New Horizons with Growth Differentiation Factor 15 in Oncology: From Cancer Cachexia and Tumour Immunity to Novel Therapeutic Strategies
Simple Summary
Abstract
1. Introduction
2. Overview of GDF-15
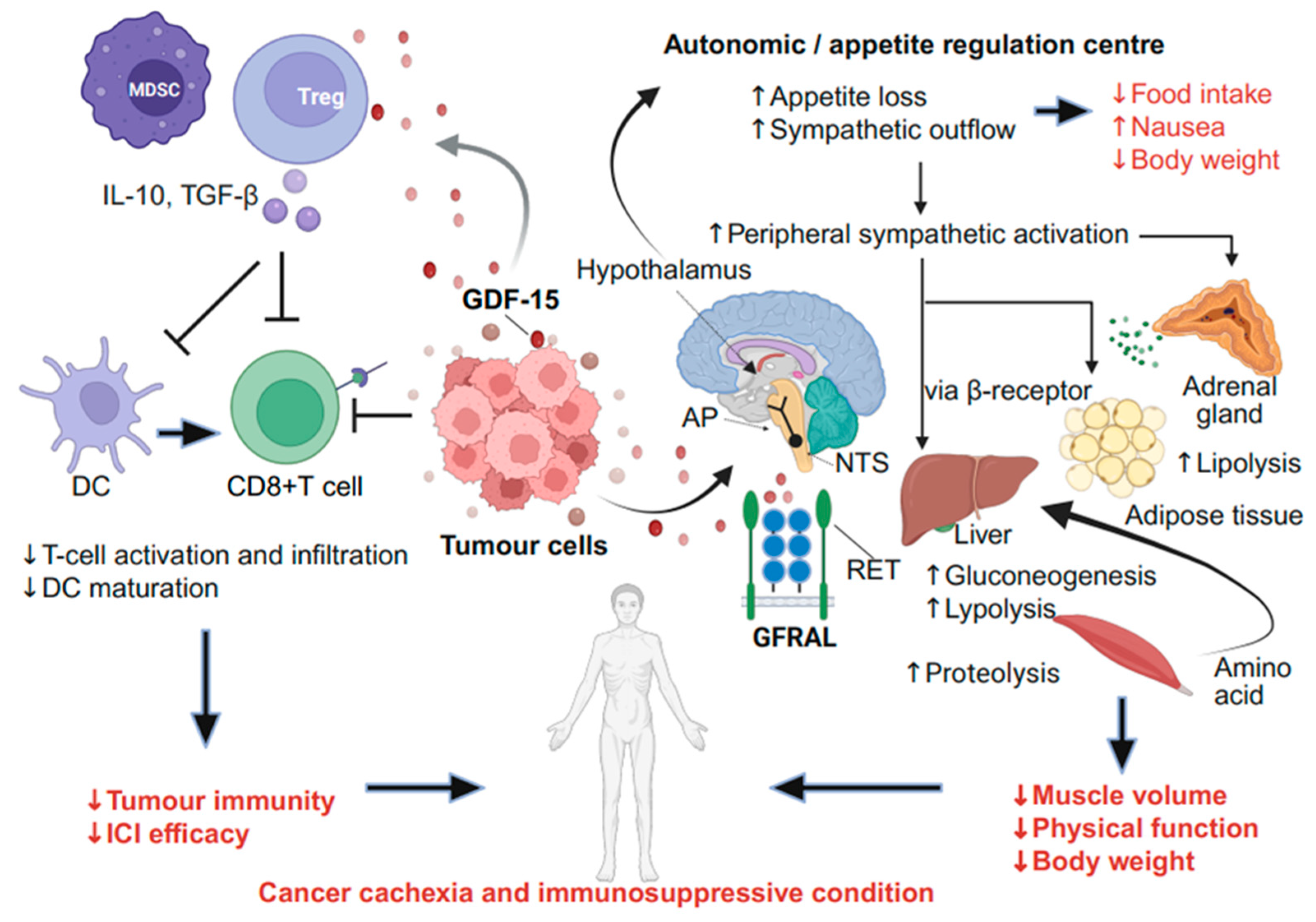
2.1. Mechanism of GDF-15-Mediated Cancer Cachexia via the Neural System
2.2. Role of GDF-15 in CINV
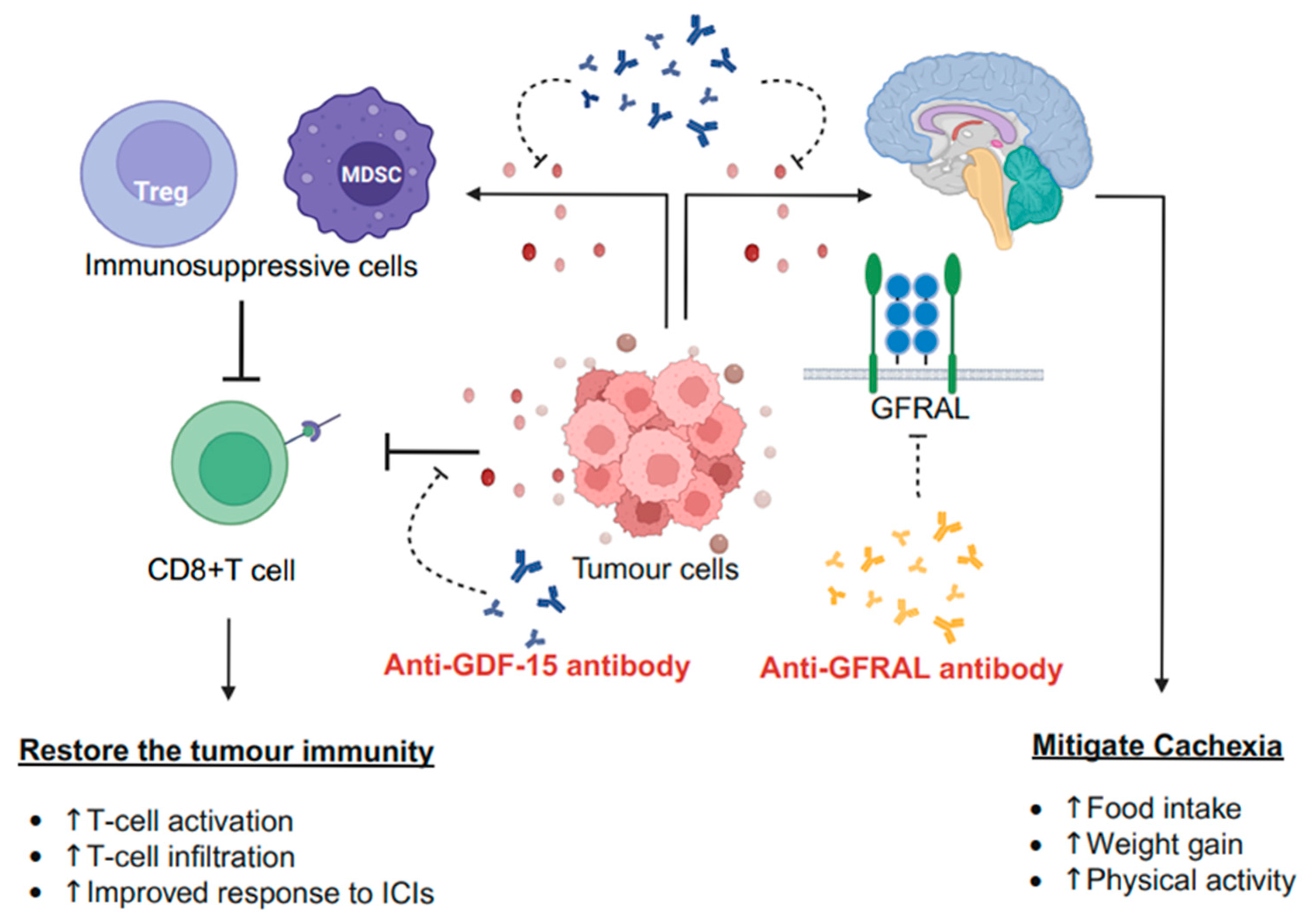
2.3. Effects of GDF-15 in Tumour Immunity and TME
2.4. Therapeutic GDF-15 Implementation
3. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT3 | 5-Hydroxytryptamine type 3 |
| AEs | Adverse events |
| AP | Area postrema |
| CINV | Chemotherapy-induced nausea and vomiting |
| CRC | Colorectal cancer |
| DCs | Dendritic cells |
| DLT | Dose-limiting toxicity |
| DoR | Duration of response |
| GDF-15 | Growth differentiation factor 15 |
| GDNF | Glial cell line-derived neurotrophic factor |
| GFRAL | Glial cell line-derived neurotrophic factor family receptor alpha-like |
| GZMB | Granzyme B |
| HPA | Hypothalamic–pituitary–adrenal |
| ICIs | Immune checkpoint inhibitors |
| IL | Interleukin |
| NSCLC | Non-small cell lung cancer |
| NTS | Nucleus tractus solitarius |
| ORR | Overall response rate |
| PDAC | Pancreatic ductal adenocarcinoma |
| QoL | Quality of life |
| RET | Rearranged during transfection |
| SNS | Sympathetic nervous system |
| TGF | Transforming growth factor |
| TME | Tumour microenvironment |
| TNF-α | Tumour necrosis factor-alpha |
| UC | Urothelial carcinoma |
References
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.S.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.; Baldwin, C.; Chasen, M.; et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines. ESMO Open 2021, 6, 100092. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Roeland, E.J.; Bohlke, K.; Baracos, V.E.; Smith, T.J.; Loprinzi, C.L. Cancer Cachexia Expert Panel Cancer cachexia: ASCO guideline rapid recommendation update. J. Clin. Oncol. 2023, 41, 4178–4179. [Google Scholar] [CrossRef]
- Bianchini, C.; Bonomo, P.; Bossi, P.; Caccialanza, R.; Fabi, A. Bridging gaps in cancer cachexia Care: Current insights and future perspectives. Cancer Treat. Rev. 2024, 125, 102717. [Google Scholar] [CrossRef]
- Breit, S.N.; Brown, D.A.; Tsai, V.W.-W. The GDF15-GFRAL pathway in health and metabolic disease: Friend or foe? Annu. Rev. Physiol. 2021, 83, 127–151. [Google Scholar] [CrossRef]
- Mullican, S.E.; Lin-Schmidt, X.; Chin, C.-N.; Chavez, J.A.; Furman, J.L.; Armstrong, A.A.; Beck, S.C.; South, V.J.; Dinh, T.Q.; Cash-Mason, T.D.; et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 2017, 23, 1150–1157. [Google Scholar] [CrossRef]
- Borner, T.; Pataro, A.M.; De Jonghe, B.C. Central mechanisms of emesis: A role for GDF15. Neurogastroenterol. Motil. 2024, 37, e14886. [Google Scholar] [CrossRef]
- Wischhusen, J.; Melero, I.; Fridman, W.H. Growth/differentiation factor-15 (GDF-15): From biomarker to novel targetable immune checkpoint. Front. Immunol. 2020, 11, 951. [Google Scholar] [CrossRef]
- Staff, A.C.; Bock, A.J.; Becker, C.; Kempf, T.; Wollert, K.C.; Davidson, B. Growth differentiation factor-15 as a prognostic biomarker in ovarian cancer. Gynecol. Oncol. 2010, 118, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Vocka, M.; Langer, D.; Fryba, V.; Petrtyl, J.; Hanus, T.; Kalousova, M.; Zima, T.; Petruzelka, L. Growth/differentiation factor 15 (GDF-15) as new potential serum marker in patients with metastatic colorectal cancer. Cancer Biomark. 2018, 21, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Weide, B.; Schäfer, T.; Martens, A.; Kuzkina, A.; Uder, L.; Noor, S.; Garbe, C.; Harter, P.N.; Mittelbronn, M.; Wischhusen, J. High GDF-15 serum levels independently correlate with poorer overall survival of patients with tumor-free stage III and unresectable stage IV melanoma. J. Investig. Dermatol. 2016, 136, 2444–2452. [Google Scholar] [CrossRef]
