ctDNA in Pancreatic Adenocarcinoma: A Critical Appraisal
Simple Summary
Abstract
1. Introduction
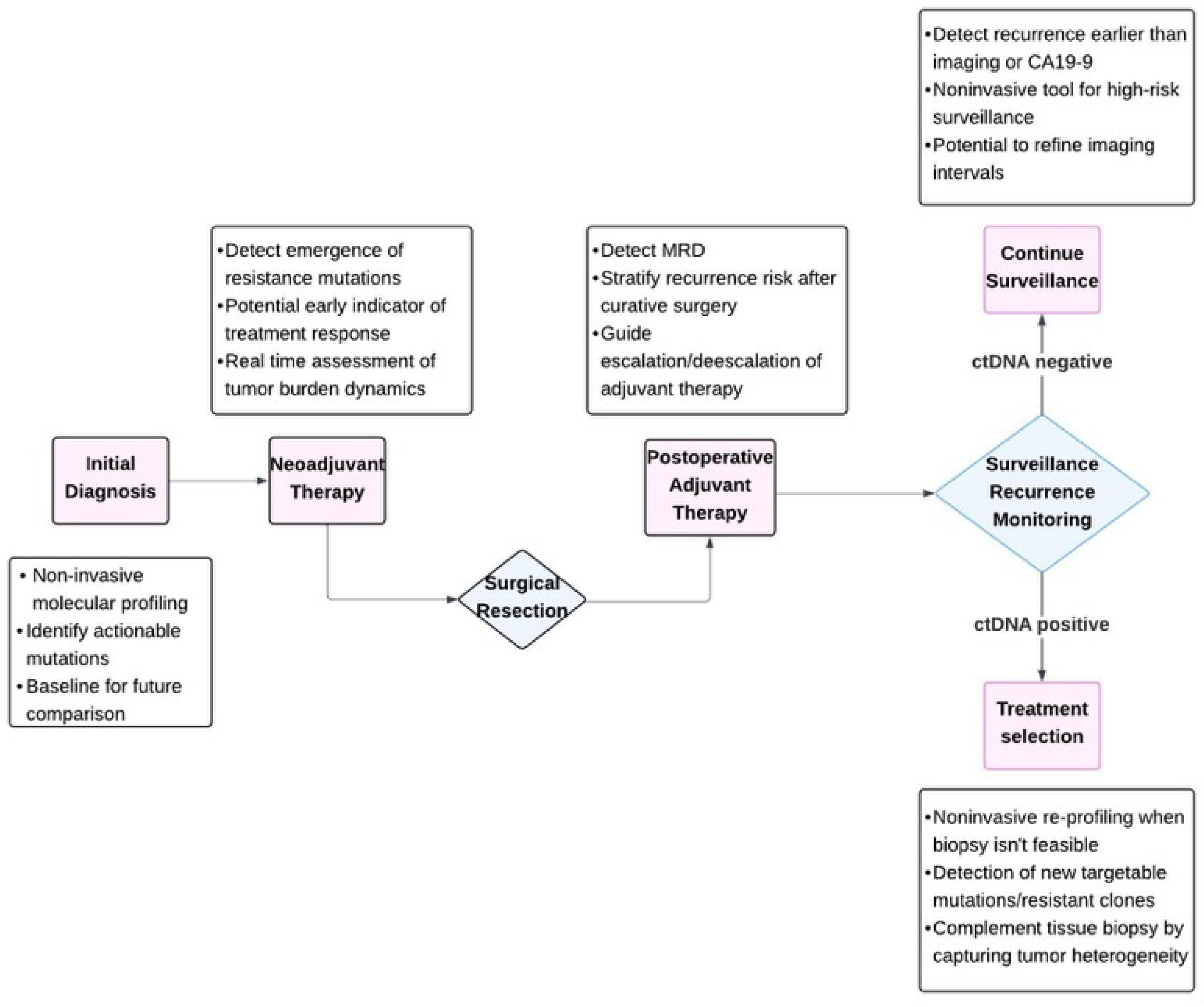
2. Current Evidence on ctDNA Clinical Utility in PDAC
2.1. ctDNA as a Novel Prognostic Marker in Advanced PDAC
2.2. ctDNA as a Tool for PDAC Screening/Early Detection
2.3. Differentiating Benign and Pancreatic Cysts with Malignant Potential
2.4. Measuring Minimal Residual Disease
2.5. ctDNA as a Tumor Marker to Monitor Chemotherapy Response
Monitoring Therapy in Neoadjuvant and Palliative Settings
2.6. Patient Selection for Precision Medicine
3. Limitations and Challenges
4. Discussion and Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| ALK | Anaplastic lymphoma kinase |
| BRCA1/2 | Breast cancer gene 1 and 2 |
| BRAF | B-Raf proto-oncogene |
| CA19-9 | Carbohydrate antigen 19-9 |
| CEA | Carcinoembryonic antigen |
| cfDNA | Cell-free DNA |
| CRC | Colorectal cancer |
| CT | Computed tomography |
| ctDNA | Circulating tumor DNA |
| ddPCR | Droplet digital polymerase chain reaction |
| EGFR | Epidermal growth factor receptor |
| FDA | U.S. Food and Drug Administration |
| FGFR2 | Fibroblast growth factor receptor 2 |
| IDH1 | Isocitrate dehydrogenase 1 |
| KRAS | Kirsten rat sarcoma viral oncogene |
| MRI | Magnetic resonance imaging |
| MRD | Minimal residual disease |
| NGS | Next-generation sequencing |
| OS | Overall survival |
| PDAC | Pancreatic ductal adenocarcinoma |
| PET | Positron emission tomography |
| PFS | Progression free survival (PFS) |
References
- Li, Q.; Feng, Z.; Miao, R.; Liu, X.; Liu, C.; Liu, Z. Prognosis and survival analysis of patients with pancreatic cancer: Retrospective experience of a single institution. World J. Surg. Oncol. 2022, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Zanini, S.; Renzi, S.; Limongi, A.R.; Bellavite, P.; Giovinazzo, F.; Bermano, G. A review of lifestyle and environment risk factors for pancreatic cancer. Eur. J. Cancer 2021, 145, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.; Ziranu, P.; Spanu, D.; Dubois, M.; Pretta, A.; Tolu, S.; Camera, S.; Liscia, N.; Mariani, S.; Persano, M.; et al. BRCA-mutant pancreatic ductal adenocarcinoma. Br. J. Cancer 2021, 125, 1321–1332. [Google Scholar] [CrossRef]
- Stejskal, P.; Goodarzi, H.; Srovnal, J.; Hajdúch, M.; van ’t Veer, L.J.; Magbanua, M.J.M. Circulating tumor nucleic acids: Biology, release mechanisms, and clinical relevance. Mol. Cancer 2023, 22, 15. [Google Scholar] [CrossRef]
- Sánchez-Herrero, E.; Serna-Blasco, R.; Robado de Lope, L.; González-Rumayor, V.; Romero, A.; Provencio, M. Circulating Tumor DNA as a Cancer Biomarker: An Overview of Biological Features and Factors That may Impact on ctDNA Analysis. Front. Oncol. 2022, 12, 943253. [Google Scholar] [CrossRef]
- Wan, J.C.M.; Massie, C.; Garcia-Corbacho, J.; Mouliere, F.; Brenton, J.D.; Caldas, C.; Pacey, S.; Baird, R.; Rosenfeld, N. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer 2017, 17, 223–238. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef]
- Newman, A.M.; Lovejoy, A.F.; Klass, D.M.; Kurtz, D.M.; Chabon, J.J.; Scherer, F.; Stehr, H.; Liu, C.L.; Bratman, S.V.; Say, C.; et al. Integrated digital error suppression for improved detection of circulating tumor DNA. Nat. Biotechnol. 2016, 34, 547–555. [Google Scholar] [CrossRef]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef]
- Zheng, J.; Qin, C.; Wang, Q.; Tian, D.; Chen, Z. Circulating tumour DNA-Based molecular residual disease detection in resectable cancers: A systematic review and meta-analysis. eBioMedicine 2024, 103, 105109. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.Y.; Singhania, R.; Fehringer, G.; Chakravarthy, A.; Roehrl, M.H.A.; Chadwick, D.; Zuzarte, P.C.; Borgida, A.; Wang, T.T.; Li, T.; et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature 2018, 563, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Eledkawy, A.; Hamza, T.; El-Metwally, S. Precision cancer classification using liquid biopsy and advanced machine learning techniques. Sci. Rep. 2024, 14, 5841. [Google Scholar] [CrossRef] [PubMed]
