Dynamic Monitoring of Recurrent Ovarian Cancer Using Serial ctDNA: A Real-World Case Series
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
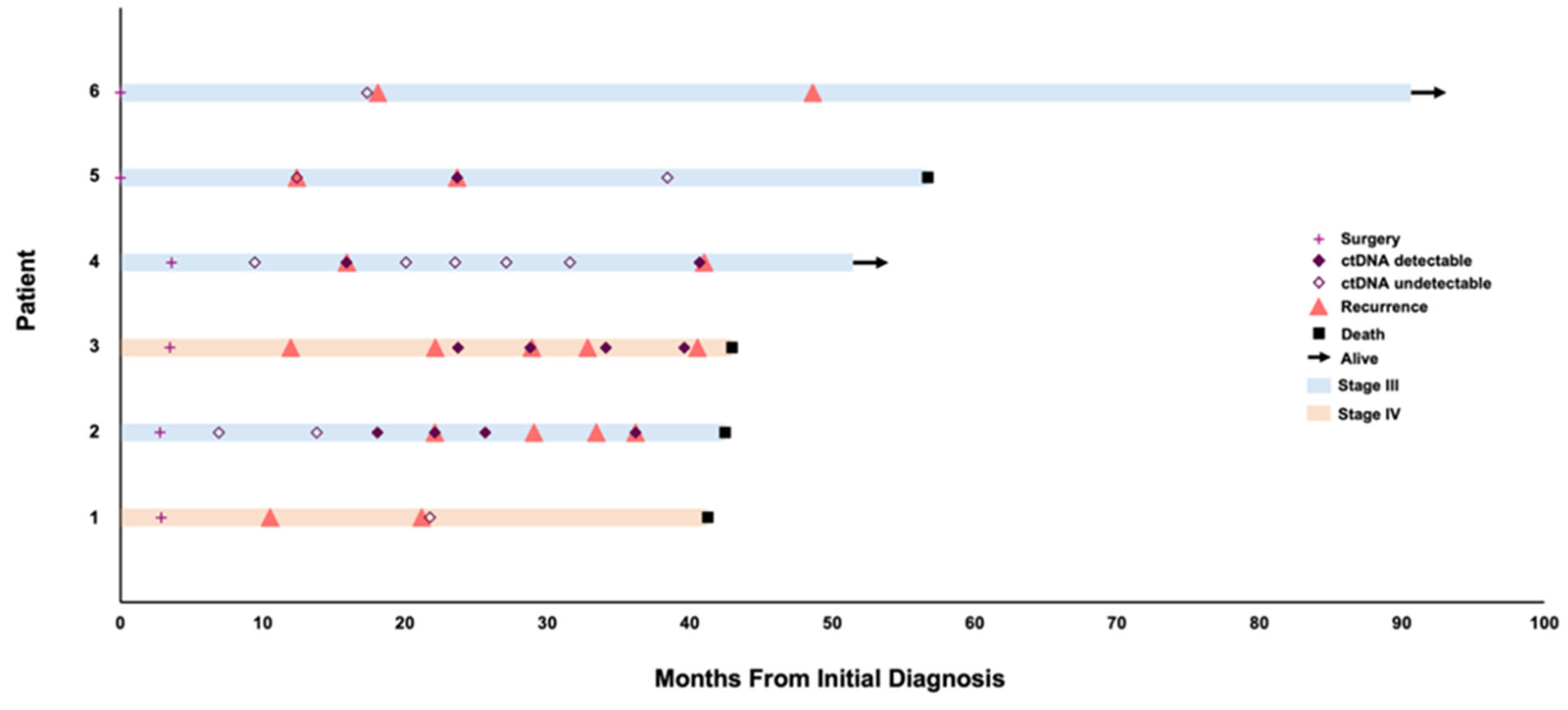
3. Results
3.1. Patient 1
3.2. Patient 2
3.3. Patient 3
3.4. Patient 4
3.5. Patient 5
3.6. Patient 6
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ctDNA | Circulating tumor DNA |
| OC | Ovarian Cancer |
| HGSC | High grade Serous Ovarian Cancer |
References
- Sant, M.; Bernat-Peguera, A.; Felip, E.; Margelí, M. Role of ctDNA in Breast Cancer. Cancers 2022, 14, 310. [Google Scholar] [CrossRef]
- Puccini, A.; Martelli, V.; Pastorino, A.; Sciallero, S.; Sobrero, A. ctDNA to Guide Treatment of Colorectal Cancer: Ready for Standard of Care? Curr. Treat. Options Oncol. 2023, 24, 76–92. [Google Scholar] [CrossRef]
- Gracie, L.; Pan, Y.; Atenafu, E.G.; Ward, D.G.; Teng, M.; Pallan, L.; Stevens, N.M.; Khoja, L. Circulating tumour DNA (ctDNA) in metastatic melanoma, a systematic review and meta-analysis. Eur. J. Cancer 2021, 158, 191–207. [Google Scholar] [CrossRef]
- Li, S.; Noor, Z.S.; Zeng, W.; Stackpole, M.L.; Ni, X.; Zhou, Y.; Yuan, Z.; Wong, W.H.; Agopian, V.G.; Dubinett, S.M.; et al. Sensitive detection of tumor mutations from blood and its application to immunotherapy prognosis. Nat. Commun. 2021, 12, 4172. [Google Scholar] [CrossRef]
- Verschoor, N.; Bos, M.K.; Oomen-de Hoop, E.; Martens, J.W.; Sleijfer, S.; Jager, A.; Beije, N. A review of trials investigating ctDNA-guided adjuvant treatment of solid tumors: The importance of trial design. Eur. J. Cancer. 2024, 207, 114159. [Google Scholar] [CrossRef]
- Moding, E.J.; Nabet, B.Y.; Alizadeh, A.A.; Diehn, M. Detecting Liquid Remnants of Solid Tumors: Circulating Tumor DNA Minimal Residual Disease. Cancer Discov. 2021, 11, 2968–2986. [Google Scholar] [CrossRef]
- Magbanua, M.J.M.; Brown Swigart, L.; Ahmed, Z.; Sayaman, R.W.; Renner, D.; Kalashnikova, E.; Hirst, G.L.; Yau, C.; Wolf, D.M.; Li, W.; et al. Clinical significance and biology of circulating tumor DNA in high-risk early-stage HER2-negative breast cancer receiving neoadjuvant chemotherapy. Cancer Cell 2023, 41, 1091–1102.e4. [Google Scholar] [CrossRef]
