with a three-coordinate reference system. The following year, in 1948, he performed an inaugural surgical procedure on a 72-year-old male patient. This procedure involved thalamotomy via electrocoagulation targeting the ventromedian and centromedian nuclei to alleviate severe trigeminal neuralgia secondary to herpes zoster ophthalmicus [10]. He continued to refine his methods by introducing ventriculography and intraoperative teleangiographic radiography. To deal with human brain variability caused by the use of external landmarks, he described a standardized anatomical coordinate system in 1952 and published his first atlases in 1957 [10]. Innovations in stereotactic technology and clinical use reached a significant milestone in the 1950s with the introduction of an arc radius stereotactic apparatus developed by the Swedish neurosurgeon Lars Leksell. The initial device for stereotactic radiosurgery (SRS) was not a Gamma Knife but rather an orthovoltage X-ray tube affixed to Leksell’s stereotactic frame. This prototype enabled the targeting of cranial pain pathways, such as the Gasserian ganglion, with submillimetric precision, marking the inception of SRS treatments globally. Subsequently, Leksell investigated the use of high-energy proton beams at Uppsala’s cyclotron, achieving remarkable dose conformity despite encountering logistical and technical obstacles. In pursuit of a more feasible source, Leksell collaborated with Börje Larsson to develop a multi-beam Cobalt-60 unit. The inaugural Gamma Knife prototype was used to treat patients in Stockholm in 1968, becoming the first commercially available SRS system. These developments eventually led to the advancements observed today in linac-based radiosurgery [13,14].
The systems’ utility increased over the years with their integration of a CT scanner, followed by the addition of a dedicated MRI adaptation in 1985 [15]. Figure 1 illustrates the evolution of stereotactic apparatuses and radiosurgery platforms, the major technical milestones in the creation of Leksell’s Gamma Knife, and the eventual use of stereotactic CT- and MRI-guided targeting.
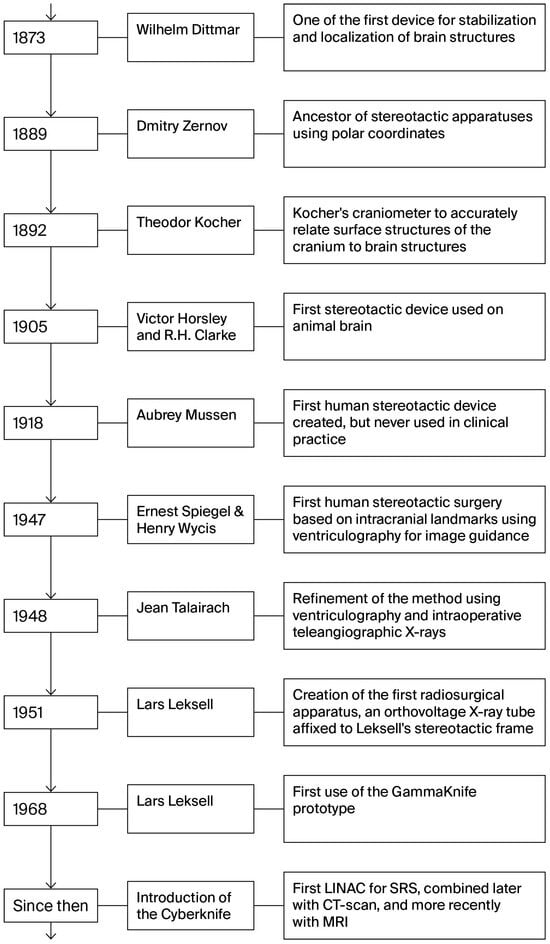
Figure 1.
Evolution of stereotactic apparatuses and radiosurgery platforms. This timeline maps the major technical milestones from the first polar coordinate devices of the late 19th century through the advent of human stereotactic frames to the creation of Leksell’s Gamma Knife and the integration of stereotactic CT- and eventual MRI-guided targeting.
4. Clinical Beginnings of Stereotactic Neurosurgery
4.1. Treatment of Movement Disorders
As previously mentioned, Wycis and Spiegel were the first to document stereotactic neurosurgery in humans in 1947. However, surgical intervention to treat movement disorders precedes stereotaxic treatment. In 1908, Horsley reported excision of the precentral cortex in a 14-year-old boy with athetoid and clonic movements of the right arm. He used electrodes to locate the area and achieved complete alleviation of spasmodic movements one year later. However, voluntary movement in the left upper limb was almost absent, accompanied by astereognosis, highlighting the need for more precise interventions [16]. Subsequent approaches developed by Putnam in 1938 targeted other structures, such as the pyramidal tract. In 1940, Meyers targeted the caudate nucleus and globus pallidus (GPi). Some improvements were reported, but not full recovery or a lower mortality rate. Subsequent approaches were developed by, for example, Talairach in 1947, who aimed to cure dyskinesia with cortical resection of Brodmann areas 4, 6, and 8 [10]. Most interventions, however, targeted the GP internus with new lesioning methods, such as with the cryogenic probe introduced by Cooper in 1955 [15]. Prior to the advent of DBS, posteroventral pallidotomy was the most common intervention for Parkinson’s disease, effectively addressing tremor, rigidity, and dystonia. Ventrolateral thalamotomy was typically reserved for patients with tremor-dominant presentations. The introduction of L-dopa in 1965 diminished the need for invasive neurosurgical interventions. However, the inability of medication to consistently deliver therapeutic outcomes without adverse effects over time, coupled with advancements in imaging technology, rekindled interest in stereotactic surgery.
4.2. Treatment of Epilepsy
Until the late 19th century, trepanation was the sole surgical intervention used in epilepsy treatment. Significant advancements occurred when Victor Horsley pioneered new treatment methods and conducted the first modern epilepsy surgery in 1886 on a 22-year-old male patient. Advancements in technologies such as electroencephalography (EEG), computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) have significantly enhanced our understanding of the mechanisms of epilepsy and localization of activity clusters [17].
4.3. Treatment of Psychiatric Conditions
The development of stereotactic frames by Wycis and Spiegel facilitated the exploration of alternative treatment methods, particularly those targeting the thalamus and hypothalamus. This innovation marked the inception of subcortical stereotactic neurosurgical procedures and serves as a foundational model for contemporary psychiatric neurosurgery [12]. Around the same time, Leksell and Talairach pioneered capsulotomy (the lesioning of the anterior limb of the internal capsule) for refractory psychiatric conditions, such as obsessive–compulsive disorders (OCDs) and anxiety disorders. Other targets were explored, such as the hypothalamus, to treat refractory aggressive behavior. Sano attempted this in 1962 and performed posteromedial hypothalamotomy with positive outcomes to reduce such behavior [18]. Additional experiments have been conducted to address various psychiatric disorders with varying degrees of success.