- Traeger, L.; Ellermann, I.; Wiethoff, H.; Ihbe, J.; Gallitz, I.; Eveslage, M.; Moritz, R.; Herrmann, E.; Schrader, A.J.; Steinbicker, A.U. Serum Hepcidin and GDF-15 levels as prognostic markers in urothelial carcinoma of the upper urinary tract and renal cell carcinoma. BMC Cancer 2019, 19, 74. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, X.; Zhang, W. GDF15 predict platinum response during first-line chemotherapy and can act as a complementary diagnostic serum biomarker with CA125 in epithelial ovarian cancer. BMC Cancer 2018, 18, 328. [Google Scholar] [CrossRef]
- Staff, A.C.; Trovik, J.; Eriksson, A.G.Z.; Wik, E.; Wollert, K.C.; Kempf, T.; Salvesen, H.B. Elevated plasma growth differentiation factor-15 correlates with lymph node metastases and poor survival in endometrial cancer. Clin. Cancer Res. 2011, 17, 4825–4833. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-B.; Jiang, X.-R.; Yu, X.-Y.; Wang, L.; He, S.; Feng, F.-Y.; Guo, L.; Jiang, W.; Lu, S. Macrophage inhibitory factor 1 acts as a potential biomarker in patients with esophageal squamous cell carcinoma and is a target for antibody-based therapy. Cancer Sci. 2014, 105, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Calvo, M.; Tarrío, N.; Reboredo, M.; Haz-Conde, M.; García, J.; Quindós, M.; Figueroa, A.; Antón-Aparicio, L.; Calvo, L.; Valladares-Ayerbes, M. Circulating levels of GDF15, MMP7 and miR-200c as a poor prognostic signature in gastric cancer. Futur. Oncol. 2014, 10, 1187–1202. [Google Scholar] [CrossRef]
- Shnaper, S.; Desbaillets, I.; Brown, D.A.; Murat, A.; Migliavacca, E.; Schluep, M.; Ostermann, S.; Hamou, M.; Stupp, R.; Breit, S.N.; et al. Elevated levels of MIC-1/GDF15 in the cerebrospinal fluid of patients are associated with glioblastoma and worse outcome. Int. J. Cancer 2009, 125, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Tian, H.; Qi, J.; Li, M.; Fu, C.; Wu, F.; Wang, Y.; Cheng, D.; Zhao, W.; et al. Macrophage inhibitory cytokine 1 (MIC-1/GDF15) as a novel diagnostic serum biomarker in pancreatic ductal adenocarcinoma. BMC Cancer 2014, 14, 578. [Google Scholar] [CrossRef]
- Wang, S.-F.; Chang, Y.-L.; Fang, W.-L.; Li, A.F.-Y.; Chen, C.-F.; Yeh, T.-S.; Hung, G.; Huang, K.; Lee, H. Growth differentiation factor 15 induces cisplatin resistance through upregulation of xCT expression and glutathione synthesis in gastric cancer. Cancer Sci. 2023, 114, 3301–3317. [Google Scholar] [CrossRef]
- Solheim, T.S.; Laird, B.J.A.; Balstad, T.R.; Stene, G.B.; Baracos, V.; Bye, A.; Dajani, O.; Hendifar, A.E.; Strasser, F.; Chasen, M.R.; et al. Results from a randomised, open-label trial of a multimodal intervention (exercise, nutrition and anti-inflammatory medication) plus standard care versus standard care alone to attenuate cachexia in patients with advanced cancer undergoing chemotherapy. J. Clin. Oncol. 2024, 42 (Suppl. S17), LBA12007. [Google Scholar] [CrossRef]
- Bootcov, M.R.; Bauskin, A.R.; Valenzuela, S.M.; Moore, A.G.; Bansal, M.; He, X.Y.; Zhang, H.P.; Donnellan, M.; Mahler, S.; Pryor, K.; et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc. Natl. Acad. Sci. USA 1997, 94, 11514–11519. [Google Scholar] [CrossRef]
- Tsai, V.W.W.; Husaini, Y.; Sainsbury, A.; Brown, D.A.; Breit, S.N. The MIC-1/GDF15-GFRAL pathway in energy homeostasis: Implications for obesity, cachexia, and other associated diseases. Cell Metab. 2018, 28, 353–368. [Google Scholar] [CrossRef]
