Antibiotic Exposure Does Not Impact Anti-BRAF/Anti-MEK Targeted Therapy Outcome in Patients with Advanced Melanoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
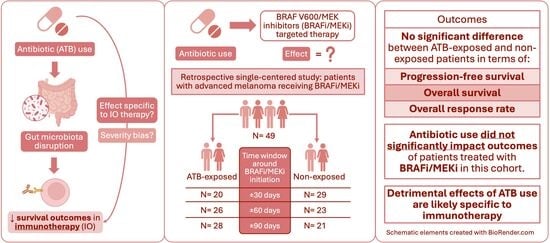
2.1. Study Population
2.2. Antibiotic Exposure Assessment
2.3. Outcomes
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Patient Characteristics
3.2. Antibiotic Use
3.3. Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BRAFi | BRAF V600 inhibitor |
| MEKi | MEK inhibitor |
| ATB | Antibiotic |
| ICI | Immune checkpoint inhibitor |
| ORR | Overall response rate |
| PFS | Progression-free survival |
| mPFS | Median progression-free survival |
| OS | Overall survival |
| mOS | Median overall survival |
| HR | Hazard ratio |
| CI | Confidence interval |
| BMT | Bone marrow transplantation |
| CAR-T | Chimeric antigen receptor T-cell therapy |
| NSCLC | Non–small cell lung cancer |
| RCC | Renal cell carcinoma |
References
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Long, G.V.; Menzies, A.M.; Nagrial, A.M.; Haydu, L.E.; Hamilton, A.L.; Mann, G.J.; Hughes, T.M.; Thompson, J.F.; Scolyer, R.A.; Kefford, R.F. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J. Clin. Oncol. 2011, 29, 1239–1246. [Google Scholar] [CrossRef]
- Castellani, G.; Buccarelli, M.; Arasi, M.B.; Rossi, S.; Pisanu, M.E.; Bellenghi, M.; Lintas, C.; Tabolacci, C. BRAF Mutations in Melanoma: Biological Aspects, Therapeutic Implications, and Circulating Biomarkers. Cancers 2023, 15, 4026. [Google Scholar] [CrossRef]
- Kong, B.Y.; Carlino, M.S.; Menzies, A.M. Biology and treatment of BRAF mutant metastatic melanoma. Melanoma Manag. 2016, 3, 33–45. [Google Scholar] [CrossRef]
- Teixido, C.; Castillo, P.; Martinez-Vila, C.; Arance, A.; Alos, L. Molecular Markers and Targets in Melanoma. Cells 2021, 10, 2320. [Google Scholar] [CrossRef]
- Long, G.V.; Flaherty, K.T.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: Long-term survival and safety analysis of a phase 3 study. Ann. Oncol. 2017, 28, 1631–1639. [Google Scholar] [CrossRef]
- Grob, J.J.; Amonkar, M.M.; Karaszewska, B.; Schachter, J.; Dummer, R.; Mackiewicz, A.; Stroyakovskiy, D.; Drucis, K.; Grange, F.; Chiarion-Sileni, V.; et al. Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): Results of a phase 3, open-label, randomised trial. Lancet Oncol. 2015, 16, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Dummer, R.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Contribution of MEK Inhibition to BRAF/MEK Inhibitor Combination Treatment of BRAF-Mutant Melanoma: Part 2 of the Randomized, Open-Label, Phase III COLUMBUS Trial. J. Clin. Oncol. 2023, 41, 4621–4631. [Google Scholar] [CrossRef]