- Pietrasz, D.; Wang-Renault, S.; Taieb, J.; Dahan, L.; Postel, M.; Durand-Labrunie, J.; Le Malicot, K.; Mulot, C.; Rinaldi, Y.; Phelip, J.-M.; et al. Prognostic value of circulating tumour DNA in metastatic pancreatic cancer patients: Post-hoc analyses of two clinical trials. Br. J. Cancer 2022, 126, 440–448. [Google Scholar] [CrossRef]
- Lapin, M.; Edland, K.H.; Tjensvoll, K.; Oltedal, S.; Austdal, M.; Garresori, H.; Rozenholc, Y.; Gilje, B.; Nordgård, O. Comprehensive ctDNA Measurements Improve Prediction of Clinical Outcomes and Enable Dynamic Tracking of Disease Progression in Advanced Pancreatic Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2023, 29, 1267–1278. [Google Scholar] [CrossRef]
- Uesato, Y.; Sasahira, N.; Ozaka, M.; Sasaki, T.; Takatsuki, M.; Zembutsu, H. Evaluation of circulating tumor DNA as a biomarker in pancreatic cancer with liver metastasis. PLoS ONE 2020, 15, e0235623. [Google Scholar] [CrossRef]
- Strijker, M.; Soer, E.C.; de Pastena, M.; Creemers, A.; Balduzzi, A.; Beagan, J.J.; Busch, O.R.; van Delden, O.M.; Halfwerk, H.; van Hooft, J.E.; et al. Circulating tumor DNA quantity is related to tumor volume and both predict survival in metastatic pancreatic ductal adenocarcinoma. Int. J. Cancer 2020, 146, 1445–1456. [Google Scholar] [CrossRef]
- Guan, S.; Deng, G.; Sun, J.; Han, Q.; Lv, Y.; Xue, T.; Ding, L.; Yang, T.; Qian, N.; Dai, G. Evaluation of circulating tumor DNA as a prognostic biomarker for metastatic pancreatic adenocarcinoma. Front. Oncol. 2022, 12, 926260. [Google Scholar] [CrossRef]
- Guven, D.C.; Sahin, T.K.; Yildirim, H.C.; Aktepe, O.H.; Dizdar, O.; Yalcin, S. A systematic review and meta-analysis of the association between circulating tumor DNA (ctDNA) and prognosis in pancreatic cancer. Crit. Rev. Oncol. Hematol. 2021, 168, 103528. [Google Scholar] [CrossRef]
- Shah, D.; Wells, A.; Cox, M.; Dawravoo, K.; Abad, J.; D’Souza, A.; Suh, G.; Bayer, R.; Chaudhry, S.; Zhang, Q.; et al. Prospective Evaluation of Circulating Tumor DNA Using Next-generation Sequencing as a Biomarker During Neoadjuvant Chemotherapy in Localized Pancreatic Cancer. Ann. Surg. 2025, 281, 997–1005. [Google Scholar] [CrossRef]
- Chen, I.; Raymond, V.M.; Geis, J.A.; Collisson, E.A.; Jensen, B.V.; Hermann, K.L.; Erlander, M.G.; Tempero, M.; Johansen, J.S. Ultrasensitive plasma ctDNA KRAS assay for detection, prognosis, and assessment of therapeutic response in patients with unresectable pancreatic ductal adenocarcinoma. Oncotarget 2017, 8, 97769–97786. [Google Scholar] [CrossRef] [PubMed]
- Pinson, J.; Henriques, J.; Beaussire, L.; Sarafan-Vasseur, N.; Sa Cunha, A.; Bachet, J.-B.; Vernerey, D.; Di Fiore, F.; Schwarz, L.; PANACHE01-PRODIGE48 Group. New Biomarkers to Define a Biological Borderline Situation for Pancreatic Adenocarcinoma: Results of an Ancillary Study of the PANACHE01-PRODIGE48 Trial. Ann. Surg. 2024, 280, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Arayici, M.E.; İnal, A.; Basbinar, Y.; Olgun, N. Evaluation of the diagnostic and prognostic clinical values of circulating tumor DNA and cell-free DNA in pancreatic malignancies: A comprehensive meta-analysis. Front. Oncol. 2024, 14, 1382369. [Google Scholar] [CrossRef]
- Kawamura, H.; Honda, M.; Takano, Y.; Kinuta, S.; Kamiga, T.; Saji, S.; Kono, K. Prognostic Role of Carcinoembryonic Antigen and Carbohydrate Antigen 19-9 in Stage IV Colorectal Cancer. Anticancer Res. 2022, 42, 3921–3928. [Google Scholar] [CrossRef]
- Botta, G.P.; Abdelrahim, M.; Drengler, R.L.; Aushev, V.N.; Esmail, A.; Laliotis, G.; Brewer, C.M.; George, G.V.; Abbate, S.M.; Chandana, S.R.; et al. Association of personalized and tumor-informed ctDNA with patient survival outcomes in pancreatic adenocarcinoma. Oncologist 2024, 29, 859–869. [Google Scholar] [CrossRef]
- Schwarz, L.; Vernerey, D.; Bachet, J.-B.; Tuech, J.-J.; Portales, F.; Michel, P.; Cunha, A.S. Resectable pancreatic adenocarcinoma neo-adjuvant FOLF(IRIN)OX-based chemotherapy-a multicenter, non-comparative, randomized, phase II trial (PANACHE01-PRODIGE48 study). BMC Cancer 2018, 18, 762. [Google Scholar] [CrossRef]