The negative perception of surgical interventions for psychiatric disorders can be attributed to their historical development. The practice of “psychosurgery” was initiated by Swiss psychiatrist Gottlieb Burckhardt, who, in 1888, conducted cerebral cortical excisions on six patients diagnosed with schizophrenia. Without surgical training, he removed parts of the cortex, believing it was the center of the psychiatric disorder. His patients showed improvements but experienced aphasia or seizures, leading to backlash from the medical community [19]. The field reemerged in 1936 when António Egas Moniz, with Almeida Lima, developed frontal lobotomies using a “leucotome,” based on Fulton and Jacobsen’s animal research. Moniz received a Nobel Prize in 1949 for his leukotomies [15,20]. James Watts standardized prefrontal lobotomy in the U.S. and helped with the popularization of Walter Freeman’s transorbital lobotomy technique, which was simpler to perform [21]. In 1949, Fulton proposed cingulotomy, which proved superior to Watts’ procedures and remains in use for OCD, depression, anxiety, and chronic pain [22,23]. However, the introduction of chlorpromazine in 1952 had the same effect as L-dopa in Parkinson’s disease, which caused a significant decrease in interest in lobotomies to treat psychiatric conditions.
Today, neurosurgical approaches for psychiatric conditions are the main focus, including a wide range of targets and techniques, whose indications are better controlled using multidisciplinary approaches.
4.4. Treatment of Chronic Pain
The application of stereotactic neurosurgery to chronic pain began in the early 20th century and focused predominantly on trigeminal neuralgia (TN). In the 1930s, Kirschner developed a frame specifically designed to access the foramen ovale for percutaneous injections into the Gasserian ganglion to alleviate facial pain. By the mid-20th century, some stereotactic techniques were also extended to the management of cancer-related pain. In 1953, pituitary gland lesioning was explored as a palliative intervention for metastatic disease [15]. The use of neurosurgery for TN diminished over time after the 1960s due to the introduction of carbamazepine. Interventions targeting the nuclei of the thalamus, especially the ventral posterolateral nucleus, have also been proposed for the treatment of intractable deafferentation pain (anesthesia dolorosa), such as in cases of trauma, amputation, or stroke.
5. Deep Brain Stimulation: Treatment Without Destruction
DBS emerged as a direct extension of stereotactic neurosurgical principles, offering a safe, reversible, and ethically acceptable procedure by modulating neural function, rather than permanently lesioning deep brain structures. Enabled by the precision of stereotactic targeting, DBS was initially used intraoperatively to confirm lesion locations. However, in the 1960s and 1970s, some neurosurgeons proposed to also use it as a therapeutic modality. Modern DBS was pioneered in 1987 by neurosurgeon Alim-Louis Benabid and neurologist Pierre Pollack in Grenoble, who published their first paper on the use of thalamic DBS for tremor, with results comparable to those of lesioning while maintaining reversibility [24]. These advances would not have been possible without stereotactic technologies, allowing for safe electrode placement in deep-seated millimeter-scale targets.
5.1. Treatment of Movement Disorders by DBS
The most well-established application of DBS is the treatment of movement disorders, particularly Parkinson’s disease, essential tremors, and dystonia. The success of this technique is based on its reliance on stereotactic frames, atlases, and MRI or CT imaging to precisely localize targets. Benabid and Pollak’s landmark use of DBS in the VIM in 1987 to alleviate tremors in Parkinson’s opened the door to broader applications [24]. By the early 1990s, stereotactically guided implantation in the STN and GPi had been validated through animal and clinical trials. These targets remain central to Parkinson’s disease treatment, with STN being better for Parkinsonian symptoms and GPi being chosen often for patients with mood, cognitive, or speech issues. Dystonia, particularly generalized forms, is also treated via GPi stimulation [25,26].
5.2. DBS in Epilepsy
From the 1950s to the 1970s, extensive research was conducted to identify precise surgical targets for epilepsy treatment [12]. Key targets identified via stereotactic imaging include the anterior nucleus of the thalamus for limbic seizures, the hippocampus for temporal lobe epilepsy, and the centromedian nucleus for generalized seizures [27]. The pivotal SANTE trial (Stimulation of the Anterior Nucleus of the Thalamus for Epilepsy) in 2010 led to FDA approval of Medtronic’s ANT-DBS system as an adjunctive therapy for adults with drug-resistant partial-onset seizures [28,29]. Advances in imaging, particularly high-resolution MRI, now allow improved direct visualization of deep nuclei, although many procedures still rely on indirect atlas-based targeting [28].
5.3. DBS in Psychiatric Disorders
DBS has brought new ethical and technical standards to psychiatric functional neurosurgery by enabling reversible functional modulation without the irreversible damage caused by ablative psychosurgery [18,20,30,31]. These procedures are dependent on precise stereotactic planning, owing to the complexity and variability of the individual limbic anatomy.
5.4. DBS in Pain Treatment
Although it is barely used nowadays and lacks FDA approval, DBS for chronic pain has historically been one of its earliest applications. Relying on stereotactic methods, DBS has helped control intractable post-amputation pain, as explored by Mazars in France and Cooper in the United States in the 1970s and 1980s [24,32].
6. Radiosurgery and Its Possibilities: The Revival of Its Role in Functional Neurosurgery
The exact mechanism of functional SRS remains debated to this day and is likely multifactorial. SRS allows for the creation of precise intracranial lesions using focused beams of ionizing radiation, thereby minimizing collateral damage. While certain effects are attributed to localized tissue damage, inflammation, and demyelination [33], other studies propose that SRS may induce neuroglial remodeling and functional modulation without significant cellular destruction, or if destruction occurs, it progresses slowly enough to allow for cerebral reorganization [34]. Some researchers propose that it is a combination of both, involving focal necrosis and peripheral neuromodulation effects. Due to the complexity of this subject, it falls beyond the scope of this review and may warrant a dedicated paper. For further exploration, the following references should be consulted with careful consideration [35,36,37].
The first Gamma Knife unit was installed in Stockholm in 1967, but its functional neurosurgical applications remained limited. It was not until the late 1980s that SRS began to gain traction in this field with the development of advanced MRI-guided planning and more precise stereotactic localization. A significant limitation of SRS compared with DBS or open lesioning is its inability to perform intraoperative electrophysiological confirmation [38]. This has led to suboptimal usage of SRS for decades as a treatment option for functional disorders [39]. While Gamma Knife remains the predominant technology in the literature, the use of Linac-based platforms is increasing, especially for trigeminal neuralgia. To our knowledge, SBRT and adaptive image-guided radiotherapy have not yet been reported for functional indications [40].
6.1. SRS for Functional Neurosurgery
The following section explores the clinical use of SRS in each of the major domains of functional neurosurgery: movement disorders, epilepsy, psychiatric disorders, and pain syndromes.
6.2. Movement Disorders
Although the first Gamma Knife was installed in 1967, only five cases of movement disorders were treated from 1968 to 1970 [39]. The use of SRS for movement disorders expanded in the mid-1980s with improved neuroimaging, better treatment planning systems, and the Gamma Knife developed by Leksell. Preclinical studies on rats and monkeys have helped us explore many parts of the brain, including the caudate nucleus and the ventral intermediate nucleus (VIM), which is still the most used target for Parkinson’s disease. VIM targeting can reduce tremor, bradykinesia, and rigidity to some extent while being safe and precise if used with MRI [41,42]. The International Stereotactic Radiosurgery Society practice guidelines (2019) recommend 130–150 Gy as an effective and well-tolerated dose range [43].