- Provencio, M.; Serna-Blasco, R.; Franco, F.; Calvo, V.; Royuela, A.; Auglytė, M.; Sánchez-Hernández, A.; Campayo, M.d.J.; García-Girón, C.; Dómine, M.; et al. Analysis of circulating tumour DNA to identify patients with epidermal growth factor receptor-positive non-small cell lung cancer who might benefit from sequential tyrosine kinase inhibitor treatment. Eur. J. Cancer 2021, 149, 61–72. [Google Scholar] [CrossRef]
- Garlan, F.; Laurent-Puig, P.; Sefrioui, D.; Siauve, N.; Didelot, A.; Sarafan-Vasseur, N.; Michel, P.; Perkins, G.; Mulot, C.; Blons, H.; et al. Early Evaluation of Circulating Tumor DNA as Marker of Therapeutic Efficacy in Metastatic Colorectal Cancer Patients (PLACOL Study). Clin. Cancer Res. 2017, 23, 5416–5425. [Google Scholar] [CrossRef]
- Kuo, A.J.; Paulson, V.A.; Hempelmann, J.A.; Beightol, M.; Todhunter, S.; Colbert, B.G.; Salipante, S.J.; Konnick, E.Q.; Pritchard, C.C.; Lockwood, C.M. Validation and implementation of a modular targeted capture assay for the detection of clinically significant molecular oncology alterations. Pract. Lab. Med. 2020, 19, e00153. [Google Scholar] [CrossRef]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.M.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A.; et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 2017, 19, 4–23. [Google Scholar] [CrossRef]
- Parkinson, C.A.; Gale, D.; Piskorz, A.M.; Biggs, H.; Hodgkin, C.; Addley, H.; Freeman, S.; Moyle, P.; Sala, E.; Sayal, K.; et al. Exploratory Analysis of TP53 Mutations in Circulating Tumour DNA as Biomarkers of Treatment Response for Patients with Relapsed High-Grade Serous Ovarian Carcinoma: A Retrospective Study. PLoS Med. 2016, 13, e1002198. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, S.W.; Lee, Y.J.; Lee, H.Y.; Lee, J.E.; Choi, E.K. Prospective study of the efficacy and utility of TP53 mutations in circulating tumor DNA as a non-invasive biomarker of treatment response monitoring in patients with high-grade serous ovarian carcinoma. J. Gynecol. Oncol. 2019, 30, e32. [Google Scholar] [CrossRef]
- Alves, M.C.; Fonseca, F.L.A.; Yamada, A.M.T.D.; Barros, L.A.D.R.; Lopes, A.; Silva, L.C.F.F.; Luz, A.S.; Cruz, F.J.S.M.; Del Giglio, A. Increased circulating tumor DNA as a noninvasive biomarker of early treatment response in patients with metastatic ovarian carcinoma: A pilot study. Tumor Biol. 2020, 42, 1010428320919198. [Google Scholar] [CrossRef]