- Sefrioui, D.; Blanchard, F.; Toure, E.; Basile, P.; Beaussire, L.; Dolfus, C.; Perdrix, A.; Paresy, M.; Antonietti, M.; Iwanicki-Caron, I.; et al. Diagnostic value of CA19.9, circulating tumour DNA and circulating tumour cells in patients with solid pancreatic tumours. Br. J. Cancer 2017, 117, 1017–1025. [Google Scholar] [CrossRef]
- Luo, G.; Jin, K.; Deng, S.; Cheng, H.; Fan, Z.; Gong, Y.; Qian, Y.; Huang, Q.; Ni, Q.; Liu, C.; et al. Roles of CA19-9 in pancreatic cancer: Biomarker, predictor and promoter. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188409. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, B.; Chen, F. Diagnostic value of serum carbohydrate antigen 19-9 in pancreatic cancer: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2022, 34, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Fahrmann, J.F.; Schmidt, C.M.; Mao, X.; Irajizad, E.; Loftus, M.; Zhang, J.; Patel, N.; Vykoukal, J.; Dennison, J.B.; Long, J.P.; et al. Lead-Time Trajectory of CA19-9 as an Anchor Marker for Pancreatic Cancer Early Detection. Gastroenterology 2021, 160, 1373–1383.e6. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.; Lundberg, E.; Jonsson, P.; Nyström, H.; Franklin, O.; Lundin, C.; Naredi, P.; Antti, H.; Sund, M.; Öhlund, D. A Cross-Sectional and Longitudinal Analysis of Pre-Diagnostic Blood Plasma Biomarkers for Early Detection of Pancreatic Cancer. Int. J. Mol. Sci. 2022, 23, 12969. [Google Scholar] [CrossRef]
- Zheng, Z.; Lu, Z.; Yan, F.; Song, Y. The role of novel biomarkers in the early diagnosis of pancreatic cancer: A systematic review and meta-analysis. PLoS ONE 2025, 20, e0322720. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Wang, Q.-L.; Zhang, J.; Supplee, J.G.; Fahrmann, J.F.; Lehmann-Werman, R.; Brais, L.K.; Nowak, J.; Yuan, C.; Loftus, M.; et al. Protein biomarkers and alternatively methylated cell-free DNA detect early stage pancreatic cancer. Gut 2024, 73, 639–648. [Google Scholar] [CrossRef]
- Lennerz, J.K.; Stenzinger, A. Allelic Ratio of KRAS Mutations in Pancreatic Cancer. Oncologist 2015, 20, e8–e9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Liao, X.; Tsai, H. KRAS mutation: The booster of pancreatic ductal adenocarcinoma transformation and progression. Front. Cell Dev. Biol. 2023, 11, 1147676. [Google Scholar] [CrossRef]
- Nitschke, C.; Markmann, B.; Walter, P.; Badbaran, A.; Tölle, M.; Kropidlowski, J.; Belloum, Y.; Goetz, M.R.; Bardenhagen, J.; Stern, L.; et al. Peripheral and Portal Venous KRAS ctDNA Detection as Independent Prognostic Markers of Early Tumor Recurrence in Pancreatic Ductal Adenocarcinoma. Clin. Chem. 2023, 69, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Taback, B.; Saha, S.; Hoon, D.S.B. Comparative analysis of mesenteric and peripheral blood circulating tumor DNA in colorectal cancer patients. Ann. N. Y. Acad. Sci. 2006, 1075, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Han, Y.; Yun, W.-G.; Kwon, W.; Kim, H.; Jeong, H.; Seo, M.-S.; Park, Y.; Cho, S.I.; Kim, H.; et al. Parallel Analysis of Pre- and Postoperative Circulating Tumor DNA and Matched Tumor Tissues in Resectable Pancreatic Ductal Adenocarcinoma: A Prospective Cohort Study. Clin. Chem. 2022, 68, 1509–1518. [Google Scholar] [CrossRef]
- Groot, V.P.; Mosier, S.; Javed, A.A.; Teinor, J.A.; Gemenetzis, G.; Ding, D.; Haley, L.M.; Yu, J.; Burkhart, R.A.; Hasanain, A.; et al. Circulating Tumor DNA as a Clinical Test in Resected Pancreatic Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 4973–4984. [Google Scholar] [CrossRef]
- Jiang, J.; Ye, S.; Xu, Y.; Chang, L.; Hu, X.; Ru, G.; Guo, Y.; Yi, X.; Yang, L.; Huang, D. Circulating Tumor DNA as a Potential Marker to Detect Minimal Residual Disease and Predict Recurrence in Pancreatic Cancer. Front. Oncol. 2020, 10, 1220. [Google Scholar] [CrossRef]
- Ueberroth, B.E.; Jones, J.C.; Bekaii-Saab, T.S. Circulating tumor DNA (ctDNA) to evaluate minimal residual disease (MRD), treatment response, and posttreatment prognosis in pancreatic adenocarcinoma. Pancreatol. Off. J. Int. Assoc. Pancreatol. IAP Al 2022, 22, 741–748. [Google Scholar] [CrossRef]
- The Circulating Cell-Free Genome Atlas (CCGA) Study. GRAIL. Available online: https://grail.com/clinical-studies/ccga-study/ (accessed on 21 April 2025).