While the Gamma Knife unit still uses a stereotactic frame attached to the patient’s head, frameless SRS techniques, such as CyberKnife and other Linac-based systems, have been investigated and have shown promising early outcomes in terms of safety and efficacy, potentially offering broader access to functional radiosurgical treatment [44,45].
A recent meta-analysis [46] suggested comparable efficacy for Gamma Knife and Linac-based thalamotomy in tremor reduction, but conclusions were limited due to the small number of Linac studies; Gamma Knife was linked to more adverse events and a longer time to improvement.
Table 1 presents some recent studies on tremor with their characteristics and results. The individual studies summarized in this table were selected from the recent systematic reviews and meta-analyses referenced in [47,48].

Table 1.
Summary of studies evaluating reduction in tremor in patients with essential tremors and/or Parkinson’s disease with stereotactic radiosurgery (SRS) treatment. RR, response rate—the maximal response in the cohort at any point; RR 12 m, response rate at 12 months of follow-up; AE, adverse effect.
6.3. Epilepsy
The first use of radiation as a potential treatment for epilepsy appeared before the invention of SRS. Talairach performed pioneering work on implanted radioactive seeds in the brain. Initially, he used it in 1954 to treat inoperable tumors with radioactive gold seeds. He then applied, around 1957, yttrium-90 implants in patients with mesial temporal lobe epilepsy (MTLE) and no space-occupying lesions. In his 1974 study, Talairach achieved promising early seizure control rates [12,55,56]. However, other researchers did not replicate these outcomes with comparable success, limiting the popularity of the SRS technique for epilepsy at the time. Functional SRS for epilepsy re-emerged in the mid-1990s through the work of Régis et al. in France, who published encouraging results using Gamma Knife [55].
To evaluate the efficacy of SRS in comparison with traditional open surgery, the ROSE trial was conducted, and its findings were published in 2018. This randomized, single-blinded, controlled trial assessed SRS for anterior temporal lobectomy in patients with pharmacoresistant unilateral MTLE. The results indicated that SRS is a viable alternative to anterior temporal lobectomy (ATL) for patients who have contraindications to or are hesitant to undergo open surgery [57]. A 2025 systematic review and meta-analysis comparing laser interstitial thermal therapy, radiofrequency ablation, and stereotactic radiosurgery for mesial temporal lobe epilepsy provides contemporary comparative outcome data and helps contextualize the ROSE trial results within evolving minimally invasive options [58]. Authors explain that the three treatment methods are comparable, even if laser therapy seems more promising. However, there is no doubt that more RCTs for SRS are necessary in the future.
Table 2 summarizes recent mesial temporal lobe epilepsy studies with key characteristics and results.

Table 2.
Summary of studies evaluating reduction in seizures in patients with MTLEs with SRS treatment. RR, response rate—the maximal response in the cohort at any point; AE, adverse effect.
In addition to MTLE, SRS has also been applied to treat hypothalamic hamartomas, which are difficult to access in open surgery. SRS has shown good results in reducing seizure frequency, but without seizure freedom, and offers a better risk–benefit ratio than surgical methods [55,62].
6.4. Psychiatric Conditions
The use of stereotactic radiosurgery for psychiatric disorders began with Lars Leksell in 1953, when he treated a patient with intractable obsessive–compulsive disorder (OCD) using a 300-kV X-ray apparatus [34,63]. The patient was a 29-year-old man with intractable OCD. This stereotactic radiosurgery operation was performed in two stages: First, an anterior gamma capsulotomy on the right side was performed with a collimator helmet with an 8 mm diameter and a maximum dose of 100 Gy to the most anterior part of the internal capsule, guided via pneumoencephalography. One month later, the same procedure was performed with a maximum dose of 120 Gy on the left side. No information was reported regarding the follow-up of this patient [64]. Similarly to other functional treatments, the application of SRS was constrained prior to the development of advanced imaging techniques. It was not until 1978 that Rylander published an inaugural outcome study following the Gamma Knife capsulotomy conducted by the Karolinska group. This study involved nine patients, five of whom were treated for OCD, yielding reasonable results [65]. In the following decades, Gamma Knife surgery for OCD was performed in Sweden, but due to a lack of radiobiology knowledge at the time, it led to excessively high doses, resulting in many adverse effects, which limited the clinical use of SRS for many years [66].
Current radiosurgical approaches most commonly target the anterior portion of the internal capsule; outcome data indicate efficacy comparable to alternative anterior capsulotomy techniques [67]. Table 3 provides an overview of recent series, reporting patient numbers, dosing, targeting, efficacy, and adverse events [68,69].

Table 3.
Summary of studies evaluating reduction in symptoms in patients with OCD with SRS treatment. FR, full response described in the literature as a greater than 35% improvement in the Y-BOCS score (* greater than or equal to a 25% improvement); Remission, defined as an endpoint Y-BOCS total score ≤ 12 or 8; VP, ventral portion; ALIC, anterior limb of the internal capsule.
6.5. Pain
For pain management, neurosurgery and later SRS were investigated for the treatment of two main entities: trigeminal neuralgia (TN) and intractable pain. In 1951, Leksell performed the first radiosurgical procedure for TN targeting the Gasserian ganglion. Like other SRS procedures, interest in the treatment of TN increased in the early 1990s with the availability of MRI, which enabled improved direct visualization of the nerve [75]. With time, the target moved closer and closer to the nerve root. In 1993, Rand targeted the cisternal segment of the nerve, while Lindquist et al. targeted the nerve at its emergence from the brainstem, the trigeminal nerve root entry zone (REZ), which is now the most used target. Doses of 70–90 Gy were demonstrated to be safe and effective in 1996 in the multicenter trial conducted by Kondziolka and a prospective controlled trial in 2006 [34,75]. However, microvascular surgeries remain the gold standard treatment for TN, proven to have lower rates of short-term and long-term pain freedom than SRS [76].
Recent studies report comparable clinical outcomes between Gamma Knife and frameless Linac-based or CyberKnife systems for trigeminal neuralgia [77,78,79], although meta-analyses continue to favor conventional procedures such as microvascular decompression or rhizotomy [80,81]. Table 4 summarizes recent series with treatment details and outcomes.

Table 4.
Summary of studies evaluating reduction in pain in patients with TN with SRS treatment. Pain-free—Grade I to IIIa on the Barrow Neurological Institute scale (* also included Grade IIIb); RG, retrogasserian target on the trigeminal nerve; REZ, nerve root entry zone.