- Lockhart, S.M.; Saudek, V.; O’Rahilly, S. GDF15: A hormone conveying somatic distress to the brain. Endocr. Rev. 2020, 41, 610–642. [Google Scholar] [CrossRef]
- Fejzo, M.; Rocha, N.; Cimino, I.; Lockhart, S.M.; Petry, C.J.; Kay, R.G.; Burling, K.; Barker, P.; George, A.L.; Yasara, N.; et al. GDF15 linked to maternal risk of nausea and vomiting during pregnancy. Nature 2024, 625, 760–767. [Google Scholar] [CrossRef]
- Sherman, P.W.; Flaxman, S.M. Nausea and vomiting of pregnancy in an evolutionary perspective. Am. J. Obstet. Gynecol. 2002, 186 (Suppl. S5), S190–S197. [Google Scholar] [CrossRef]
- Wang, D.; Day, E.A.; Townsend, L.K.; Djordjevic, D.; Jørgensen, S.B.; Steinberg, G.R. GDF15: Emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat. Rev. Endocrinol. 2021, 17, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Tsai, V.W.-W.; Manandhar, R.; Jørgensen, S.B.; Lee-Ng, K.K.M.; Zhang, H.P.; Marquis, C.P.; Jiang, L.; Husaini, Y.; Lin, S.; Sainsbury, A.; et al. The anorectic actions of the TGFβ cytokine MIC-1/GDF15 require an intact brainstem area postrema and nucleus of the solitary tract. PLoS ONE 2014, 9, e100370. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, J.; Yin, L.; Yang, J.; Zheng, Y.; Zhang, M.; Ni, B.; Wang, H. Upregulated GDF-15 expression facilitates pancreatic ductal adenocarcinoma progression through orphan receptor GFRAL. Aging 2020, 12, 22564–22581. [Google Scholar] [CrossRef]
- Fichtner, K.; Kalwa, H.; Lin, M.-M.; Gong, Y.; Müglitz, A.; Kluge, M.; Krügel, U. GFRAL is widely distributed in the brain and peripheral tissues of mice. Nutrients 2024, 16, 734. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.; Lightman, S. The human stress response. Nat. Rev. Endocrinol. 2019, 15, 525–534. [Google Scholar] [CrossRef]
- Eddy, A.C.; Trask, A.J. Growth differentiation factor-15 and its role in diabetes and cardiovascular disease. Cytokine Growth Factor Rev. 2021, 57, 11–18. [Google Scholar] [CrossRef]
- Delrue, C.; Speeckaert, R.; Delanghe, J.R.; Speeckaert, M.M. Growth differentiation factor 15 (GDF-15) in kidney diseases. Adv. Clin. Chem. 2023, 114, 1–46. [Google Scholar] [PubMed]
- Depotte, L.; Nay, P.; Borg, C.; Meurisse, A.; Henriques, J.; Bennouna, J.; De La Fouchardière, C.; Tougeron, D.; Mazard, T.; Chibaudel, B.; et al. Interplay between sarcopenia, GDF-15, and the efficacy of nivolumab plus ipilimumab in patients with mismatch repair deficient metastatic colorectal cancer: Final survival analysis of the phase II GERCOR NIPICOL study. J. Immunother. Cancer. 2025, 13, e011220. [Google Scholar] [CrossRef] [PubMed]
- Roth, P.; Junker, M.; Tritschler, I.; Mittelbronn, M.; Dombrowski, Y.; Breit, S.N.; Tabatabai, G.; Wick, W.; Weller, M.; Wischhusen, J. GDF-15 contributes to proliferation and immune escape of malignant gliomas. Clin. Cancer Res. 2010, 16, 3851–3859. [Google Scholar] [CrossRef] [PubMed]
- Haake, M.; Haack, B.; Schäfer, T.; Harter, P.N.; Mattavelli, G.; Eiring, P.; Vashist, N.; Wedekink, F.; Genssler, S.; Fischer, B.; et al. Tumor-derived GDF-15 blocks LFA-1 dependent T cell recruitment and suppresses responses to anti-PD-1 treatment. Nat. Commun. 2023, 14, 4253. [Google Scholar] [CrossRef]
- Lerner, L.; Hayes, T.G.; Tao, N.; Krieger, B.; Feng, B.; Wu, Z.; Nicoletti, R.; Chiu, M.I.; Gyuris, J.; Garcia, J.M. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients: GDF15 in cancer-related weight loss. J. Cachexia Sarcopenia Muscle 2015, 6, 317–324. [Google Scholar] [CrossRef]
- Elecsys GDF-15 [Internet]. Available online: https://assets.roche.com/f/173850/x/ecd2b5c2aa/gdf15-07028172190-en-can.pdf (accessed on 1 March 2025).
