Molecular Classification Guides Fertility-Sparing Treatment for Endometrial Cancer and Atypical Hyperplasia Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Fertility-Sparing Treatment
2.3. Follow-Up and Evaluation of Treatment Efficacy
2.4. Molecular Classification Procedure
2.5. Post-Treatment Management
2.6. Statistical Analysis
3. Results
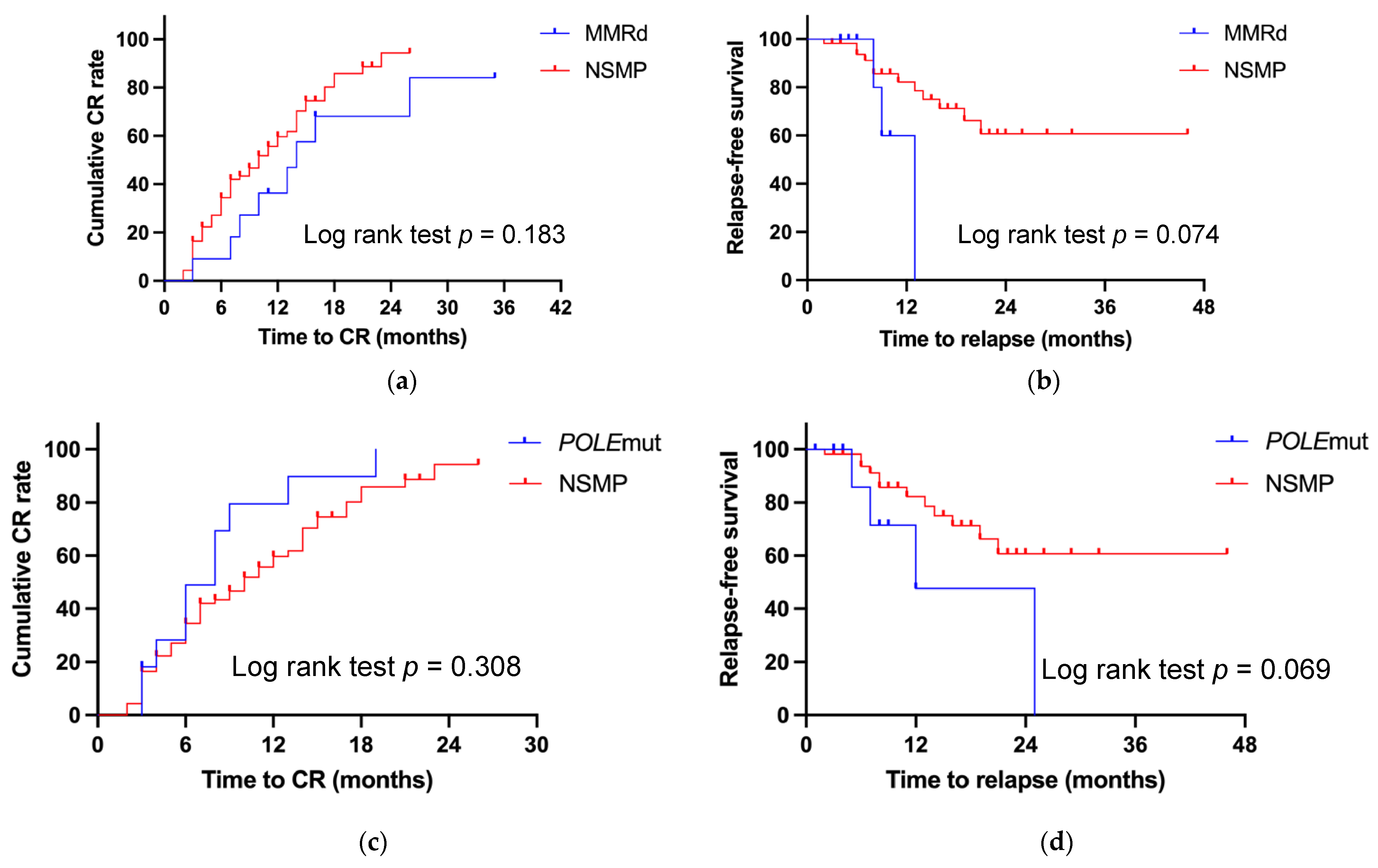
3.1. Outcomes for Patients with MMRd
3.2. Outcomes for Patients with POLEmut
3.3. Outcomes for Patients with p53abn
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, J.Y.; Nam, J.H. Progestins in the Fertility-Sparing Treatment and Retreatment of Patients With Primary and Recurrent Endometrial Cancer. Oncologist 2015, 20, 270–278. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 1, 16–41. [Google Scholar] [CrossRef]
- Greenwald, Z.R.; Huang, L.N.; Wissing, M.D.; Franco, E.L.; Gotlieb, W.H. Does hormonal therapy for fertility preservation affect the survival of young women with early-stage endometrial cancer? Cancer 2017, 9, 1545–1554. [Google Scholar] [CrossRef]
- Baxter, E.; Brennan, D.J.; McAlpine, J.N.; Mueller, J.J.; Amant, F.; van Gent, M.D.J.M.; Huntsman, D.G.; Coleman, R.L.; Westin, S.N.; Yates, M.S.; et al. Improving response to progestin treatment of low-grade endometrial cancer. Int. J. Gynecol. Cancer 2020, 30, 1811–1823. [Google Scholar] [CrossRef]
- Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; Benz, C.C.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 7447, 67–73. [Google Scholar] [CrossRef]
- Concin, N.; Creutzberg, C.L.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.A.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ES-GO/ESTRO/ESP Guidelines for the management of patients with endometrial carcinoma. Virchows Arch. 2021, 2, 153–190. [Google Scholar] [CrossRef]
- Dagher, C.; Manning-Geist, B.; Ellenson, L.H.; Weigelt, B.; Rios-Doria, E.; Barry, D.; Abu-Rustum, N.R.; Leitao, M.M., Jr.; Mueller, J.J. Mo-lecular subtyping in endometrial cancer: A promising strategy to guide fertility preservation. Gynecol. Oncol. 2023, 180–187. [Google Scholar] [CrossRef]
- Chung, Y.S.; Woo, H.Y.; Lee, J.-Y.; Park, E.; Nam, E.J.; Kim, S.; Kim, S.W.; Kim, Y.T. Mismatch repair status influences response to fertility-sparing treatment of endometrial cancer. Am. J. Obstet. Gynecol. 2021, 224, 370.e1–370.e13. [Google Scholar] [CrossRef]
- Raffone, A.; Catena, U.; Travaglino, A.; Masciullo, V.; Spadola, S.; Della Corte, L.; Piermattei, A.; Insabato, L.; Zannoni, G.F.; Scambia, G.; et al. Mismatch repair-deficiency specifically predicts recurrence of atypical endometrial hyperplasia and early endometrial carcinoma after conservative treatment: A multi-center study. Gynecol. Oncol. 2021, 161, 795–801. [Google Scholar] [CrossRef]
- Ran, X.; Hu, T.; Li, Z. Molecular Classification in Patients with Endometrial Cancer After Fertility-Preserving Treatment: Application of ProMisE Classifier and Combination of Prognostic Evidence. Front. Oncol. 2022, 12, 810631. [Google Scholar] [CrossRef] [PubMed]