- Elkrief, A.; El Raichani, L.; Richard, C.; Messaoudene, M.; Belkaid, W.; Malo, J.; Belanger, K.; Miller, W.; Jamal, R.; Letarte, N.; et al. Antibiotics are associated with decreased progression-free survival of advanced melanoma patients treated with immune checkpoint inhibitors. Oncoimmunology 2019, 8, e1568812. [Google Scholar] [CrossRef]
- Elkrief, A.; Mendez-Salazar, E.O.; Maillou, J.; Vanderbilt, C.M.; Gogia, P.; Desilets, A.; Messaoudene, M.; Kelly, D.; Ladanyi, M.; Hellmann, M.D.; et al. Antibiotics are associated with worse outcomes in lung cancer patients treated with chemotherapy and immunotherapy. npj Precis. Oncol. 2024, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Derosa, L.; Hellmann, M.D.; Spaziano, M.; Halpenny, D.; Fidelle, M.; Rizvi, H.; Long, N.; Plodkowski, A.J.; Arbour, K.C.; Chaft, J.E.; et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 2018, 29, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Angrish, M.D.; Agha, A.; Pezo, R.C. Association of Antibiotics and Other Drugs with Clinical Outcomes in Metastatic Melanoma Patients Treated with Immunotherapy. J. Skin. Cancer 2021, 2021, 9120162. [Google Scholar] [CrossRef]
- Cortellini, A.; Di Maio, M.; Nigro, O.; Leonetti, A.; Cortinovis, D.L.; Aerts, J.G.; Guaitoli, G.; Barbieri, F.; Giusti, R.; Ferrara, M.G.; et al. Differential influence of antibiotic therapy and other medications on oncological outcomes of patients with non-small cell lung cancer treated with first-line pembrolizumab versus cytotoxic chemotherapy. J. Immunother. Cancer 2021, 9, e002421. [Google Scholar] [CrossRef]
- Elkrief, A.; Routy, B.; Derosa, L.; Bolte, L.; Wargo, J.A.; McQuade, J.L.; Zitvogel, L. Gut Microbiota in Immuno-Oncology: A Practical Guide for Medical Oncologists With a Focus on Antibiotics Stewardship. Am. Soc. Clin. Oncol. Educ. Book 2025, 45, e472902. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Weyer-Fahlbusch, S.S.; Overbeck, J.; Abu Rached, N.; Becker, J.C.; Susok, L. Impaired Overall Survival of Melanoma Patients Due to Antibiotic Use Prior to Immune Checkpoint Inhibitor Therapy: Systematic Review and Meta-Analysis. Cancers 2025, 17, 1872. [Google Scholar] [CrossRef]
- Fidelle, M.; Rauber, C.; Alves Costa Silva, C.; Tian, A.-L.; Lahmar, I.; de La Varende, A.-L.M.; Zhao, L.; Thelemaque, C.; Lebhar, I.; Messaoudene, M.; et al. A microbiota-modulated checkpoint directs immunosuppressive intestinal T cells into cancers. Science 2023, 380, eabo2296. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.-M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef]
- Hadi, D.K.; Baines, K.J.; Jabbarizadeh, B.; Miller, W.H.; Jamal, R.; Ernst, S.; Logan, D.; Belanger, K.; Esfahani, K.; Elkrief, A.; et al. Improved survival in advanced melanoma patients treated with fecal microbiota transplantation using healthy donor stool in combination with anti-PD1: Final results of the MIMic phase 1 trial. J. Immunother. Cancer 2025, 13, e012659. [Google Scholar] [CrossRef]