- Groarke, J.D.; Crawford, J.; Collins, S.M.; Lubaczewski, S.; Roeland, E.J.; Naito, T.; Hendifar, A.E.; Fallon, M.; Takayama, K.; Asmis, T.; et al. Ponsegromab for the treatment of cancer cachexia. N. Engl. J. Med. 2024, 391, 2291–2303. [Google Scholar] [CrossRef]
- Suzuki, H.; Mitsunaga, S.; Ikeda, M.; Aoyama, T.; Yoshizawa, K.; Yoshimatsu, H.; Kawai, N.; Masuda, M.; Miura, T.; Ochiai, A. Clinical and tumor characteristics of patients with high serum levels of growth differentiation factor 15 in advanced pancreatic cancer. Cancers 2021, 13, 4842. [Google Scholar] [CrossRef]
- Skipworth, R.J.E.; Deans, D.A.C.; Tan, B.H.L.; Sangster, K.; Paterson-Brown, S.; Brown, D.A.; Hunter, M.; Breit, S.N.; Ross, J.A.; Fearon, K.C.H. Plasma MIC-1 correlates with systemic inflammation but is not an independent determinant of nutritional status or survival in oesophago-gastric cancer. Br. J. Cancer 2010, 102, 665–672. [Google Scholar] [CrossRef]
- Xue, H.; Lü, B.; Zhang, J.; Wu, M.; Huang, Q.; Wu, Q.; Sheng, H.; Wu, D.; Hu, J.; Lai, M. Identification of serum biomarkers for colorectal cancer metastasis using a differential secretome approach. J. Proteome Res. 2010, 9, 545–555. [Google Scholar] [CrossRef]
- Molfino, A.; Amabile, M.I.; Imbimbo, G.; Rizzo, V.; Pediconi, F.; Catalano, C.; Emiliani, A.; Belli, R.; Ramaccini, C.; Parisi, C.; et al. Association between growth differentiation factor-15 (GDF-15) serum levels, anorexia and low muscle mass among cancer patients. Cancers 2020, 13, 99. [Google Scholar] [CrossRef]
- Hsu, J.-Y.; Crawley, S.; Chen, M.; Ayupova, D.A.; Lindhout, D.A.; Higbee, J.; Kutach, A.; Joo, W.; Gao, Z.; Fu, D.; et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature 2017, 550, 255–259. [Google Scholar] [CrossRef]
- Miller, A.D.; Leslie, R.A. The area postrema and vomiting. Front. Neuroendocrinol. 1994, 15, 301–320. [Google Scholar] [CrossRef] [PubMed]
- Grill, H.J.; Hayes, M.R. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012, 16, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.P.; Dube, M.G.; Pu, S.; Xu, B.; Horvath, T.L.; Kalra, P.S. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr. Rev. 1999, 20, 68–100. [Google Scholar]
- Roh, E.; Song, D.K.; Kim, M.-S. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp. Mol. Med. 2016, 48, e216. [Google Scholar] [CrossRef]
- Cimino, I.; Kim, H.; Tung, Y.C.L.; Pedersen, K.; Rimmington, D.; Tadross, J.A.; Kohnke, S.N.; Neves-Costa, A.; Barros, A.; Joaquim, S.; et al. Activation of the hypothalamic-pituitary-adrenal axis by exogenous and endogenous GDF15. Proc. Natl. Acad. Sci. USA 2021, 118, e2106868118. [Google Scholar] [CrossRef]
- Olson, B.; Diba, P.; Korzun, T.; Marks, D.L. Neural mechanisms of cancer cachexia. Cancers 2021, 13, 3990. [Google Scholar] [CrossRef] [PubMed]
- Stewart Coats, A.J.; Ho, G.F.; Prabhash, K.; von Haehling, S.; Tilson, J.; Brown, R.; Anker, S.D.; Bunga, S.; Tan, S.B.; Socinski, M.A.; et al. Espindolol for the treatment and prevention of cachexia in patients with stage III/IV non-small cell lung cancer or colorectal cancer: A randomized, double-blind, placebo-controlled, international multicentre phase II study (the ACT-ONE trial): Espindolol for the treatment and prevention of cachexia. J. Cachexia Sarcopenia Muscle 2016, 7, 355–365. [Google Scholar]
- Wang, D.; Townsend, L.K.; DesOrmeaux, G.J.; Frangos, S.M.; Batchuluun, B.; Dumont, L.; Kuhre, R.E.; Ahmadi, E.; Hu, S.; Rebalka, I.A.; et al. GDF15 promotes weight loss by enhancing energy expenditure in muscle. Nature 2023, 619, 143–150. [Google Scholar] [CrossRef]
- Suriben, R.; Chen, M.; Higbee, J.; Oeffinger, J.; Ventura, R.; Li, B.; Mondal, K.; Gao, Z.; Ayupova, D.; Taskar, P.; et al. Antibody-mediated inhibition of GDF15-GFRAL activity reverses cancer cachexia in mice. Nat. Med. 2020, 26, 1264–1270. [Google Scholar] [CrossRef]
- Lerner, L.; Gyuris, J.; Nicoletti, R.; Gifford, J.; Krieger, B.; Jatoi, A. Growth differentiating factor-15 (GDF-15): A potential biomarker and therapeutic target for cancer-associated weight loss. Oncol. Lett. 2016, 12, 4219–4223. [Google Scholar] [CrossRef]
- Lerner, L.; Tao, J.; Liu, Q.; Nicoletti, R.; Feng, B.; Krieger, B.; Mazsa, E.; Siddiquee, Z.; Wang, R.; Huang, L.; et al. MAP3K11/GDF15 axis is a critical driver of cancer cachexia: MAP3K11/GDF15 axis is a driver of cancer cachexia. J. Cachexia Sarcopenia Muscle 2016, 7, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Paval, D.R.; Patton, R.; McDonald, J.; Skipworth, R.J.E.; Gallagher, I.J.; Laird, B.J. A systematic review examining the relationship between cytokines and cachexia in incurable cancer. J. Cachexia Sarcopenia Muscle 2022, 13, 824–838. [Google Scholar] [CrossRef] [PubMed]
- van Norren, K.; Dwarkasing, J.T.; Witkamp, R.F. The role of hypothalamic inflammation, the hypothalamic-pituitary-adrenal axis and serotonin in the cancer anorexia-cachexia syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 396–401. [Google Scholar] [CrossRef]