- Britton, H.; Huang, L.; Lum, A.; Leung, S.; Shum, K.; Kale, M.; Burleigh, A.; Senz, J.; Yang, W.; McConechy, M.; et al. Molecular classification defines outcomes and opportunities in young women with endometrial carcinoma. Gynecol. Oncol. 2019, 153, 487–495. [Google Scholar] [CrossRef]
- Rodolakis, A.; Scambia, G.; Planchamp, F.; Acien, M.; Di Spiezio Sardo, A.; Farrugia, M.; Grynberg, M.; Pakiz, M.; Pavlakis, K.; Vermeulen, N.; et al. ESGO/ESHRE/ESGE Guidelines for the fertility-sparing treatment of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2023, 2, 208–222. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, M.; Zhang, L.; Wang, T.; Wang, B.; Xue, Y.; Xu, Z.; Shao, W.; Chen, X.; Wang, C. Outcomes of fertility preservation treatments in patients with endometrial cancer with different molecular classifications based on an NGS panel. Front. Oncol. 2023, 13, 1282356. [Google Scholar] [CrossRef]
- Zhou, R.; Lu, Q.; Liu, G.; Wang, Y.; Wang, J. Chinese expert consensus on fertility-preserving treatment for young women with early stage well differentiated endometrial cancer. Gynecol. Obstet. Clin. Med. 2021, 1, 49–53. [Google Scholar] [CrossRef]
- Höhn, A.K.; Brambs, C.E.; Hiller, G.G.R.; May, D.; Schmoeckel, E.; Horn, L.-C. 2020 WHO Classification of Female Genital Tumors. Geburtshilfe Und Frauenheilkd. 2021, 81, 1145–1153. [Google Scholar] [CrossRef]
- Rao, Q.; Liao, J.; Li, Y.; Zhang, X.; Xu, G.; Zhu, C.; Tian, S.; Chen, Q.; Zhou, H.; Zhang, B. Application of NGS molecular classification in the diagnosis of endometrial carcinoma: A supplement to traditional pathological diagnosis. Cancer Med. 2022, 12, 5409–5419. [Google Scholar] [CrossRef]
- León-Castillo, A.; Britton, H.; McConechy, M.K.; McAlpine, J.N.; Nout, R.; Kommoss, S.; Brucker, S.Y.; Carlson, J.W.; Epstein, E.; Rau, T.T.; et al. Interpretation of somatic POLE mutations in endometrial carcinoma. J. Pathol. 2019, 250, 323–335. [Google Scholar] [CrossRef]
- Raffone, A.; Travaglino, A.; Gabrielli, O.; Micheli, M.; Zuccalà, V.; Bitonti, G.; Camastra, C.; Gargiulo, V.; Insabato, L.; Zullo, F. Clinical features of ProMisE groups identify different phenotypes of patients with endometrial cancer. Arch. Gynecol. Obstet. 2021, 303, 1393–1400. [Google Scholar] [CrossRef]
- Vermij, L.; Jobsen, J.J.; León-Castillo, A.; Brinkhuis, M.; Roothaan, S.; Powell, M.E.; de Boer, S.M.; Khaw, P.; Mileshkin, L.R.; Fyles, A.; et al. Prognostic refinement of NSMP high-risk endometrial cancers using oestrogen receptor immunohistochemistry. Br. J. Cancer 2023, 128, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.; Huvila, J.; Chiu, D.; Thompson, E.F.; Scott, S.; Salvador, S.; Vicus, D.; Helpman, L.; Gotlieb, W.; Kean, S.; et al. Grade and Estrogen Receptor Expression Identify a Subset of No Specific Molecular Profile Endometrial Carcinomas at a Very Low Risk of Disease-Specific Death. Mod. Pathol. 2023, 36, 100085. [Google Scholar] [CrossRef] [PubMed]