- Metselaar-Albers, M.; Meijerman, I.; Engels, F.; Haanen, J.; Beijnen, J.; Lalmohamed, A. No detrimental association between antibiotic use and immune checkpoint inhibitor therapy: An observational cohort study comparing patients with ICI-treated and TKI-treated melanoma and NSCLC. J. Immunother. Cancer 2024, 12, e008269. [Google Scholar] [CrossRef]
- Tinsley, N.; Zhou, C.; Nahm, S.; Rack, S.; Tan, G.; Lorigan, P.; Blackhall, F.; Cook, N. Antibiotic use reduces efficacy of tyrosine kinase inhibitors in patients with advanced melanoma and non-small-cell lung cancer. ESMO Open 2022, 7, 100430. [Google Scholar] [CrossRef]
- Lalani, A.A.; Xie, W.; Braun, D.A.; Kaymakcalan, M.; Bossé, D.; Steinharter, J.A.; Martini, D.J.; Simantov, R.; Lin, X.; Wei, X.X.; et al. Effect of Antibiotic Use on Outcomes with Systemic Therapies in Metastatic Renal Cell Carcinoma. Eur. Urol. Oncol. 2020, 3, 372–381. [Google Scholar] [CrossRef]
- Wilmott, J.S.; Long, G.V.; Howle, J.R.; Haydu, L.E.; Sharma, R.N.; Thompson, J.F.; Kefford, R.F.; Hersey, P.; Scolyer, R.A. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin. Cancer Res. 2012, 18, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Kakavand, H.; Wilmott, J.S.; Menzies, A.M.; Vilain, R.; Haydu, L.E.; Yearley, J.H.; Thompson, J.F.; Kefford, R.F.; Hersey, P.; Long, G.V.; et al. PD-L1 Expression and Tumor-Infiltrating Lymphocytes Define Different Subsets of MAPK Inhibitor-Treated Melanoma Patients. Clin. Cancer Res. 2015, 21, 3140–3148. [Google Scholar] [CrossRef] [PubMed]
- Guardamagna, M.; Berciano-Guerrero, M.A.; Villaescusa-Gonzalez, B.; Perez-Ruiz, E.; Oliver, J.; Lavado-Valenzuela, R.; Rueda-Dominguez, A.; Barragán, I.; Queipo-Ortuño, M.I. Gut Microbiota and Therapy in Metastatic Melanoma: Focus on MAPK Pathway Inhibition. Int. J. Mol. Sci. 2022, 23, 11990. [Google Scholar] [CrossRef] [PubMed]
- Darwin, A.; Xie, J.; Smith, M. Antibiotic use: Impact on the microbiome and cellular therapy outcomes. Blood Adv. 2025, 9, 3356–3367. [Google Scholar] [CrossRef]
- Shono, Y.; Docampo, M.D.; Peled, J.U.; Perobelli, S.M.; Velardi, E.; Tsai, J.J.; Slingerland, A.E.; Smith, O.M.; Young, L.F.; Gupta, J.; et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci. Transl. Med. 2016, 8, 339ra71. [Google Scholar] [CrossRef]
- Stein-Thoeringer, C.K.; Saini, N.Y.; Zamir, E.; Blumenberg, V.; Schubert, M.-L.; Mor, U.; Fante, M.A.; Schmidt, S.; Hayase, E.; Hayase, T.; et al. A non-antibiotic-disrupted gut microbiome is associated with clinical responses to CD19-CAR-T cell cancer immunotherapy. Nat. Med. 2023, 29, 906–916. [Google Scholar] [CrossRef]