- Emmerson, P.J.; Wang, F.; Du, Y.; Liu, Q.; Pickard, R.T.; Gonciarz, M.D.; Coskun, T.; Hamang, M.J.; Sindelar, D.K.; Ballman, K.K.; et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat. Med. 2017, 23, 1215–1219. [Google Scholar] [CrossRef]
- Suzuki, H.; Asakawa, A.; Amitani, H.; Nakamura, N.; Inui, A. Cancer cachexia--pathophysiology and management. J. Gastroenterol. 2013, 48, 574–594. [Google Scholar] [CrossRef]
- Macia, L.; Tsai, V.W.; Nguyen, A.D.; Johnen, H.; Kuffner, T.; Shi, Y.C.; Lin, S.; Herzog, H.; Brown, D.A.; Breit, S.N.; et al. Macrophage inhibitory cytokine 1 (MIC-1/GDF15) decreases food intake, body weight and improves glucose tolerance in mice on normal & obesogenic diets. PLoS ONE 2012, 7, e34868. [Google Scholar]
- Li, J.; Hu, X.; Xie, Z.; Li, J.; Huang, C.; Huang, Y. Overview of growth differentiation factor 15 (GDF15) in metabolic diseases. Biomed. Pharmacother. 2024, 176, 116809. [Google Scholar] [CrossRef]
- Johnen, H.; Lin, S.; Kuffner, T.; Brown, D.A.; Tsai, V.W.; Bauskin, A.R.; Wu, L.; Pankhurst, G.; Jiang, L.; Junankar, S.; et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat. Med. 2007, 13, 1333–1340. [Google Scholar] [CrossRef]
- Martin, A.; Gallot, Y.S.; Freyssenet, D. Molecular mechanisms of cancer cachexia-related loss of skeletal muscle mass: Data analysis from preclinical and clinical studies. J. Cachexia Sarcopenia Muscle 2023, 14, 1150–1167. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, P.J. Chemotherapy-induced nausea and vomiting. N. Engl. J. Med. 2008, 358, 2482–2494. [Google Scholar] [CrossRef]
- Altena, R.; Fehrmann, R.S.N.; Boer, H.; de Vries, E.G.E.; Meijer, C.; Gietema, J.A. Growth differentiation factor 15 (GDF-15) plasma levels increase during bleomycin- and cisplatin-based treatment of testicular cancer patients and relate to endothelial damage. PLoS ONE 2015, 10, e0115372. [Google Scholar] [CrossRef]
- Worth, A.A.; Shoop, R.; Tye, K.; Feetham, C.H.; D’Agostino, G.; Dodd, G.T.; Reimann, F.; Gribble, F.M.; Beebe, E.C.; Dunbar, J.D.; et al. The cytokine GDF15 signals through a population of brainstem cholecystokinin neurons to mediate anorectic signalling. eLife 2020, 9, e55164. [Google Scholar] [CrossRef]
- Borner, T.; Shaulson, E.D.; Ghidewon, M.Y.; Barnett, A.B.; Horn, C.C.; Doyle, R.P. GDF15 induces anorexia through nausea and Emesis. Cell Metab. 2020, 31, 351–362.e5. [Google Scholar] [CrossRef] [PubMed]
- Borner, T.; Tinsley, I.C.; Milliken, B.T.; Doebley, S.A.; Najjar, N.R.; Kerwood, D.J.; De Jonghe, B.C.; Hayes, M.R.; Doyle, R.P. Creation of a peptide antagonist of the GFRAL-RET receptor complex for the treatment of GDF15-induced malaise. J. Med. Chem. 2023, 66, 11237–11249. [Google Scholar] [CrossRef]
- Breen, D.M.; Kim, H.; Bennett, D.; Calle, R.A.; Collins, S.; Esquejo, R.M.; He, T.; Joaquim, S.; Joyce, A.; Lambert, M.; et al. GDF-15 neutralization alleviates platinum-based chemotherapy-induced Emesis, anorexia, and weight loss in mice and nonhuman primates. Cell Metab. 2020, 32, 938–950.e6. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, N.M.; Peterson, J.M.; Talbert, E.E.; Ladner, K.J.; Rajasekera, P.V.; Schmidt, C.R.; Dillhoff, M.E.; Swanson, B.J.; Haverick, E.; Kladney, R.D.; et al. NF-κB regulates GDF-15 to suppress macrophage surveillance during early tumor development. J. Clin. Investig. 2017, 127, 3796–3809. [Google Scholar] [CrossRef]
- Deng, J.; Pan, T.; Wang, D.; Hong, Y.; Liu, Z.; Zhou, X.; An, Z.; Li, L.; Alfano, G.; Li, G.; et al. The MondoA-dependent TXNIP/GDF15 axis predicts oxaliplatin response in colorectal adenocarcinomas. EMBO Mol. Med. 2024, 16, 2080–2108. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, W.; Song, Y.; Wang, L.; Zhang, K.; Yang, J.; Zhang, W.; Su, H.; Zhang, Y. Growth differentiation factor-15 suppresses maturation and function of dendritic cells and inhibits tumor-specific immune response. PLoS ONE 2013, 8, e78618. [Google Scholar] [CrossRef]
- Salminen, A. GDF15/MIC-1: A stress-induced immunosuppressive factor which promotes the aging process. Biogerontology 2024, 26, 19. [Google Scholar] [CrossRef]
- Melero, I.; de Miguel Luken, M.; de Velasco, G.; Garralda, E.; Martín-Liberal, J.; Joerger, M.; Alonso, G.; Goebeler, M.E.; Schuler, M.; König, D.; et al. Neutralizing GDF-15 can overcome anti-PD-1 and anti-PD-L1 resistance in solid tumours. Nature 2024, 637, 1218–1227. [Google Scholar] [CrossRef]
- Østensen, M.; Villiger, P.M.; Förger, F. Interaction of pregnancy and autoimmune rheumatic disease. Autoimmun. Rev. 2012, 11, A437–A446. [Google Scholar] [CrossRef]
- Shitara, K.; Van Cutsem, E.; Bang, Y.J.; Fuchs, C.; Wyrwicz, L.; Lee, K.W.; Kudaba, I.; Garrido, M.; Chung, H.C.; Lee, J.; et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: The KEYNOTE-062 phase 3 randomized clinical trial: The KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020, 6, 1571–1580. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Cho, B.C.; Takahashi, M.; Okada, M.; Lin, C.Y.; Chin, K.; Kadowaki, S.; Ahn, M.J.; Hamamoto, Y.; Doki, Y.; et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 1506–1517. [Google Scholar] [CrossRef]