- Merker, J.D.; Oxnard, G.R.; Compton, C.; Diehn, M.; Hurley, P.; Lazar, A.J.; Lindeman, N.; Lockwood, C.M.; Rai, A.J.; Schilsky, R.L.; et al. Circulating Tumor DNA Analysis in Patients with Cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1631–1641. [Google Scholar] [CrossRef]
- Sellahewa, R.; Moghaddam, S.M.; Lundy, J.; Jenkins, B.J.; Croagh, D. Circulating Tumor DNA Is an Accurate Diagnostic Tool and Strong Prognostic Marker in Pancreatic Cancer. Pancreas 2023, 52, e188–e195. [Google Scholar] [CrossRef]
- Abdallah, R.; Taly, V.; Zhao, S.; Pietrasz, D.; Bachet, J.-B.; Basile, D.; Mas, L.; Zaanan, A.; Laurent-Puig, P.; Taieb, J. Plasma circulating tumor DNA in pancreatic adenocarcinoma for screening, diagnosis, prognosis, treatment and follow-up: A systematic review. Cancer Treat. Rev. 2020, 87, 102028. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Ding, X.-Q.; Zhu, H.; Wang, R.-X.; Pan, X.-R.; Tong, J.-H. KRAS Mutant Allele Fraction in Circulating Cell-Free DNA Correlates with Clinical Stage in Pancreatic Cancer Patients. Front. Oncol. 2019, 9, 1295. [Google Scholar] [CrossRef]
- Gonda, T.A.; Cahen, D.L.; Farrell, J.J. Pancreatic Cysts. N. Engl. J. Med. 2024, 391, 832–843. [Google Scholar] [CrossRef]
- Laquière, A.E.; Lagarde, A.; Napoléon, B.; Bourdariat, R.; Atkinson, A.; Donatelli, G.; Pol, B.; Lecomte, L.; Curel, L.; Urena-Campos, R.; et al. Genomic profile concordance between pancreatic cyst fluid and neoplastic tissue. World J. Gastroenterol. 2019, 25, 5530–5542. [Google Scholar] [CrossRef] [PubMed]
- Elta, G.H.; Enestvedt, B.K.; Sauer, B.G.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018, 113, 464–479. [Google Scholar] [CrossRef]
- Chidharla, A.; Rapoport, E.; Agarwal, K.; Madala, S.; Linares, B.; Sun, W.; Chakrabarti, S.; Kasi, A. Circulating Tumor DNA as a Minimal Residual Disease Assessment and Recurrence Risk in Patients Undergoing Curative-Intent Resection with or without Adjuvant Chemotherapy in Colorectal Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 10230. [Google Scholar] [CrossRef]
- Hoang, T.; Choi, M.K.; Oh, J.H.; Kim, J. Utility of circulating tumor DNA to detect minimal residual disease in colorectal cancer: A systematic review and network meta-analysis. Int. J. Cancer 2025, 157, 593–799. [Google Scholar] [CrossRef]
- Ryoo, S.-B.; Heo, S.; Lim, Y.; Lee, W.; Cho, S.H.; Ahn, J.; Kang, J.-K.; Kim, S.Y.; Kim, H.-P.; Bang, D.; et al. Personalised circulating tumour DNA assay with large-scale mutation coverage for sensitive minimal residual disease detection in colorectal cancer. Br. J. Cancer 2023, 129, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Slater, S.; Bryant, A.; Aresu, M.; Begum, R.; Chen, H.-C.; Peckitt, C.; Lazaro-Alcausi, R.; Carter, P.; Anandappa, G.; Khakoo, S.; et al. Tissue-Free Liquid Biopsies Combining Genomic and Methylation Signals for Minimal Residual Disease Detection in Patients with Early Colorectal Cancer from the UK TRACC Part B Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2024, 30, 3459–3469. [Google Scholar] [CrossRef]
- Tarazona, N.; Gimeno-Valiente, F.; Gambardella, V.; Zuñiga, S.; Rentero-Garrido, P.; Huerta, M.; Roselló, S.; Martinez-Ciarpaglini, C.; Carbonell-Asins, J.A.; Carrasco, F.; et al. Targeted next-generation sequencing of circulating-tumor DNA for tracking minimal residual disease in localized colon cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1804–1812. [Google Scholar] [CrossRef]
- Negro, S.; Pulvirenti, A.; Trento, C.; Indraccolo, S.; Ferrari, S.; Scarpa, M.; Urso, E.D.L.; Bergamo, F.; Pucciarelli, S.; Deidda, S.; et al. Circulating Tumor DNA as a Real-Time Biomarker for Minimal Residual Disease and Recurrence Prediction in Stage II Colorectal Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 2486. [Google Scholar] [CrossRef]
- Henriksen, T.V.; Demuth, C.; Frydendahl, A.; Nors, J.; Nesic, M.; Rasmussen, M.H.; Reinert, T.; Larsen, O.H.; Jaensch, C.; Løve, U.S.; et al. Unraveling the potential clinical utility of circulating tumor DNA detection in colorectal cancer-evaluation in a nationwide Danish cohort. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2024, 35, 229–239. [Google Scholar] [CrossRef]
- Parikh, A.R.; Chee, B.H.; Tsai, J.; Rich, T.A.; Price, K.S.; Patel, S.A.; Zhang, L.; Ibrahim, F.; Esquivel, M.; Van Seventer, E.E.; et al. Minimal Residual Disease using a Plasma-Only Circulating Tumor DNA Assay to Predict Recurrence of Metastatic Colorectal Cancer Following Curative Intent Treatment. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2024, 30, 2964–2973. [Google Scholar] [CrossRef]