- Yin, L.; Lv, B.; Ge, J.; Qi, Y.; Xia, J.; Ma, S.; Wang, Y.; Liu, Y.; Zhou, D.; Cao, J.; et al. The impact of antibiotic use on outcomes of relapsed/refractory multiple myeloma patients treated with CAR-T therapy. Front. Immunol. 2025, 16, 1566016. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Dai, A.; Ghilardi, G.; Amelsberg, K.V.; Devlin, S.M.; Pajarillo, R.; Slingerland, J.B.; Beghi, S.; Herrera, P.S.; Giardina, P.; et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat. Med. 2022, 28, 713–723. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | ABX Within 30 Days n = 20 1 | No ABX Within 30 Days n = 29 1 | p-Value 2 |
|---|---|---|---|
| Age | 62.7 [51.1, 67.0] | 57.3 [44.1, 63.8] | 0.5 |
| Sex | >0.9 | ||
| Female | 7 (35.0%) | 10 (34.5%) | |
| Male | 13 (65.0%) | 19 (65.5%) | |
| Stage | 0.7 | ||
| 2 or 3 (unresectable) | 2 (10.0%) | 5 (17.2%) | |
| 4 (advanced) | 18 (90.0%) | 24 (82.8%) | |
| BRAF status | >0.9 | ||
| V600E | 19 (95.0%) | 26 (92.9%) | |
| V600K | 1 (5.0%) | 2 (7.1%) | |
| Missing | 0 | 1 | |
| ECOG | 0.038 | ||
| ≥2 | 8 (40.0%) | 3 (11.5%) | |
| 0–1 | 12 (60.0%) | 23 (88.5%) | |
| Missing | 0 | 3 | |
| Melanoma type | 0.5 | ||
| Cutaneous | 16 (80.0%) | 26 (89.7%) | |
| Mucosal | 1 (5.0%) | 0 (0.0%) | |
| Unknown primary | 3 (15.0%) | 3 (10.3%) | |
| Liver metastasis | 8 (40.0%) | 14 (48.3%) | 0.8 |
| Brain metastasis | 7 (35.0%) | 7 (24.1%) | 0.5 |
| LDH value | 276.0 [187.5, 380.5] | 329.0 [192.0, 451.0] | 0.7 |
| Missing | 0 | 4 | |
| Line of therapy | >0.9 | ||
| First-line | 6 (30.0%) | 10 (34.5%) | |
| Second-line or beyond | 14 (70.0%) | 19 (65.5%) | |
| Targeted therapy regimen | 0.2 | ||
| Dabrafenib/trametinib | 18 (90.0%) | 24 (82.8%) | |
| Encorafenib/binimetinib | 1 (5.0%) | 5 (17.2%) | |
| Vemurafenib | 1 (5.0%) | 0 (0.0%) |
| Characteristic | ABX Within 60 Days n = 26 1 | No ABX Within 60 Days n = 23 1 | p-Value 2 |
|---|---|---|---|
| Age | 60.8 [49.2, 66.5] | 56.7 [44.1, 66.4] | 0.7 |
| Sex | 0.6 | ||
| Female | 8 (30.8%) | 9 (39.1%) | |
| Male | 18 (69.2%) | 14 (60.9%) | |
| Stage | >0.9 | ||
| 2 or 3 (unresectable) | 4 (15.4%) | 3 (13.0%) | |
| 4 (advanced) | 22 (84.6%) | 20 (87.0%) | |
| BRAF status | 0.6 | ||
| V600E | 24 (96.0%) | 21 (91.3%) | |
| V600K | 1 (4.0%) | 2 (8.7%) | |
| Missing | 1 | 0 | |
| ECOG | 0.045 | ||
| ≥2 | 9 (36.0%) | 2 (9.5%) | |
| 0–1 | 16 (64.0%) | 19 (90.5%) | |
| Missing | 1 | 2 | |
| Melanoma type | 0.7 | ||
| Cutaneous | 21 (80.8%) | 21 (91.3%) | |
| Mucosal | 1 (3.8%) | 0 (0.0%) | |
| Unknown primary | 4 (15.4%) | 2 (8.7%) | |
| Liver metastasis | 10 (38.5%) | 12 (52.2%) | 0.4 |
| Brain metastasis | 9 (34.6%) | 5 (21.7%) | 0.4 |
| LDH value | 276.0 [178.0, 399.0] | 329.0 [211.0, 451.0] | 0.6 |
| Missing | 2 | 2 | |
| Line of therapy | 0.5 | ||
| First-line | 7 (26.9%) | 9 (39.1%) | |
| Second-line or beyond | 19 (73.1%) | 14 (60.9%) | |
| Targeted therapy regimen | >0.9 | ||
| Dabrafenib/trametinib | 22 (84.6%) | 20 (87.0%) | |