- Tewari, K.S.; Monk, B.J.; Vergote, I.; Miller, A.; de Melo, A.C.; Kim, H.S.; Kim, Y.M.; Lisyanskaya, A.; Samouëlian, V.; Lorusso, D.; et al. Survival with cemiplimab in recurrent cervical cancer. N. Engl. J. Med. 2022, 386, 544–555. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbé, C.; Linette, G.P.; Milella, M.; et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef]
- Sharma, P.; Goswami, S.; Raychaudhuri, D.; Siddiqui, B.A.; Singh, P.; Nagarajan, A.; Liu, J.; Subudhi, S.K.; Poon, C.; Gant, K.L.; et al. Immune checkpoint therapy-current perspectives and future directions. Cell 2023, 186, 1652–1669. [Google Scholar] [CrossRef]
- Sun, Q.; Hong, Z.; Zhang, C.; Wang, L.; Han, Z.; Ma, D. Immune checkpoint therapy for solid tumours: Clinical dilemmas and future trends. Signal Transduct. Target. Ther. 2023, 8, 320. [Google Scholar] [CrossRef]
- Tran, S.D.; Forrest, N.J.; Guggilla, V.; Perottino, G.M.; Johnson, J.L.; Sosman, J.; Roy, I.; Walunas, T.L. Weight and blood-based markers of cachexia predict disability, hospitalization and worse survival in cancer immunotherapy patients. J. Cachexia Sarcopenia Muscle 2025, 16, e13685. [Google Scholar] [CrossRef] [PubMed]
- Kawachi, H.; Yamada, T.; Tamiya, M.; Negi, Y.; Kijima, T.; Goto, Y.; Nakao, A.; Shiotsu, S.; Tanimura, K.; Takeda, T.; et al. Clinical impact of cancer cachexia on the outcome of patients with non-small cell lung cancer with PD-L1 tumor proportion scores of ≥50% receiving pembrolizumab monotherapy versus immune checkpoint inhibitor with chemotherapy. Oncoimmunology 2025, 14, 2442116. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, B.; Wang, X.; Zhang, H.; Wang, C.; Fan, B.; Wang, L. Effect of longitudinal changes of cachexia on the efficacy and toxicity of immune checkpoint inhibitors in esophageal squamous cell cancer (ESCC) patients. Nutrition 2024, 124, 112462. [Google Scholar] [CrossRef]
- Yu, Y.; Yan, L.; Huang, T.; Wu, Z.; Liu, J. Cancer cachexia reduces the efficacy of immune checkpoint inhibitors in cancer patients. Aging 2024, 16, 5354–5369. [Google Scholar] [CrossRef]
- Takenaka, Y.; Oya, R.; Takemoto, N.; Inohara, H. Predictive impact of sarcopenia in solid cancers treated with immune checkpoint inhibitors: A meta-analysis. J. Cachexia Sarcopenia Muscle 2021, 12, 1122–1135. [Google Scholar] [CrossRef]
- Groarke, J.D.; Crawford, J.; Collins, S.M.; Lubaczewski, S.L.; Breen, D.M.; Harrington, M.A.; Jacobs, I.; Qiu, R.; Revkin, J.; Rossulek, M.I.; et al. Phase 2 study of the efficacy and safety of ponsegromab in patients with cancer cachexia: PROACC-1 study design. J. Cachexia Sarcopenia Muscle 2024, 15, 1054–1061. [Google Scholar] [CrossRef]
- Crawford, J.; Calle, R.A.; Collins, S.M.; Weng, Y.; Lubaczewski, S.L.; Buckeridge, C.; Wang, E.Q.; Harrington, M.A.; Tarachandani, A.; Rossulek, M.I.; et al. A phase Ib first-in-patient study assessing the safety, tolerability, pharmacokinetics, and pharmacodynamics of ponsegromab in participants with cancer and cachexia. Clin. Cancer Res. 2024, 30, 489–497. [Google Scholar] [CrossRef]
- Carneiro, B.A.; Diab, M.; Van Tine, B.A.; Shields, A.F.; Abdul Razak, A.; Hilton, J.F.; Santana-Davila, R.; Sanai, E.; Zeron-Medina, J.; Bragulat, V.; et al. Abstract CT116: First-in-human study of AZD8853, an anti-growth and differentiation factor 15 (GDF15) antibody, in patients (pts) with advanced/metastatic solid tumors. Cancer Res. 2023, 83 (Suppl. S8), CT116. [Google Scholar] [CrossRef]
- Carneiro, B.A.; Gbolahan, O.B.; Abdul Razak, A.A.; Hilton, J.F.; Lambert, A.W.; Hood, J.; Pluta, M.; Bragulat, V.; Sanai, E.; Kumar, R.; et al. First-in-human study to evaluate the safety and efficacy of anti-GDF15 antibody AZD8853 in patients with advanced/metastatic solid tumors. Cancer Res. Commun. 2025, 5, 896–905. [Google Scholar] [CrossRef]
- Chavan, S.S.; Pavlov, V.A.; Tracey, K.J. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity 2017, 46, 927–942. [Google Scholar] [CrossRef]
- Qiao, G.; Chen, M.; Bucsek, M.J.; Repasky, E.A.; Hylander, B.L. Adrenergic signaling: A targetable checkpoint limiting development of the antitumor immune response. Front. Immunol. 2018, 9, 164. [Google Scholar] [CrossRef]
- Jain, R.; Kim, E.J.; Lenz, H.-J.; Messersmith, W.A.; Picozzi, V.J.; Beg, M.S.; Weinberg, B.A.; Mahalingam, D.; Tran-Muchowski, C.; Yuan, N.; et al. 550P Initial results of a phase Ia/Ib study of NGM120, a first-in-class anti-GDNF family receptor alpha like (GFRAL) antibody in patients (pts) with advanced solid tumors. Ann. Oncol. 2021, 32, S610–S611. [Google Scholar] [CrossRef]
- Andrews, P.L.; Bhandari, P. The 5-hydroxytryptamine receptor antagonists as antiemetics: Preclinical evaluation and mechanism of action. Eur. J. Cancer. 1993, 29 (Suppl. S1), S11–S16. [Google Scholar] [CrossRef]