The first SRS thalamotomies for intractable pain were performed by Leksell in 1968 using a Gamma Knife, which showed, as opposed to other functional disorders, an immediate effect in approximately two-thirds of the patients. This rapid pain reduction was likely due to the high doses of radiation, sometimes exceeding 250 Gy, which caused severe side effects. In 1972, Backlund first attempted pituitary SRS for the treatment of malignant pain [89]. In the 1980s, research on ventromedial thalamotomy was explored in cancer patients, using SRS as a pain-relieving method, but the effect was variable and not maintained for a sufficient amount of time [90].
6.6. Linking Historical Evolution to Current Radiotherapy Paradigms
The historical evolution of stereotactic radiosurgery, from early lesioning and frame-based stereotaxis to modern image-guided, frameless systems, directly informs its present-day context. Innovations in target localization, dose conformality, and motion management that originated in functional radiosurgery have been translated into SBRT and adaptive image-guided radiotherapy, enabling higher ablative doses, hypofractionation, and on-table plan adaptation while preserving precision and normal-tissue sparing. Explicitly linking these developments clarifies how past technical and conceptual advances underpin current clinical capabilities and helps clinicians appreciate the rationale for adopting newer platforms and workflows. Recent advances in other radiation-based disciplines illustrate how integrated, personalized care can reshape therapeutic paradigms, for example, total neoadjuvant therapy [91], organ-preservation strategies [92], and AI-driven treatment personalization in rectal cancer [93]. By analogy, stereotactic radiosurgery is positioned to follow a similar trajectory toward greater individualization and function preservation through combined modality strategies [94], refined patient selection supported by radiomics and predictive analytics [95], adaptive image-guided planning including MR-guided adaptive radiotherapy techniques [96], and the incorporation of predictive analytics and machine learning to optimize dose, fractionation, and target selection for each patient [97].
7. Ethical Challenges
Stereotactic radiosurgery for functional brain disorders carries unique ethical challenges that echo the history of ablative psychosurgery. Unlike Deep Brain Stimulation, SRS effects are irreversible once the radiation induces tissue damage. Therefore, patients must be counseled extensively on both the potential benefits and permanence of their treatment. The historical context of stereotaxy emphasizes the need to acknowledge the legacy of psychosurgery abuse in building trust and avoiding repeat missteps. Stereotactic professional societies should continue to champion consensus guidelines for target selection and radiation dosing to reduce practice variability and enhance patient safety.
8. Conclusions
Stereotaxy has undergone remarkable evolution since the late 19th century, driven by innovations in stereotactic targeting and imaging technologies. It epitomizes the culmination of over a century of advancements in stereotactic and radio- and neurosurgery, achieved through the contributions of numerous researchers from various countries. Stereotactic radiosurgery (SRS) has evolved significantly since its inception in 1951 as a noninvasive alternative to functional neurosurgery. Although initially limited by imaging constraints, advances in MRI-guided planning have expanded SRS applications beyond tumors to functional disorders, such as tremor, epilepsy, OCD, and trigeminal neuralgia.
SRS is a noninvasive option for carefully selected patients who have failed medical therapy or cannot undergo surgery, delivered with precise, image-guided stereotactic targeting. Multidisciplinary collaboration between neurosurgeons and neurologists is essential. As imaging and delivery systems continue to advance, radiation oncologists should remain aware of the emerging functional applications of SRS. These innovations may expand treatment options for patients with neurological and psychiatric disorders.
Because the current literature is largely retrospective, high-quality prospective studies and randomized trials are necessary to establish whether SRS offers meaningful advantages over standard treatments.
Author Contributions
M.G. and D.T. conceptualized the review topic, led the literature search, and drafted the initial manuscript. M.L. and G.L.M. contributed to the historical analysis and critically revised the manuscript for intellectual content. M.L. assisted with clinical interpretation and provided expert input on the stereotactic techniques. All authors have read and agreed to the published version of the manuscript.
Funding
MG received support from Tersera Canada.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gildenberg, P.L.; Krauss, J.K. History of Stereotactic Surgery. In Textbook of Stereotactic and Functional Neurosurgery; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Ganz, J.C. The journey from proton to gamma knife. Prog Brain Res. 2014, 215, 67–75. [Google Scholar] [CrossRef]
- Davidson, B.; Hamani, C.; Huang, Y.; Jones, R.M.; Meng, Y.; Giacobbe, P.; Lipsman, N. Magnetic Resonance-Guided Focused Ultrasound Capsulotomy for Treatment-Resistant Psychiatric Disorders. Oper. Neurosurg. 2020, 19, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Kondziolka, D. Functional radiosurgery. Neurosurgery 1999, 44, 12–20; discussion 12–20. [Google Scholar] [CrossRef]
- Schultke, E. Theodor Kocher’s craniometer. Neurosurgery 2009, 64, 1001–1004; discussion 1004–1005. [Google Scholar] [CrossRef] [PubMed]
- Blomstedt, P.; Olivecrona, M.; Sailer, A.; Hariz, M.I. Dittmar and the history of stereotaxy; or rats, rabbits, and references. Neurosurgery 2007, 60, 198–201; discussion 201–192. [Google Scholar] [CrossRef]
- Fodstad, H.; Hariz, M.; Ljunggren, B. History of Clarke’s stereotactic instrument. Stereotact. Funct. Neurosurg. 1991, 57, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Horsley, V.; Clarke, R.H. The structure and functions of the cerebellum examined by a new method. Brain 1908, 31, 45–124. [Google Scholar] [CrossRef]