- Rustin, G.J.S.; van der Burg, M.E.L.; Griffin, C.L.; Guthrie, D.; Lamont, A.; Jayson, G.C.; Kristensen, G.; Mediola, C.; Coens, C.; Qian, W.; et al. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): A randomised trial. Lancet 2010, 376, 1155–1163. [Google Scholar] [CrossRef]
- Tie, J.; Wang, Y.; Tomasetti, C.; Li, L.; Springer, S.; Kinde, I.; Silliman, N.; Tacey, M.; Wong, H.-L.; Christie, M.; et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci. Transl. Med. 2016, 8, 346ra92. [Google Scholar] [CrossRef]
- Reinert, T.; Henriksen, T.V.; Christensen, E.; Sharma, S.; Salari, R.; Sethi, H.; Knudsen, M.; Nordentoft, I.K.; Wu, H.-T.; Tin, A.S.; et al. Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients With Stages I to III Colorectal Cancer. JAMA Oncol. 2019, 5, 1124–1131. [Google Scholar] [CrossRef]
- Tran, H.T.; Heeke, S.; Sujit, S.; Vokes, N.; Zhang, J.; Aminu, M.; Lam, V.; Vaporciyan, A.; Swisher, S.; Godoy, M.; et al. Circulating tumor DNA and radiological tumor volume identify patients at risk for relapse with resected, early-stage non-small-cell lung cancer. Ann. Oncol. 2024, 35, 183–189. [Google Scholar] [CrossRef]
- Qiu, B.; Guo, W.; Zhang, F.; Lv, F.; Ji, Y.; Peng, Y.; Chen, X.; Bao, H.; Xu, Y.; Shao, Y.; et al. Dynamic recurrence risk and adjuvant chemotherapy benefit prediction by ctDNA in resected NSCLC. Nat. Commun. 2021, 12, 6770. [Google Scholar] [CrossRef]
- Magbanua, M.J.M.; Swigart, L.B.; Wu, H.T.; Hirst, G.; Yau, C.; Wolf, D.; Tin, A.; Salari, R.; Shchegrova, S.; Pawar, H.; et al. Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival. Ann. Oncol. 2021, 32, 229–239. [Google Scholar] [CrossRef]
- Zhou, Q.; Gampenrieder, S.P.; Frantal, S.; Rinnerthaler, G.; Singer, C.F.; Egle, D.; Pfeiler, G.; Bartsch, R.; Wette, V.; Pichler, A.; et al. Persistence of ctDNA in Patients with Breast Cancer During Neoadjuvant Treatment Is a Significant Predictor of Poor Tumor Response. Clin. Cancer Res. 2022, 28, 697–707. [Google Scholar] [CrossRef]
- Tie, J.; Cohen, J.D.; Lahouel, K.; Lo, S.N.; Wang, Y.; Kosmider, S.; Wong, R.; Shapiro, J.; Lee, M.; Harris, S.; et al. Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer. N. Engl. J. Med. 2022, 386, 2261–2272. [Google Scholar] [CrossRef]
- Kotani, D.; Oki, E.; Nakamura, Y.; Yukami, H.; Mishima, S.; Bando, H.; Shirasu, H.; Yamazaki, K.; Watanabe, J.; Kotaka, M.; et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat. Med. 2023, 29, 127–134. [Google Scholar] [CrossRef]