- Luan, H.H.; Wang, A.; Hilliard, B.K.; Carvalho, F.; Rosen, C.E.; Ahasic, A.M.; Herzog, E.L.; Kang, I.; Pisani, M.A.; Yu, S.; et al. GDF15 is an inflammation-induced central mediator of tissue tolerance. Cell 2019, 178, 1231–1244.e11. [Google Scholar] [CrossRef]
- Kamiya, A.; Hiyama, T.; Fujimura, A.; Yoshikawa, S. Sympathetic and parasympathetic innervation in cancer: Therapeutic implications. Clin. Auton. Res. 2021, 31, 165–178. [Google Scholar] [CrossRef]
- Cole, S.W.; Nagaraja, A.S.; Lutgendorf, S.K.; Green, P.A.; Sood, A.K. Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer 2015, 15, 563–572. [Google Scholar] [CrossRef]
- Mimeault, M.; Johansson, S.L.; Batra, S.K. Marked improvement of cytotoxic effects induced by docetaxel on highly metastatic and androgen-independent prostate cancer cells by downregulating macrophage inhibitory cytokine-1. Br. J. Cancer 2013, 108, 1079–1091. [Google Scholar] [CrossRef]
- Lin, H.; Luo, Y.; Gong, T.; Fang, H.; Li, H.; Ye, G.; Zhang, Y.; Zhong, M. GDF15 induces chemoresistance to oxaliplatin by forming a reciprocal feedback loop with Nrf2 to maintain redox homeostasis in colorectal cancer. Cell Oncol. 2024, 47, 1149–1165. [Google Scholar] [CrossRef]
- Jatoi, A.; Dakhil, S.R.; Nguyen, P.L.; Sloan, J.A.; Kugler, J.W.; Rowland, K.M., Jr.; Soori, G.S.; Wender, D.B.; Fitch, T.R.; Novotny, P.J.; et al. A placebo-controlled double blind trial of etanercept for the cancer anorexia/weight loss syndrome: Results from N00C1 from the North Central Cancer Treatment Group. Cancer 2007, 110, 1396–1403. [Google Scholar] [CrossRef]
- Wiedenmann, B.; Malfertheiner, P.; Friess, H.; Ritch, P.; Arseneau, J.; Mantovani, G.; Caprioni, F.; Van Cutsem, E.; Richel, D.; DeWitte, M.; et al. A multicenter, phase II study of infliximab plus gemcitabine in pancreatic cancer cachexia. J. Support. Oncol. 2008, 6, 18–25. [Google Scholar] [PubMed]
- Jatoi, A.; Ritter, H.L.; Dueck, A.; Nguyen, P.L.; Nikcevich, D.A.; Luyun, R.F.; Mattar, B.I.; Loprinzi, C.L. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9). Lung Cancer 2010, 68, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Yennurajalingam, S.; Willey, J.S.; Palmer, J.L.; Allo, J.; Del Fabbro, E.; Cohen, E.N.; Tin, S.; Reuben, J.M.; Bruera, E. The role of thalidomide and placebo for the treatment of cancer-related anorexia-cachexia symptoms: Results of a double-blind placebo-controlled randomized study. J. Palliat. Med. 2012, 15, 1059–1064. [Google Scholar] [CrossRef]
- Tamayo-Torres, E.; Garrido, A.; de Cabo, R.; Carretero, J.; Gómez-Cabrera, M.C. Molecular mechanisms of cancer cachexia. Role of exercise training. Mol. Asp. Med. 2024, 99, 101293. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Brown, L.R.; Laird, B.J.A.; Wigmore, S.J.; Skipworth, R.J.E. Understanding cancer cachexia and its implications in upper gastrointestinal cancers. Curr. Treat. Options Oncol. 2022, 23, 1732–1747. [Google Scholar] [CrossRef]
- Akaoka, M.; Haruki, K.; Yamahata, Y.; Okazaki, K.; Furukawa, K.; Tsunematsu, M.; Shirai, Y.; Onda, S.; Matsumoto, M.; Ikegami, T. The prognostic impact of perioperative dynamic changes in cachexia index in patients with hepatocellular carcinoma. Ann. Gastroenterol. Surg. 2024, 8, 917–926. [Google Scholar] [CrossRef]
- Wang, S.-L.; Zhang, F.-M.; Chen, C.-B.; Dong, Q.-T.; Liu, S.; Yu, Z.; Shen, X.; Zhuang, C.-L. Comparison between AWGC-cachexia and GLIM-malnutrition in patients with gastric cancer. Eur. J. Surg. Oncol. 2024, 50, 108580. [Google Scholar] [CrossRef]
- Brown, L.R.; Thomson, G.G.; Gardner, E.; Chien, S.; McGovern, J.; Dolan, R.D.; McSorley, S.T.; Forshaw, M.J.; McMillan, D.C.; Wigmore, S.J.; et al. Cachexia index for prognostication in surgical patients with locally advanced oesophageal or gastric cancer: Multicentre cohort study. Br. J. Surg. 2024, 111, znae098. [Google Scholar] [CrossRef]
- Brown, L.R.; Sayers, J.; Yule, M.S.; Drake, T.M.; Dolan, R.D.; McMillan, D.C.; Laird, B.J.A.; Wigmore, S.J.; Skipworth, R.J.E. The prognostic impact of pre-treatment cachexia in resectional surgery for oesophagogastric cancer: A meta-analysis and meta-regression. Br. J. Surg. 2023, 110, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, N.; Nakayama, K.; Ishibashi, T.; Katayama, S.; Kyo, S. Effect of muscle loss but not fat loss during primary debulking surgery and chemotherapy on prognosis of patients with ovarian cancer. J. Clin. Med. 2022, 11, 3184. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Fan, G.H.; Hastie, D.J.; Addonizio, E.A.; Suh, J.; Prakasam, V.N.; Karagozian, R. The clinical impact of malnutrition on the postoperative outcomes of patients undergoing colorectal resection surgery for colon or rectal cancer: Propensity score matched analysis of 2011–2017 US hospitals. Surg. Oncol. 2021, 38, 101587. [Google Scholar] [CrossRef]