- Malla, M.; Loree, J.M.; Kasi, P.M.; Parikh, A.R. Using Circulating Tumor DNA in Colorectal Cancer: Current and Evolving Practices. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 2846–2857. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Kasi, A.K.; Parikh, A.R.; Mahipal, A. Finding Waldo: The Evolving Paradigm of Circulating Tumor DNA (ctDNA)-Guided Minimal Residual Disease (MRD) Assessment in Colorectal Cancer (CRC). Cancers 2022, 14, 3078. [Google Scholar] [CrossRef]
- Sogbe, M.; Aliseda, D.; Sangro, P.; de la Torre-Aláez, M.; Sangro, B.; Argemi, J. Prognostic value of circulating tumor DNA in different cancer types detected by ultra-low-pass whole-genome sequencing: A systematic review and patient-level survival data meta-analysis. Carcinogenesis 2025, 46, bgae073. [Google Scholar] [CrossRef] [PubMed]
- Gracie, L.; Pan, Y.; Atenafu, E.G.; Ward, D.G.; Teng, M.; Pallan, L.; Stevens, N.M.; Khoja, L. Circulating tumour DNA (ctDNA) in metastatic melanoma, a systematic review and meta-analysis. Eur. J. Cancer 2021, 158, 191–207. [Google Scholar] [CrossRef]
- Gandini, S.; Zanna, I.; De Angelis, S.P.; Cocorocchio, E.; Queirolo, P.; Lee, J.H.; Carlino, M.S.; Mazzarella, L.; Achutti Duso, B.; Palli, D.; et al. Circulating tumour DNA and melanoma survival: A systematic literature review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 157, 103187. [Google Scholar] [CrossRef]
- Fan, G.; Zhang, K.; Yang, X.; Ding, J.; Wang, Z.; Li, J. Prognostic value of circulating tumor DNA in patients with colon cancer: Systematic review. PLoS ONE 2017, 12, e0171991. [Google Scholar] [CrossRef]
- Ocaña, A.; Díez-González, L.; García-Olmo, D.C.; Templeton, A.J.; Vera-Badillo, F.; José Escribano, M.; Serrano-Heras, G.; Corrales-Sánchez, V.; Seruga, B.; Andrés-Pretel, F.; et al. Circulating DNA and Survival in Solid Tumors. Cancer Epidemiol. Biomark. Prev. 2016, 25, 399–406. [Google Scholar] [CrossRef]
- Yanala, U.R.; Martos, M.P.; Dickey, E.M.; Corona, A.M.; Ezenwajiaku, N.; Pizzolato, J.F.; Terrero, G.; Hester, C.A.; Merchant, N.B.; Datta, J.; et al. Utility of circulating tumor DNA (ctDNA) for the detection of minimal residual disease (MRD) after curative-intent therapy for patients with localized pancreatic adenocarcinoma (PDAC): A single institution series and meta-analysis. J. Clin. Oncol. 2024, 42 (Suppl. 3), 695. [Google Scholar] [CrossRef]
- Lee, B.; Lipton, L.; Cohen, J.; Tie, J.; Javed, A.A.; Li, L.; Goldstein, D.; Burge, M.; Cooray, P.; Nagrial, A.; et al. Circulating tumor DNA as a potential marker of adjuvant chemotherapy benefit following surgery for localized pancreatic cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1472–1478. [Google Scholar] [CrossRef]
- Lee, J.-S.; Rhee, T.-M.; Pietrasz, D.; Bachet, J.-B.; Laurent-Puig, P.; Kong, S.-Y.; Takai, E.; Yachida, S.; Shibata, T.; Lee, J.W.; et al. Circulating tumor DNA as a prognostic indicator in resectable pancreatic ductal adenocarcinoma: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 16971. [Google Scholar] [CrossRef]
- Vidal, L.; Pando, E.; Blanco, L.; Fabregat-Franco, C.; Castet, F.; Sierra, A.; Macarulla, T.; Balsells, J.; Charco, R.; Vivancos, A. Liquid biopsy after resection of pancreatic adenocarcinoma and its relation to oncological outcomes. Systematic review and meta-analysis. Cancer Treat. Rev. 2023, 120, 102604. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Mizuma, M.; Motoi, F.; Ohtsuka, H.; Nakagawa, K.; Morikawa, T.; Unno, M. Prognostic impact of postoperative circulating tumor DNA as a molecular minimal residual disease marker in patients with pancreatic cancer undergoing surgical resection. J. Hepato-Biliary-Pancreat. Sci. 2023, 30, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Z.-X.; Song, B. Role of imaging in evaluating the response after neoadjuvant treatment for pancreatic ductal adenocarcinoma. World J. Gastroenterol. 2021, 27, 3037–3049. [Google Scholar] [CrossRef] [PubMed]
- Soloff, E.V.; Al-Hawary, M.M.; Desser, T.S.; Fishman, E.K.; Minter, R.M.; Zins, M. Imaging Assessment of Pancreatic Cancer Resectability After Neoadjuvant Therapy: AJR Expert Panel Narrative Review. AJR Am. J. Roentgenol. 2022, 218, 570–581. [Google Scholar] [CrossRef]
- Panda, A.; Garg, I.; Truty, M.J.; Kline, T.L.; Johnson, M.P.; Ehman, E.C.; Suman, G.; Anaam, D.A.; Kemp, B.J.; Johnson, G.B.; et al. Borderline Resectable and Locally Advanced Pancreatic Cancer: FDG PET/MRI and CT Tumor Metrics for Assessment of Pathologic Response to Neoadjuvant Therapy and Prediction of Survival. AJR Am. J. Roentgenol. 2021, 217, 730–740. [Google Scholar] [CrossRef]
- Vitello, D.J.; Shah, D.; Wells, A.; Masnyk, L.; Cox, M.; Janczewski, L.M.; Abad, J.; Dawravoo, K.; D’Souza, A.; Suh, G.; et al. Mutant KRAS in Circulating Tumor DNA as a Biomarker in Localized Pancreatic Cancer in Patients Treated with Neoadjuvant Chemotherapy. Ann. Surg. 2024. [Google Scholar] [CrossRef]