- Bertrand, G. Stereotactic surgery at McGill: The early years. Neurosurgery 2004, 54, 1244–1251; discussion 1242–1251. [Google Scholar] [CrossRef]
- Zanello, M.; Duriez, P.; Savoureux, A.S.; Vinckier, F.; Chretien, F.; Gavaret, M.; Gorwood, P.; Gaillard, R.; Pallud, J. Jean Talairach (1911–2007). An untold story of the pioneer of stereotactic and functional neurosurgery. Neurochirurgie 2022, 68, 398–408. [Google Scholar] [CrossRef]
- Spiegel, E.A.; Wycis, H.T.; Marks, M.; Lee, A.J. Stereotaxic Apparatus for Operations on the Human Brain. Science 1947, 106, 349–350. [Google Scholar] [CrossRef]
- Lehman, R.M.; Augustine, J.R. Evolution and rebirth of functional stereotaxy in the subthalamus. World Neurosurg. 2013, 80, 521–533. [Google Scholar] [CrossRef]
- Leksell, L. The stereotaxic method and radiosurgery of the brain. Acta Chir. Scand. 1951, 102, 316–319. [Google Scholar]
- Lunsford, L.D.; Flickinger, J.C.; Steiner, L. The gamma knife. JAMA 1988, 259, 2544. [Google Scholar] [CrossRef] [PubMed]
- Dagi, T.F. Stereotactic surgery. Neurosurg. Clin. N. Am. 2001, 12, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Horsley, V. The Linacre Lecture on the function of the so-called motor area of the brain: Delivered to the Master and Fellows of St. John’s College, Cambridge, May 6th, 1909. Br. Med. J. 1909, 2, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Pooya, A.A.; Rostami, C. History of surgery for temporal lobe epilepsy. Epilepsy Behav. 2017, 70 Pt A, 57–60. [Google Scholar] [CrossRef]
- Barbosa, D.A.N.; de Oliveira-Souza, R.; Monte Santo, F.; de Oliveira Faria, A.C.; Gorgulho, A.A.; De Salles, A.A.F. The hypothalamus at the crossroads of psychopathology and neurosurgery. Neurosurg. Focus 2017, 43, E15. [Google Scholar] [CrossRef]
- Stone, J.L. Dr. Gottlieb Burckhardt--the pioneer of psychosurgery. J. Hist. Neurosci. 2001, 10, 79–92. [Google Scholar] [CrossRef]
- Zanello, M.; Pallud, J.; Baup, N.; Peeters, S.; Turak, B.; Krebs, M.O.; Oppenheim, C.; Gaillard, R.; Devaux, B. History of psychosurgery at Sainte-Anne Hospital, Paris, France, through translational interactions between psychiatrists and neurosurgeons. Neurosurg. Focus 2017, 43, E9. [Google Scholar] [CrossRef]
- Lichterman, B.L.; Schulder, M.; Liu, B.; Yang, X.; Taira, T. A comparative history of psychosurgery. Prog. Brain Res. 2022, 270, 1–31. [Google Scholar] [CrossRef]
- Lapidus, K.A.; Kopell, B.H.; Ben-Haim, S.; Rezai, A.R.; Goodman, W.K. History of psychosurgery: A psychiatrist’s perspective. World Neurosurg. 2013, 80, S27.e1–S27.e16. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.P.; Alterman, R.L.; Goodrich, J.T. Contemporary psychosurgery and a look to the future. J. Neurosurg. 2001, 95, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Cavallieri, F.; Mulroy, E.; Moro, E. The history of deep brain stimulation. Parkinsonism Relat. Disord. 2024, 121, 105980. [Google Scholar] [CrossRef]
- Miocinovic, S.; Somayajula, S.; Chitnis, S.; Vitek, J.L. History, applications, and mechanisms of deep brain stimulation. JAMA Neurol. 2013, 70, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Pycroft, L.; Stein, J.; Aziz, T. Deep brain stimulation: An overview of history, methods, and future developments. Brain Neurosci. Adv. 2018, 2, 2398212818816017. [Google Scholar] [CrossRef]
- de Oliveira, T.; Cukiert, A. Deep Brain Stimulation for Treatment of Refractory Epilepsy. Neurol. India 2021, 69, 42–44. [Google Scholar] [CrossRef]
- Remore, L.G.; Omidbeigi, M.; Tsolaki, E.; Bari, A.A. Deep brain stimulation of thalamic nuclei for the treatment of drug-resistant epilepsy: Are we confident with the precise surgical target? Seizure 2023, 105, 22–28. [Google Scholar] [CrossRef]
- Yassin, A.; Al-Kraimeen, L.; Qarqash, A.; AbuShukair, H.; Ababneh, O.; Al-Aomar, S.; Abu-Rub, M.; Alsherbini, K. Deep brain stimulation targets in drug-resistant epilepsy: Systematic review and meta-analysis of effectiveness and predictors of response. Seizure 2024, 122, 144–152. [Google Scholar] [CrossRef]
- Alonso, P.; Cuadras, D.; Gabriels, L.; Denys, D.; Goodman, W.; Greenberg, B.D.; Jimenez-Ponce, F.; Kuhn, J.; Lenartz, D.; Mallet, L.; et al. Deep Brain Stimulation for Obsessive-Compulsive Disorder: A Meta-Analysis of Treatment Outcome and Predictors of Response. PLoS ONE 2015, 10, e0133591. [Google Scholar] [CrossRef]
- Cleary, D.R.; Ozpinar, A.; Raslan, A.M.; Ko, A.L. Deep brain stimulation for psychiatric disorders: Where we are now. Neurosurg. Focus 2015, 38, E2. [Google Scholar] [CrossRef] [PubMed]
- Frizon, L.A.; Yamamoto, E.A.; Nagel, S.J.; Simonson, M.T.; Hogue, O.; Machado, A.G. Deep Brain Stimulation for Pain in the Modern Era: A Systematic Review. Neurosurgery 2020, 86, 191–202. [Google Scholar] [CrossRef]
- Martinez-Alvarez, R. Radiosurgery for Behavioral Disorders. Prog. Neurol. Surg. 2019, 34, 289–297. [Google Scholar] [CrossRef]
- Regis, J. Gamma knife for functional diseases. Neurotherapeutics 2014, 11, 583–592. [Google Scholar] [CrossRef]
- Regis, J.; Carron, R.; Park, M. Is radiosurgery a neuromodulation therapy?: A 2009 Fabrikant award lecture. J. Neurooncol. 2010, 98, 155–162. [Google Scholar] [CrossRef]
- Regis, J. Radiosurgery as neuromodulation therapy! Acta Neurochir. Suppl. 2013, 116, 121–126. [Google Scholar] [CrossRef]
- Schneider, M.B.; Walcott, B.; Adler, J.R., Jr. Neuromodulation via Focal Radiation: Radiomodulation Update. Cureus 2021, 13, e14700. [Google Scholar] [CrossRef] [PubMed]
- Stancanello, J.; Romanelli, P.; Modugno, N.; Cerveri, P.; Ferrigno, G.; Uggeri, F.; Cantore, G. Atlas-based identification of targets for functional radiosurgery. Med. Phys. 2006, 33, 1603–1611. [Google Scholar] [CrossRef]