- Tie, J.; Wang, Y.; Lo, S.N.; Lahouel, K.; Cohen, J.D.; Wong, R.; Shapiro, J.D.; Harris, S.J.; Khattak, A.; Burge, M.E.; et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer: 5-year outcomes of the randomized DYNAMIC trial. Nat. Med. 2025, 31, 1509–1518. [Google Scholar] [CrossRef]
- You, B.; Robelin, P.; Tod, M.; Louvet, C.; Lotz, J.-P.; Abadie-Lacourtoisie, S.; Fabbro, M.; Desauw, C.; Bonichon-Lamichhane, N.; Kurtz, J.-E.; et al. CA-125 ELIMination Rate Constant K (KELIM) Is a Marker of Chemosensitivity in Patients with Ovarian Cancer: Results from the Phase II CHIVA Trial. Clin. Cancer Res. 2020, 26, 4625–4632. [Google Scholar] [CrossRef]
- Piedimonte, S.; Kim, R.; Bernardini, M.Q.; Atenafu, E.G.; Clark, M.; Lheureux, S.; May, T. Validation of the KELIM score as a predictor of response to neoadjuvant treatment in patients with advanced high grade serous ovarian cancer. Gynecol. Oncol. 2022, 167, 417–422. [Google Scholar] [CrossRef]

| Case | Variant Detected in ctDNA |
|---|---|
| 1 | N/A |
| 2 | TP53 p.A161T |
| 3 | TP53 p.R337C |
| 4 | ATM p.L1611I |
| 5 | TP53 p.V147Lfs*23 * |
| 6 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rios-Doria, E.; Reichel, J.B.; Radke, M.R.; Manhardt, E.; Rubin-Saika, M.; Lockwood, C.; Swisher, E.M.; Banda, K. Dynamic Monitoring of Recurrent Ovarian Cancer Using Serial ctDNA: A Real-World Case Series. Curr. Oncol. 2025, 32, 585. https://doi.org/10.3390/curroncol32100585
Rios-Doria E, Reichel JB, Radke MR, Manhardt E, Rubin-Saika M, Lockwood C, Swisher EM, Banda K. Dynamic Monitoring of Recurrent Ovarian Cancer Using Serial ctDNA: A Real-World Case Series. Current Oncology. 2025; 32(10):585. https://doi.org/10.3390/curroncol32100585
Chicago/Turabian StyleRios-Doria, Eric, Jonathan B. Reichel, Marc R. Radke, Enna Manhardt, Mayumi Rubin-Saika, Christina Lockwood, Elizabeth M. Swisher, and Kalyan Banda. 2025. "Dynamic Monitoring of Recurrent Ovarian Cancer Using Serial ctDNA: A Real-World Case Series" Current Oncology 32, no. 10: 585. https://doi.org/10.3390/curroncol32100585
APA StyleRios-Doria, E., Reichel, J. B., Radke, M. R., Manhardt, E., Rubin-Saika, M., Lockwood, C., Swisher, E. M., & Banda, K. (2025). Dynamic Monitoring of Recurrent Ovarian Cancer Using Serial ctDNA: A Real-World Case Series. Current Oncology, 32(10), 585. https://doi.org/10.3390/curroncol32100585