- Kitahata, Y.; Kawai, M.; Hirono, S.; Okada, K.-I.; Miyazawa, M.; Motobayashi, H.; Ueno, M.; Hayami, S.; Miyamoto, A.; Yamaue, H. Circulating Tumor DNA as a Potential Prognostic Marker in Patients with Borderline-Resectable Pancreatic Cancer Undergoing Neoadjuvant Chemotherapy Followed by Pancreatectomy. Ann. Surg. Oncol. 2022, 29, 1596–1605. [Google Scholar] [CrossRef]
- Lyskjær, I.; Kronborg, C.S.; Rasmussen, M.H.; Sørensen, B.S.; Demuth, C.; Rosenkilde, M.; Johansen, A.F.B.; Knudsen, M.; Vang, S.; Krag, S.R.P.; et al. Correlation between early dynamics in circulating tumour DNA and outcome from FOLFIRI treatment in metastatic colorectal cancer. Sci. Rep. 2019, 9, 11542. [Google Scholar] [CrossRef]
- Kim, S.; Lim, Y.; Kang, J.-K.; Kim, H.-P.; Roh, H.; Kim, S.Y.; Lee, D.; Bang, D.; Jeong, S.-Y.; Park, K.J.; et al. Dynamic changes in longitudinal circulating tumour DNA profile during metastatic colorectal cancer treatment. Br. J. Cancer 2022, 127, 898–907. [Google Scholar] [CrossRef]
- Ghidini, M.; Hahne, J.C.; Senti, C.; Heide, T.; Proszek, P.Z.; Shaikh, R.; Carter, P.; Hubank, M.; Trevisani, F.; Garrone, O.; et al. Circulating Tumor DNA Dynamics and Clinical Outcome in Metastatic Colorectal Cancer Patients Undergoing Front-Line Chemotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2025, 31, 707–718. [Google Scholar] [CrossRef]
- Sugimori, M.; Sugimori, K.; Tsuchiya, H.; Suzuki, Y.; Tsuyuki, S.; Kaneta, Y.; Hirotani, A.; Sanga, K.; Tozuka, Y.; Komiyama, S.; et al. Quantitative monitoring of circulating tumor DNA in patients with advanced pancreatic cancer undergoing chemotherapy. Cancer Sci. 2020, 111, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Day, R.; Cocadiz, J.A.; Parackal, S.; Mitchell, W.; Black, M.A.; Lawrence, B.; Fitzgerald, S.; Print, C.; Jackson, C.; et al. Circulating tumor DNA is a sensitive marker for routine monitoring of treatment response in advanced colorectal cancer. Carcinogenesis 2020, 41, 1507–1517. [Google Scholar] [CrossRef]
- Gouda, M.A.; Huang, H.J.; Piha-Paul, S.A.; Call, S.G.; Karp, D.D.; Fu, S.; Naing, A.; Subbiah, V.; Pant, S.; Dustin, D.J.; et al. Longitudinal Monitoring of Circulating Tumor DNA to Predict Treatment Outcomes in Advanced Cancers. JCO Precis. Oncol. 2022, 6, e2100512. [Google Scholar] [CrossRef]
- Pellini, B.; Madison, R.W.; Childress, M.A.; Miller, S.T.; Gjoerup, O.; Cheng, J.; Huang, R.S.P.; Krainock, M.; Gupta, P.; Zou, W.; et al. Circulating Tumor DNA Monitoring on Chemo-immunotherapy for Risk Stratification in Advanced Non-Small Cell Lung Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2023, 29, 4596–4605. [Google Scholar] [CrossRef]
- Satoi, S.; Yamamoto, T.; Matsui, Y. Conversion surgery in patients with initially unresectable pancreatic ductal adenocarcinoma: Where do we stand in 2018? J. Pancreatol. 2018, 1, 25–29. [Google Scholar] [CrossRef]
- Botrus, G.; Kosirorek, H.; Sonbol, M.B.; Kusne, Y.; Uson Junior, P.L.S.; Borad, M.J.; Ahn, D.H.; Kasi, P.M.; Drusbosky, L.M.; Dada, H.; et al. Circulating Tumor DNA-Based Testing and Actionable Findings in Patients with Advanced and Metastatic Pancreatic Adenocarcinoma. Oncologist 2021, 26, 569–578. [Google Scholar] [CrossRef]
- Keane, F.; Saadat, L.V.; O’Connor, C.A.; Chou, J.F.; Bowman, A.S.; Xu, F.; Crowley, F.; Debnath, N.; Schoenfeld, J.D.; Singhal, A.; et al. Clinical utility and tissue concordance of circulating tumor DNA in pancreatic ductal adenocarcinoma. J. Natl. Cancer Inst. 2025, 117, djaf139. [Google Scholar] [CrossRef]
- Sivapalan, L.; Thorn, G.J.; Gadaleta, E.; Kocher, H.M.; Ross-Adams, H.; Chelala, C. Longitudinal profiling of circulating tumour DNA for tracking tumour dynamics in pancreatic cancer. BMC Cancer 2022, 22, 369. [Google Scholar] [CrossRef]
- Sivapalan, L.; Kocher, H.M.; Ross-Adams, H.; Chelala, C. Molecular profiling of ctDNA in pancreatic cancer: Opportunities and challenges for clinical application. Pancreatol. Off. J. Int. Assoc. Pancreatol. IAP Al 2021, 21, 363–378. [Google Scholar] [CrossRef]
- Hwang, S.; Woo, S.; Kang, B.; Kang, H.; Kim, J.S.; Lee, S.H.; Kwon, C.I.; Kyung, D.S.; Kim, H.-P.; Kim, G.; et al. Concordance of ctDNA and tissue genomic profiling in advanced biliary tract cancer. J. Hepatol. 2025, 82, 649–657. [Google Scholar] [CrossRef]
- Awosika, J.A.; Monge, C.; Greten, T.F. Integration of circulating tumor DNA in biliary tract cancer: The emerging landscape. Hepatic Oncol. 2024, 11, 2403334. [Google Scholar] [CrossRef]
- Labiano, I.; Huerta, A.E.; Alsina, M.; Arasanz, H.; Castro, N.; Mendaza, S.; Lecumberri, A.; Gonzalez-Borja, I.; Guerrero-Setas, D.; Patiño-Garcia, A.; et al. Building on the clinical applicability of ctDNA analysis in non-metastatic pancreatic ductal adenocarcinoma. Sci. Rep. 2024, 14, 16203. [Google Scholar] [CrossRef]