- Lindquist, C.; Kihlstrom, L.; Hellstrand, E. Functional neurosurgery--a future for the gamma knife? Stereotact. Funct. Neurosurg. 1991, 57, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Kostjuchenko, V.V.; Golanov, A.V. LINAC-based radiosurgery for functional and psychiatric disorders: Problems and their solutions. Prog. Brain Res. 2022, 270, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, A.; Raju, S.S.; Kooshkabadi, A.; Monaco, E., 3rd; Flickinger, J.C.; Lunsford, L.D. Stereotactic radiosurgery for essential tremor: Retrospective analysis of a 19-year experience. Mov. Disord. 2017, 32, 769–777. [Google Scholar] [CrossRef]
- Friehs, G.M.; Ojakangas, C.L.; Schrottner, O.; Ott, E.; Pendl, G. Radiosurgical lesioning of the caudate nucleus as a treatment for parkinsonism: A preliminary report. Neurol. Res. 1997, 19, 97–103. [Google Scholar] [CrossRef]
- Martinez-Moreno, N.E.; Sahgal, A.; De Salles, A.; Hayashi, M.; Levivier, M.; Ma, L.; Paddick, I.; Regis, J.; Ryu, S.; Slotman, B.J.; et al. Stereotactic radiosurgery for tremor: Systematic review. J. Neurosurg. 2019, 130, 589–600. [Google Scholar] [CrossRef]
- Goc, B.; Roch-Zniszczol, A.; Larysz, D.; Zarudzki, L.; Stapor-Fudzinska, M.; Rozek, A.; Wozniak, G.; Boczarska-Jedynak, M.; Miszczyk, L.; Napieralska, A. The Effectiveness and Toxicity of Frameless CyberKnife Based Radiosurgery for Parkinson’s Disease-Phase II Study. Biomedicines 2023, 11, 288. [Google Scholar] [CrossRef]
- Khattab, M.H.; Cmelak, A.J.; Sherry, A.D.; Luo, G.; Wang, L.; Yu, H.; Hedera, P.; Phibbs, F.T.; Lindsell, C.J.; Neimat, J.; et al. Noninvasive Thalamotomy for Refractory Tremor by Frameless Radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 121–130. [Google Scholar] [CrossRef]
- Chintapalli, R.; Chang, S.; Kaprealian, T.; Savjani, R.; Tenn, S.; Bari, A. Gamma knife versus linear accelerator thalamotomy for essential tremor and Parkinson’s disease: A systematic review and meta-analysis. J. Clin. Neurosci. 2025, 133, 111050. [Google Scholar] [CrossRef]
- Bilski, M.; Szklener, K.; Szklener, S.; Rudzinska, A.; Kluz, N.; Klas, J.; Rodzajewska, A.; Kurylo, W.; Korga, M.; Baranowska, I.; et al. Stereotactic radiosurgery in the treatment of essential tremor—A systematic review. Front. Neurol. 2024, 15, 1370091. [Google Scholar] [CrossRef]
- Goncalves, O.R.; Lima, H.F.; Borges, C.E.S.; Junior, A.; Batista, S.; Romeiro, P.; Fukunaga, C.K.; Goes, B.G.; Monteiro, G.A.; Chen, H.C.; et al. Efficacy and safety of stereotactic radiosurgery with gamma knife machine in patients with essential tremor: A systematic review and single-arm meta-analysis. Neurosurg. Rev. 2024, 47, 862. [Google Scholar] [CrossRef]
- Perez-Sanchez, J.R.; Martinez-Alvarez, R.; Martinez Moreno, N.E.; Torres Diaz, C.; Rey, G.; Parees, I.; Del Barrio, A.A.; Alvarez-Linera, J.; Kurtis, M.M. Gamma Knife(R) stereotactic radiosurgery as a treatment for essential and parkinsonian tremor: Long-term experience. Neurologia 2020, 38, 188–196. [Google Scholar] [CrossRef]
- Ochiai, T. Gamma Knife Thalamotomy for a Medically Refractory Tremors: Longitudinal Evaluation of Clinical Effects and MRI Response Patterns. Acta Neurochir. Suppl. 2021, 128, 127–132. [Google Scholar] [CrossRef]
- Luo, G.; Cameron, B.D.; Wang, L.; Yu, H.; Neimat, J.S.; Hedera, P.; Phibbs, F.; Bradley, E.B.; Cmelak, A.J.; Kirschner, A.N. Targeting for stereotactic radiosurgical thalamotomy based on tremor treatment response. J. Neurosurg. 2022, 136, 1387–1394. [Google Scholar] [CrossRef]
- Ankrah, N.K.; Thomas, E.M.; Bredel, M.; Middlebrooks, E.H.; Walker, H.; Fiveash, J.B.; Guthrie, B.L.; Popple, R.A.; Roper, J.; Brinkerhoff, S. Frameless LINAC-Based Stereotactic Radiosurgery is Safe and Effective for Essential and Parkinsonian Tremor. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117 (Suppl. S2), S173. [Google Scholar] [CrossRef]
- Horisawa, S.; Hayashi, M.; Tamura, N.; Kohara, K.; Nonaka, T.; Hanada, T.; Kawamata, T.; Taira, T. Gamma Knife Thalamotomy for Essential Tremor: A Retrospective Analysis. World Neurosurg. 2023, 175, e90–e96. [Google Scholar] [CrossRef]
- Tuleasca, C.; Carey, G.; Barriol, R.; Touzet, G.; Dubus, F.; Luc, D.; Carriere, N.; Reyns, N. Impact of biologically effective dose on tremor decrease after stereotactic radiosurgical thalamotomy for essential tremor: A retrospective longitudinal analysis. Neurosurg. Rev. 2024, 47, 73. [Google Scholar] [CrossRef]
- Quigg, M.; Rolston, J.; Barbaro, N.M. Radiosurgery for epilepsy: Clinical experience and potential antiepileptic mechanisms. Epilepsia 2012, 53, 7–15. [Google Scholar] [CrossRef]
- Regis, J.; Rey, M.; Bartolomei, F.; Vladyka, V.; Liscak, R.; Schrottner, O.; Pendl, G. Gamma knife surgery in mesial temporal lobe epilepsy: A prospective multicenter study. Epilepsia 2004, 45, 504–515. [Google Scholar] [CrossRef]
- Barbaro, N.M.; Quigg, M.; Ward, M.M.; Chang, E.F.; Broshek, D.K.; Langfitt, J.T.; Yan, G.; Laxer, K.D.; Cole, A.J.; Sneed, P.K.; et al. Radiosurgery versus open surgery for mesial temporal lobe epilepsy: The randomized, controlled ROSE trial. Epilepsia 2018, 59, 1198–1207. [Google Scholar] [CrossRef]
- Mohsen, Y.; Sarhan, K.; Alawadi, I.S.; Elmahdi, R.R.; Kozaa, Y.A.; Gomaa, M.A.; Serag, I.; Shahein, M. Efficacy and safety of laser interstitial thermal therapy versus radiofrequency ablation and stereotactic radiosurgery in the treatment of intractable mesial temporal lobe epilepsy: A systematic review and meta-analysis. Neurosurg. Rev. 2025, 48, 71. [Google Scholar] [CrossRef]
- Usami, K.; Kawai, K.; Koga, T.; Shin, M.; Kurita, H.; Suzuki, I.; Saito, N. Delayed complication after Gamma Knife surgery for mesial temporal lobe epilepsy. J. Neurosurg. 2012, 116, 1221–1225. [Google Scholar] [CrossRef]
- Kawamura, T.; Onishi, H.; Kohda, Y.; Hirose, G. Serious adverse effects of gamma knife radiosurgery for mesial temporal lobe epilepsy. Neurol. Med. Chir. 2012, 52, 892–898. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zhang, X.D.; Han, Y.M.; Shi, X.F.; Lan, Z.B.; Men, X.X.; Pan, Y.W. Clinical efficacy of gamma knife and surgery treatment of mesial temporal lobe epilepsy and their effects on EF-Tumt and EF-Tsmt expression. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1774–1779. Available online: https://www.europeanreview.org/wp/wp-content/uploads/1774-1779-Clinical-efficacy-of-gamma-knife-and-surgery-treatment-of-mesial-temporal-lobe-epilepsy.pdf (accessed on 24 October 2025).