- Anker, M.S.; Holcomb, R.; Muscaritoli, M.; von Haehling, S.; Haverkamp, W.; Jatoi, A.; Morley, J.E.; Strasser, F.; Landmesser, U.; Coats, A.J.S.; et al. Orphan disease status of cancer cachexia in the USA and in the European Union: A systematic review. J. Cachexia Sarcopenia Muscle 2019, 10, 22–34. [Google Scholar] [CrossRef] [PubMed]
| Trial Identifier | Agent (Agent Type) | Target Molecule | Study Status | Study Design | Number of Patients | Target Population | Treatment | Inclusion Criteria by GDF-15 Levels | Primary Endpoint | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| NCT04815551 | AV-380 (mAB) | GDF-15 | Completed | I, randomised, double-blind, placebo-controlled | 56 | Healthy volunteers | AV-380 vs. placebo | No | Safety, PK/PD analysis | Not reported |
| NCT05865535 | Recruiting | I, nonrandomised | 30 | CRC, PDAC | AV-380 + standard chemotherapy | Yes (serum GDF-15 levels ≥ 1200 pg/mL) | Safety, PK/PD analysis | Not reported. | ||
| NCT05397171 [91] | AZD8853 (mAB) | GDF-15 | Terminated | I/II, nonrandomised | 16 (Part A) | NSCLC, CRC, UC | AZD8853 | No | Safety | No DLT or safety concern was observed. No radiological tumour response. Only Part A was initiated. The entire Master Protocol was terminated. |
| NCT03392116 | NGM120 (mAb) | GFRAL | Completed | I, randomised, double-blind, placebo-controlled | 92 | Healthy volunteers | NGM120 vs. placebo | No | Safety | Not reported. |
| NCT04068896 [95] | Completed | I/II, randomised, double-blind, placebo-controlled | 75 | PDAC, CRPC | Chemotherapy plus NGM120 or placebo | Yes (serum GDF-15 levels ≥ 1300 pg/mL [prostate cancer, part 3]) | Safety | (Phase 1a/1b, PDAC with GEM + nab-PTX) No safety concern. Body weight gain was observed in monotherapy and combination with chemotherapy. DCR: 25% (5/20) with no objective response. | ||
| NCT03974776 | Ponsegromab (mAb) | GDF-15 | Completed | I, randomised, double-blind, placebo-controlled | 8 | Healthy volunteer (Japanese only) | Ponsegromab vs. placebo | No | Safety | Not reported |
| NCT04299048 [90] | Completed | IB, nonrandomised | 10 | NSCLC, PDAC, CRC | Ponsegromab | Yes (serum GDF-15 levels ≥ 1500 pg/mL) | Safety | A favourable safety profile was demonstrated. Body weight gain and improved physical activities, and appetite were observed. Inhibition of serum GDF-15 was confirmed at the lower detection limit of the assay. | ||
| NCT05546476 [38] | Active, not recruiting | II, randomised, double-blind, placebo (PROACC-1 study) | 187 | NSCLC, PDAC, CRC with elevated serum GDF-15 | Ponsegromab vs. placebo | Yes (serum GDF-15 levels ≥ 1500 pg/mL) | Change from baseline in body weight at week 12 | Significant body weight gain was observed. Improved appetite, cachexia symptoms, and physical activity were also observed (400 mg group). | ||
| NCT03599063 [89] | Completed | I, randomised, double-blind, placebo-controlled | 63 | Healthy volunteer | Ponsegromab vs. placebo | No | Safety | A favourable safety profile was demonstrated. Inhibition of serum GDF-15 was confirmed at the lower detection limit of the assay. | ||
| NCT04803305 [89] | Completed | I, randomised, double-blind, placebo-controlled | 18 | NSCLC, PDAC, CRC, prostate, breast | Ponsegromab vs. placebo | Yes (serum GDF-15 levels ≥ 1500 pg/mL) | Safety | A favourable safety profile was demonstrated. Inhibition of serum GDF-15 was confirmed at the lower detection limit of the assay. | ||
| NCT04725474 [73] | Visugromab (CTL002) (mAB) | GDF-15 | Active, not recruiting | I/II, nonrandomised (GDFFATHER-2) | 155 | Advanced cancer with refractoriness to ICIs (NSCLC, UC, others) | Visugromab (CTL002) plus nivolumab | No | Safety, antitumour activity | No DLT was observed. ORR: NSCLC, 4/27 (14.8%), including 2 CR; UC, 5/27 (18.5%), including 1 CR |
| NCT06059547 | Recruiting | II, randomised (GDFFATHER-NEO) | 30 | T2-T4aN0M0 MIBC (cisplatin-ineligible) | Visugromab (CTL-002) plus nivolumab vs. nivolumab | No | Antitumour activities (pCR rate, radiological tumour response rate) | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugiyama, K.; Starling, N.; Chau, I. New Horizons with Growth Differentiation Factor 15 in Oncology: From Cancer Cachexia and Tumour Immunity to Novel Therapeutic Strategies. Curr. Oncol. 2025, 32, 604. https://doi.org/10.3390/curroncol32110604
Sugiyama K, Starling N, Chau I. New Horizons with Growth Differentiation Factor 15 in Oncology: From Cancer Cachexia and Tumour Immunity to Novel Therapeutic Strategies. Current Oncology. 2025; 32(11):604. https://doi.org/10.3390/curroncol32110604
Chicago/Turabian StyleSugiyama, Keiji, Naureen Starling, and Ian Chau. 2025. "New Horizons with Growth Differentiation Factor 15 in Oncology: From Cancer Cachexia and Tumour Immunity to Novel Therapeutic Strategies" Current Oncology 32, no. 11: 604. https://doi.org/10.3390/curroncol32110604
APA StyleSugiyama, K., Starling, N., & Chau, I. (2025). New Horizons with Growth Differentiation Factor 15 in Oncology: From Cancer Cachexia and Tumour Immunity to Novel Therapeutic Strategies. Current Oncology, 32(11), 604. https://doi.org/10.3390/curroncol32110604