- Bayle, A.; Belcaid, L.; Aldea, M.; Vasseur, D.; Peyraud, F.; Nicotra, C.; Geraud, A.; Sakkal, M.; Seknazi, L.; Cerbone, L.; et al. Clinical utility of circulating tumor DNA sequencing with a large panel: A National Center for Precision Medicine (PRISM) study. Ann. Oncol. 2023, 34, 389–396. [Google Scholar] [CrossRef]
- Takai, E.; Totoki, Y.; Nakamura, H.; Kato, M.; Shibata, T.; Yachida, S. Clinical Utility of Circulating Tumor DNA for Molecular Assessment and Precision Medicine in Pancreatic Cancer. In Circulating Nucleic Acids in Serum and Plasma–CNAPS IX; Gahan, P.B., Fleischhacker, M., Schmidt, B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 13–17. [Google Scholar] [CrossRef]
- Andersen, L.; Kisistók, J.; Henriksen, T.V.; Bramsen, J.B.; Reinert, T.; Øgaard, N.; Mattesen, T.B.; Birkbak, N.J.; Andersen, C.L. Exploring the biology of ctDNA release in colorectal cancer. Eur. J. Cancer Oxf. Engl. 1990 2024, 207, 114186. [Google Scholar] [CrossRef]
- Bredno, J.; Lipson, J.; Venn, O.; Aravanis, A.M.; Jamshidi, A. Clinical correlates of circulating cell-free DNA tumor fraction. PLoS ONE 2021, 16, e0256436. [Google Scholar] [CrossRef]
- Cho, M.-S.; Park, C.H.; Lee, S.; Park, H.S. Clinicopathological parameters for circulating tumor DNA shedding in surgically resected non-small cell lung cancer with EGFR or KRAS mutation. PLoS ONE 2020, 15, e0230622. [Google Scholar] [CrossRef]
- Koudahl Conrad, J.B.; Vesterman Henriksen, T.; Berg Nors, J.; Heilskov Rasmussen, M.; Worm Ørntoft, M.-B.; Hallundbæk Schlesinger, N.; Vadgaard Andersen, P.; Andersson Gotschalck, K.; Andersen, C.L. The role of renal and liver function in clinical ctDNA testing. PLoS ONE 2025, 20, e0319194. [Google Scholar] [CrossRef]
- Pommergaard, H.C.; Yde, C.W.; Ahlborn, L.B.; Andersen, C.L.; Henriksen, T.V.; Hasselby, J.P.; Rostved, A.A.; Sørensen, C.L.; Rohrberg, K.S.; Nielsen, F.C.; et al. Personalized circulating tumor DNA in patients with hepatocellular carcinoma: A pilot study. Mol. Biol. Rep. 2022, 49, 1609–1616. [Google Scholar] [CrossRef]
- Yu, L.; Lopez, G.; Rassa, J.; Wang, Y.; Basavanhally, T.; Browne, A.; Huang, C.-P.; Dorsey, L.; Jen, J.; Hersey, S. Direct comparison of circulating tumor DNA sequencing assays with targeted large gene panels. PLoS ONE 2022, 17, e0266889. [Google Scholar] [CrossRef]
- Deveson, I.W.; Gong, B.; Lai, K.; LoCoco, J.S.; Richmond, T.A.; Schageman, J.; Zhang, Z.; Novoradovskaya, N.; Willey, J.C.; Jones, W.; et al. Evaluating the analytical validity of circulating tumor DNA sequencing assays for precision oncology. Nat. Biotechnol. 2021, 39, 1115–1128. [Google Scholar] [CrossRef]
- Boscolo Bielo, L.; Trapani, D.; Repetto, M.; Crimini, E.; Valenza, C.; Belli, C.; Criscitiello, C.; Marra, A.; Subbiah, V.; Curigliano, G. Variant allele frequency: A decision-making tool in precision oncology? Trends Cancer 2023, 9, 1058–1068. [Google Scholar] [CrossRef]
- Dang, D.K.; Park, B.H. Circulating tumor DNA: Current challenges for clinical utility. J. Clin. Investig. 2022, 132, e154941. [Google Scholar] [CrossRef]
- Douglas, M.P.; Ragavan, M.V.; Chen, C.; Kumar, A.; Gray, S.W.; Blakely, C.M.; Phillips, K.A. Private Payer and Medicare Coverage Policies for Use of Circulating Tumor DNA Tests in Cancer Diagnostics and Treatment. J. Natl. Compr. Cancer Netw. 2023, 21, 609–616.e4. Available online: https://jnccn.org/view/journals/jnccn/21/6/article-p609.xml (accessed on 17 June 2025). [CrossRef]
- Duffy, M.J.; Crown, J. Circulating Tumor DNA as a Biomarker for Monitoring Patients with Solid Cancers: Comparison with Standard Protein Biomarkers. Clin. Chem. 2022, 68, 1381–1390. [Google Scholar] [CrossRef]
- Arisi, M.F.; Dotan, E.; Fernandez, S.V. Circulating Tumor DNA in Precision Oncology and Its Applications in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 4441. [Google Scholar] [CrossRef]
- Corcoran, R.B.; Chabner, B.A. Application of Cell-free DNA Analysis to Cancer Treatment. N. Engl. J. Med. 2018, 379, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, P.A.; Wind, T.T.; van Kruchten, M.; Schuuring, E.; Hospers, G.A.P.; van der Wekken, A.J.; de Groot, D.-J.; Schröder, C.P.; Fehrmann, R.S.N.; Reyners, A.K.L. Clinical utility of circulating tumor DNA as a response and follow-up marker in cancer therapy. Cancer Metastasis Rev. 2020, 39, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Rajdev, L.; King, G.G.; Lieu, C.H.; Cohen, S.A.; Pant, S.; Uboha, N.V.; Deming, D.; Malla, M.; Kasi, A.; Klute, K.; et al. Incorporating Circulating Tumor DNA Testing into Clinical Trials: A Position Paper by the National Cancer Institute GI Oncology Circulating Tumor DNA Working Group. JCO Precis. Oncol. 2025, 9, e2400489. [Google Scholar] [CrossRef]
- Antolino, L. Mutation of K-RAS, CDKN2A, SMAD4 and TP53 in Pancreatic Cancer: Role of Liquid Biopsy in Preoperative Diagnosis. Clinicaltrials.Gov. 2019. Available online: https://clinicaltrials.gov/study/NCT03524677 (accessed on 17 June 2025).