| Encorafenib/binimetinib | 3 (11.5%) | 3 (13.0%) | |
| Vemurafenib | 1 (3.8%) | 0 (0.0%) |
| Characteristic | ABX Within 90 Days n = 28 1 | No ABX Within 90 Days n = 21 1 | p-Value 2 |
|---|---|---|---|
| Age | 60.3 [46.2, 66.1] | 58.2 [47.4, 66.4] | >0.9 |
| Sex | 0.8 | ||
| Female | 9 (32.1%) | 8 (38.1%) | |
| Male | 19 (67.9%) | 13 (61.9%) | |
| Stage | >0.9 | ||
| 2 or 3 (unresectable) | 4 (14.3%) | 3 (14.3%) | |
| 4 (advanced) | 24 (85.7%) | 18 (85.7%) | |
| BRAF status | 0.6 | ||
| V600E | 26 (96.3%) | 19 (90.5%) | |
| V600K | 1 (3.7%) | 2 (9.5%) | |
| Missing | 1 | 0 | |
| ECOG | 0.092 | ||
| ≥2 | 9 (33.3%) | 2 (10.5%) | |
| 0–1 | 18 (66.7%) | 17 (89.5%) | |
| Missing | 1 | 2 | |
| Melanoma type | 0.8 | ||
| Cutaneous | 23 (82.1%) | 19 (90.5%) | |
| Mucosal | 1 (3.6%) | 0 (0.0%) | |
| Unknown primary | 4 (14.3%) | 2 (9.5%) | |
| Liver metastasis | 12 (42.9%) | 10 (47.6%) | 0.8 |
| Brain metastasis | 10 (35.7%) | 4 (19.0%) | 0.3 |
| LDH value | 276.0 [181.0, 385.0] | 349.0 [211.0, 460.0] | 0.5 |
| Missing | 2 | 2 | |
| Line of therapy | 0.2 | ||
| First-line | 7 (25.0%) | 9 (42.9%) | |
| Second-line or beyond | 21 (75.0%) | 12 (57.1%) | |
| Targeted therapy regimen | >0.9 | ||
| Dabrafenib/trametinib | 24 (85.7%) | 18 (85.7%) | |
| Encorafenib/binimetinib | 3 (10.7%) | 3 (14.3%) | |
| Vemurafenib | 1 (3.6%) | 0 (0.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.S.; Wang, Q.Y.; Moise, A.E.; Syed, H.C.; Malo, J.; Soberano, S.; Belkaid, W.; Messaoudene, M.; Bélanger, K.; Desilets, A.; et al. Antibiotic Exposure Does Not Impact Anti-BRAF/Anti-MEK Targeted Therapy Outcome in Patients with Advanced Melanoma. Curr. Oncol. 2025, 32, 630. https://doi.org/10.3390/curroncol32110630
Wang YS, Wang QY, Moise AE, Syed HC, Malo J, Soberano S, Belkaid W, Messaoudene M, Bélanger K, Desilets A, et al. Antibiotic Exposure Does Not Impact Anti-BRAF/Anti-MEK Targeted Therapy Outcome in Patients with Advanced Melanoma. Current Oncology. 2025; 32(11):630. https://doi.org/10.3390/curroncol32110630
Chicago/Turabian StyleWang, Yu Shi, Qing Yin Wang, Alexia Erika Moise, Hamida Claudia Syed, Julie Malo, Spencer Soberano, Wiam Belkaid, Meriem Messaoudene, Karl Bélanger, Antoine Desilets, and et al. 2025. "Antibiotic Exposure Does Not Impact Anti-BRAF/Anti-MEK Targeted Therapy Outcome in Patients with Advanced Melanoma" Current Oncology 32, no. 11: 630. https://doi.org/10.3390/curroncol32110630
APA StyleWang, Y. S., Wang, Q. Y., Moise, A. E., Syed, H. C., Malo, J., Soberano, S., Belkaid, W., Messaoudene, M., Bélanger, K., Desilets, A., Jamal, R., Routy, B., & Elkrief, A. (2025). Antibiotic Exposure Does Not Impact Anti-BRAF/Anti-MEK Targeted Therapy Outcome in Patients with Advanced Melanoma. Current Oncology, 32(11), 630. https://doi.org/10.3390/curroncol32110630