- McGonigal, A.; Sahgal, A.; De Salles, A.; Hayashi, M.; Levivier, M.; Ma, L.; Martinez, R.; Paddick, I.; Ryu, S.; Slotman, B.J.; et al. Radiosurgery for epilepsy: Systematic review and International Stereotactic Radiosurgery Society (ISRS) practice guideline. Epilepsy Res. 2017, 137, 123–131. [Google Scholar] [CrossRef]
- Tripathi, M.; Mukherjee, K.; Chhabra, R.; Radotra, I.; Singh, A.P.; Radotra, B. Gamma knife for obsessive compulsive disorder: Can it be detrimental? Turk. Neurosurg. 2014, 24, 583–586. Available online: https://pubmed.ncbi.nlm.nih.gov/25050687/ (accessed on 6 July 2025).
- Leveque, M.; Carron, R.; Regis, J. Radiosurgery for the treatment of psychiatric disorders: A review. World Neurosurg. 2013, 80, S32.e1–S32.e9. [Google Scholar] [CrossRef]
- Sadashiva, N.; Tripathi, M.; De Salles, A. Contemporary Role of Stereotactic Radiosurgery for Psychiatric Disorders. Neurol. India 2023, 71 (Suppl. S1), S31–S38. [Google Scholar] [CrossRef]
- Martinez-Alvarez, R.; Torres-Diaz, C. Modern Gamma Knife radiosurgery for management of psychiatric disorders. Prog. Brain Res. 2022, 270, 171–183. [Google Scholar] [CrossRef]
- Gupta, R.; Chen, J.W.; Hughes, N.C.; Hamo, M.; Jean-Baptiste, S.; Paulo, D.L.; Chanbour, H.; Fan, R.; Ye, F.; Vadali, A.; et al. Benefits of stereotactic radiosurgical anterior capsulotomy for obsessive-compulsive disorder: A meta-analysis. J. Neurosurg. 2024, 141, 394–405. [Google Scholar] [CrossRef]
- Laseca-Zaballa, G.; Lubrini, G.; Perianez, J.A.; Simon-Martinez, V.; Martin Bejarano, M.; Torres-Diaz, C.; Martinez Moreno, N.; Alvarez-Linera, J.; Martinez Alvarez, R.; Rios-Lago, M. Cognitive outcomes following functional neurosurgery in refractory OCD patients: A systematic review. Neurosurg. Rev. 2023, 46, 145. [Google Scholar] [CrossRef]
- Miguel, E.C.; Lopes, A.C.; McLaughlin, N.C.R.; Noren, G.; Gentil, A.F.; Hamani, C.; Shavitt, R.G.; Batistuzzo, M.C.; Vattimo, E.F.Q.; Canteras, M.; et al. Evolution of gamma knife capsulotomy for intractable obsessive-compulsive disorder. Mol. Psychiatry 2019, 24, 218–240. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Noren, G.; Greenberg, B.D.; Marsland, R.; McLaughlin, N.C.; Malloy, P.J.; Salloway, S.P.; Strong, D.R.; Eisen, J.L.; Jenike, M.A.; et al. Gamma Ventral Capsulotomy in Intractable Obsessive-Compulsive Disorder. Biol. Psychiatry 2018, 84, 355–364. [Google Scholar] [CrossRef]
- Gupta, A.; Shepard, M.J.; Xu, Z.; Maiti, T.; Martinez-Moreno, N.; Silverman, J.; Iorio-Morin, C.; Martinez-Alvarez, R.; Barnett, G.; Mathieu, D.; et al. An International Radiosurgery Research Foundation Multicenter Retrospective Study of Gamma Ventral Capsulotomy for Obsessive Compulsive Disorder. Neurosurgery 2019, 85, 808–816. [Google Scholar] [CrossRef]
- Peker, S.; Samanci, M.Y.; Yilmaz, M.; Sengoz, M.; Ulku, N.; Ogel, K. Efficacy and Safety of Gamma Ventral Capsulotomy for Treatment-Resistant Obsessive-Compulsive Disorder: A Single-Center Experience. World Neurosurg. 2020, 141, e941–e952. [Google Scholar] [CrossRef]
- Ekmekci Ertek, I.; Ucar, O.; Emre Yaman, M.; Hakan Emmez, O.; Candansayar, S. Treatment Outcomes of Gamma-Knife Radio Surgery in Refractory Obsessive-Compulsive Disorder. Psychiatry Clin. Psychopharmacol. 2021, 31, 401–407. [Google Scholar] [CrossRef]
- Pattankar, S.; Sankhe, M.; Chavda, K. Efficacy of Gamma Knife Radiosurgery in Refractory Obsessive-Compulsive Disorder: An Indian Experience. J. Neurosci. Rural Pract. 2022, 13, 23–31. [Google Scholar] [CrossRef]
- Tuleasca, C.; Regis, J.; Sahgal, A.; De Salles, A.; Hayashi, M.; Ma, L.; Martinez-Alvarez, R.; Paddick, I.; Ryu, S.; Slotman, B.J.; et al. Stereotactic radiosurgery for trigeminal neuralgia: A systematic review. J. Neurosurg. 2019, 130, 733–757. [Google Scholar] [CrossRef]
- Lu, V.M.; Duvall, J.B.; Phan, K.; Jonker, B.P. First treatment and retreatment of medically refractive trigeminal neuralgia by stereotactic radiosurgery versus microvascular decompression: A systematic review and Meta-analysis. Br. J. Neurosurg. 2018, 32, 355–364. [Google Scholar] [CrossRef]
- Shields, L.B.E.; Malkawi, A.; Daniels, M.W.; Rao, A.J.; Plato, B.M.; Yao, T.L.; Howe, J.N.; Spalding, A.C. Frameless image-guided linear accelerator (LINAC) stereotactic radiosurgery for medically refractory trigeminal neuralgia: Clinical outcomes in 116 patients. Surg. Neurol. Int. 2024, 15, 181. [Google Scholar] [CrossRef]
- De La Pena, N.M.; Singh, R.; Anderson, M.L.; Koester, S.W.; Sio, T.T.; Ashman, J.B.; Vora, S.A.; Patel, N.P. High-Dose Frameless Stereotactic Radiosurgery for Trigeminal Neuralgia: A Single-Institution Experience and Systematic Review. World Neurosurg. 2022, 167, e432–e443. [Google Scholar] [CrossRef]
- Guillemette, A.; Heymann, S.; Roberge, D.; Menard, C.; Fournier-Gosselin, M.P. CyberKnife radiosurgery for trigeminal neuralgia: A retrospective review of 168 cases. Neurosurg. Focus 2022, 53, E4. [Google Scholar] [CrossRef]
- Akkara, Y.; Singh, J.M.; Thorne, L.; Hill, C.S. Stereotactic Radiosurgery versus Neuroablative Techniques for Medically Refractory Trigeminal Neuralgia: A Systematic Review and Meta-Analysis of Outcomes. Stereotact. Funct. Neurosurg. 2025, 103, 154–165. [Google Scholar] [CrossRef]