- Westphalen, B. Prognostic Role of Circulating Tumor DNA in Resectable Pancreatic Cancer. Clinicaltrials.Gov. 2025. Available online: https://clinicaltrials.gov/study/NCT04246203 (accessed on 17 June 2025).
- Grunvald, M.W.; Jacobson, R.A.; Kuzel, T.M.; Pappas, S.G.; Masood, A. Current Status of Circulating Tumor DNA Liquid Biopsy in Pancreatic Cancer. Int. J. Mol. Sci. 2020, 21, 7651. [Google Scholar] [CrossRef]
- National Cancer Institute (NCI) APOLLO: A Randomized Phase II Double-Blind Study of Olaparib Versus Placebo Following Curative Intent Therapy in Patients with Resected Pancreatic Cancer and a Pathogenic BRCA1, BRCA2 or PALB2 Mutation; clinicaltrials.gov. 2025. Available online: https://clinicaltrials.gov/study/NCT04858334 (accessed on 17 June 2025).
- University Hospital, Essen Identification of Novel Inflammation-related Biomarkers for Early Detection of Anthracycline-induced Cardiotoxicity in Breast Cancer Patients; clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/study/NCT05298072 (accessed on 17 June 2025).
| Biomarker | Disease Stage | Sensitivity (%) | Specificity (%) | Notes and References |
|---|---|---|---|---|
| CA 19-9 | All stages | 72–80 | 86–93 | Best validated; sensitivity drops in Lewis antigen-negative patients; lower specificity in benign disease [26,27,28] |
| CA 19-9 | Pre-diagnosis | 50–64 | 99 | Increases in CA19-9 up to two years to diagnosis; 64% sensitivity at 99% specificity to differentiate resectable PDAC from healthy controls; Sensitivity dropped in patients with benign pancreatic disease when specificity was kept at 99% [29,30] |
| ctDNA (KRAS mutations) | All stages | 48–65 | 75–94 | Sensitivity lower than CA19-9; specificity high; performance improves with stage [7,22,26,31] |
| ctDNA (KRAS mutations) | Stages I and II | 48–50 | 94 | Sensitivity limited in early stage; specificity remains high [7,31] |
| ctDNA | Locally advanced and metastatic | >75–94 | 94–99 | Sensitivity increases with tumor burden; high specificity [7,20,22] |
| CA19-9 + ctDNA | All stages | 78 | 91 | Combination improves diagnostic accuracy over either alone [26,31,32] |
| Study | Type of Study | Patient Number | Timing of MRD Monitoring | Biomarkers Measured | Key Findings |
|---|---|---|---|---|---|
| Groot VP et al. [38] | Prospective single institution cohort study | 59 | Preop, immediate postop, serial follow-up | ctDNA (KRAS mutations) | Postop ctDNA positivity predicts recurrence with median lead time of 84 days, ctDNA levels dropped significantly after resection. |
| Yanala UR et al. [64] | Single institution case series and meta-analysis | 171 | End-of-treatment (after completion of all curative-intent surgery and chemotherapy), surveillance | ctDNA (tumor-informed NGS), CA19-9 | End of treatment ctDNA positivity has high specificity and positive predictive value for recurrence and is associated with significantly worse recurrence free survival. |
| Lee B et al. [65] | Prospective multicenter biomarker trial | 81 | Preop, postop (timing not specified) | ctDNA (KRAS mutations), CA19-9 | Postop ctDNA positivity is associated with 100% recurrence and poor OS, even with adjuvant chemotherapy. |
| Botta GP et al. [24] | Retrospective real-world data analysis | 298 | Periop (2–12 weeks postop), surveillance (>12 weeks postop or postadjuvant) | ctDNA (tumor informed NGS) | Positive ctDNA detection is significantly associated with shorter disease-free survival. Surveillance ctDNA is the most significant prognostic factor for recurrence. |
| Lee JS et al. [66] | Meta-analysis | 375 | Preop, postop (timing not specified) | ctDNA | Positive ctDNA is associated with poor overall survival (HR 3.66) and is also associated with a higher recurrence risk. |
| Vidal L et al. [67] | Meta-analysis | 413 | Preop, postop (timing not specified) | ctDNA, CTCs, mRNA | Perioperative ctDNA positivity is associated with worse prognosis. Surgical resection increases ctDNA clearance. |
| Hata T et al. [68] | Prospective single institution cohort study | 66 | Preop, immediate postop | ctDNA (KRAS mutations by droplet PCR) | Detectable postop ctDNA were more likely to develop hepatic recurrence. Preoperative ctDNA did not affect long term outcomes. Postoperative ctDNA has an independent recurrence risk. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojha, S.; Sessions, W.; Zhou, Y.; Aung, K.L. ctDNA in Pancreatic Adenocarcinoma: A Critical Appraisal. Curr. Oncol. 2025, 32, 589. https://doi.org/10.3390/curroncol32110589
Ojha S, Sessions W, Zhou Y, Aung KL. ctDNA in Pancreatic Adenocarcinoma: A Critical Appraisal. Current Oncology. 2025; 32(11):589. https://doi.org/10.3390/curroncol32110589
Chicago/Turabian StyleOjha, Sujata, William Sessions, Yuhang Zhou, and Kyaw L. Aung. 2025. "ctDNA in Pancreatic Adenocarcinoma: A Critical Appraisal" Current Oncology 32, no. 11: 589. https://doi.org/10.3390/curroncol32110589
APA StyleOjha, S., Sessions, W., Zhou, Y., & Aung, K. L. (2025). ctDNA in Pancreatic Adenocarcinoma: A Critical Appraisal. Current Oncology, 32(11), 589. https://doi.org/10.3390/curroncol32110589