- Khaboushan, A.S.; Maroufi, S.F.; Jarrah, N.; Moafi, M.; Sabahi, M.; Borghei-Razavi, H.; Sheehan, J.P. Comparison of stereotactic radiosurgery and rhizotomy for trigeminal neuralgia: A systematic review and Meta-Analysis. Neurosurg. Rev. 2025, 48, 613. [Google Scholar] [CrossRef]
- Debono, B.; Lotterie, J.A.; Sol, J.C.; Bousquet, P.; Duthil, P.; Monfraix, S.; Lazorthes, Y.; Sabatier, J.; Latorzeff, I. Dedicated Linear Accelerator Radiosurgery for Classic Trigeminal Neuralgia: A Single-Center Experience with Long-Term Follow-Up. World Neurosurg. 2019, 121, e775–e785. [Google Scholar] [CrossRef]
- Koca, S.; Distel, L.; Lubgan, D.; Weissmann, T.; Lambrecht, U.; Lang-Welzenbach, M.; Eyupoglu, I.; Bischoff, B.; Buchfelder, M.; Semrau, S.; et al. Time course of pain response and toxicity after whole-nerve-encompassing LINAC-based stereotactic radiosurgery for trigeminal neuralgia-a prospective observational study. Strahlenther. Onkol. 2019, 195, 745–755. [Google Scholar] [CrossRef]
- Barzaghi, L.R.; Albano, L.; Scudieri, C.; Gigliotti, C.R.; Nadin, F.; Del Vecchio, A.; Mortini, P. Gamma Knife Radiosurgery for Trigeminal Neuralgia: Role of Trigeminal Length and Pontotrigeminal Angle on Target Definition and on Clinical Effects. World Neurosurg. 2020, 142, e140–e150. [Google Scholar] [CrossRef]
- Kienzler, J.C.; Tenn, S.; Chivukula, S.; Chu, F.I.; Sparks, H.D.; Agazaryan, N.; Kim, W.; Salles, A.; Selch, M.; Gorgulho, A.; et al. Linear accelerator-based radiosurgery for trigeminal neuralgia: Comparative outcomes of frame-based and mask-based techniques. J. Neurosurg. 2022, 137, 217–226. [Google Scholar] [CrossRef]
- Okunlola, A.I.; Pattankar, S.; Warade, A.; Khandhar, A.; Mistry, V.; Misra, B.K. Safety and Efficacy of Gamma Knife Radiosurgery for the Management of Trigeminal Neuralgia: A Retrospective and Cross-Sectional Study. Neurol. India 2023, 71 (Suppl. S1), S161–S167. [Google Scholar] [CrossRef]
- Orlev, A.; Feghali, J.; Kimchi, G.; Sun, L.; Pierre, C.; Gragnaniello, C.; Cotrutz, C.; Loiselle, C.; Vermeulen, S.; Litvack, Z. TN-RS: A novel scoring system predicts Gamma Knife Radiosurgery outcome for trigeminal neuralgia patients. Acta Neurochir. 2023, 165, 3895–3903. [Google Scholar] [CrossRef]
- Sato, D.; Hayashi, M.; Horiba, A.; Horisawa, S.; Kawamata, T. Long-Term Results of Gamma Knife Radiosurgery for Trigeminal Neuralgia. World Neurosurg. 2023, 171, e787–e791. [Google Scholar] [CrossRef]
- Roberts, D.G.; Pouratian, N. Stereotactic Radiosurgery for the Treatment of Chronic Intractable Pain: A Systematic Review. Oper. Neurosurg. 2017, 13, 543–551. [Google Scholar] [CrossRef]
- Steiner, L.; Forster, D.; Leksell, L.; Meyerson, B.A.; Boethius, J. Gammathalamotomy in intractable pain. Acta Neurochir. 1980, 52, 173–184. [Google Scholar] [CrossRef]
- Tian, F.; Dai, H.; Sha, D.; Yu, Y.; Jing, H.; Sun, C.; Shang, L.; Liu, Y.; Feng, R.; Li, J.; et al. Total neoadjuvant treatment with short-course radiotherapy followed by sintilimab plus capecitabine–oxaliplatin versus short-course radiotherapy followed by capecitabine–oxaliplatin in patients with locally advanced rectal cancer (SPRING-01): A single-centre, open-label, phase 2, randomised controlled trial. Lancet Oncol. 2025, 26, 1043–1054. [Google Scholar] [CrossRef]
- Gani, C.; Fokas, E.; Polat, B.; Ott, O.J.; Diefenhardt, M.; Königsrainer, A.; Böke, S.; Kirschniak, A.; Bachmann, R.; Wichmann, D.; et al. Organ preservation after total neoadjuvant therapy for locally advanced rectal cancer (CAO/ARO/AIO-16): An open-label, multicentre, single-arm, phase 2 trial. Lancet Gastroenterol. Hepatol. 2025, 10, 562–572. [Google Scholar] [CrossRef]
- Kawamura, M.; Kamomae, T.; Yanagawa, M.; Kamagata, K.; Fujita, S.; Ueda, D.; Matsui, Y.; Fushimi, Y.; Fujioka, T.; Nozaki, T.; et al. Revolutionizing radiation therapy: The role of AI in clinical practice. J. Radiat. Res. 2024, 65, 1–9. [Google Scholar] [CrossRef]
- Spaas, M.; Sundahl, N.; Kruse, V.; Rottey, S.; De Maeseneer, D.; Duprez, F.; Lievens, Y.; Surmont, V.; Brochez, L.; Reynders, D.; et al. Checkpoint inhibitors in combination with stereotactic body radiotherapy in patients with advanced solid tumors: The CHEERS phase 2 randomized clinical trial. JAMA Oncol. 2023, 9, 1205–1213. [Google Scholar] [CrossRef]
- Wei, Q.; Chen, Z.; Tang, Y.; Chen, W.; Zhong, L.; Mao, L.; Hu, S.; Wu, Y.; Deng, K.; Yang, W.; et al. External validation and comparison of MR-based radiomics models for predicting pathological complete response in locally advanced rectal cancer: A two-centre, multi-vendor study. Eur. Radiol. 2023, 33, 1906–1917. [Google Scholar] [CrossRef]
- Westerhoff, J.M.; Daamen, L.A.; Christodouleas, J.P.; Blezer, E.L.A.; Choudhury, A.; Westley, R.L.; Erickson, B.A.; Fuller, C.D.; Hafeez, S.; van der Heide, U.A.; et al. Safety and Tolerability of Online Adaptive High-Field Magnetic Resonance–Guided Radiotherapy. JAMA Netw. Open 2024, 7, e2410819. [Google Scholar] [CrossRef]
- Appelt, A.; Elhaminia, B.; Gooya, A.; Gilbert, A.; Nix, M. Deep Learning for Radiotherapy Outcome Prediction Using Dose Data—A Review. Clin. Oncol. 2022, 34, e87–e96. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).