- Kurnit, K.C.; Kim, G.N.; Fellman, B.M.; Urbauer, D.L.; Mills, G.B.; Zhang, W.; Broaddus, R.R. CTNNB1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod. Pathol. 2017, 30, 1032–1041. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, N.; Li, L.; Wang, Z.; Zhou, R.; Shen, D.; Wang, J. Characteristics of molecular classification in 52 endometrial cancer and atypical hyperplasia patients receiving fertility-sparing treatment. Gynecol. Obstet. Clin. Med. 2023, 3, 38–43. [Google Scholar] [CrossRef]
- Zakhour, M.; Cohen, J.G.; Gibson, A.; Walts, A.E.; Karimian, B.; Baltayan, A.; Aoyama, C.; Garcia, L.; Dhaliwal, S.K.; Elashoff, D.; et al. Ab-normal mismatch repair and other clinicopathologic predictors of poor response to progestin treatment in young women with en-dometrial complex atypical hyperplasia and well-differentiated endometrial adenocarcinoma: A consecutive case series. Bjog 2017, 10, 1576–1583. [Google Scholar] [CrossRef]
- Peng, H.; Jiang, J.; Li, L.; Hao, Z.; Lian, H.; Du, H.; Wang, W. Mismatch repair deficiency and abnormal p53 expression has significant predictive value for progesterone resistance and endometrial tumorigenesis in patients with endometrial atypical hyperplasia receiving fertility-preserving treatment. Gynecol. Oncol. 2024, 186, 154–160. [Google Scholar] [CrossRef]
- Puechl, A.M.; Spinosa, D.; Berchuck, A.; Secord, A.A.; Drury, K.E.; Broadwater, G.; Wong, J.; Whitaker, R.; Devos, N.; Corcoran, D.L.; et al. Molecular Classification to Prognosticate Response in Medically Managed Endometrial Cancers and Endometrial Intraepithelial Neoplasia. Cancers 2021, 13, 2847. [Google Scholar] [CrossRef]
- Talhouk, A.; McAlpine, J.N. New classification of endometrial cancers: The development and potential applications of genomic-based classification in research and clinical care. Gynecol. Oncol. Res. Pract. 2016, 3, 14. [Google Scholar] [CrossRef]
- Peng, H.; Jiang, J.; Li, X. Endometrial Cancer Following Levonorgestrel-Releasing Intrauterine System Insertion in Young Women with Atypical Hyperplasia: Two Case Reports and Literature Review. Reprod. Sci. 2022, 29, 3278–3284. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.A.; Uccella, S.; Franchi, M.; Scambia, G.; Fanfani, F.; Fagotti, A.; Pavone, M.; Raspagliesi, F.; Bogani, G. Performance of molecular classification in predicting oncologic outcomes of fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer. Int. J. Gynecol. Cancer 2024, 35, 100016. [Google Scholar] [CrossRef]
| Variable | POLEmut n = 11 | MMRd n = 11 | NSMP n = 92 | p53abn n = 4 | p Value |
|---|---|---|---|---|---|
| Pathology, n (%) | 0.867 | ||||
| AH | 1 (9.1) | 1 (9.1) | 6 (6.5) | 0 | |
| EC G1 | 7 (63.6) | 9 (81.8) | 73 (79.3) | 3 (75) | |
| EC G2 | 3 (27.3) | 1 (9.1) | 13 (14.1) | 1 (25) | |
| Age (years) | 37 (30–42) | 37 (30–39) | 33 (29–37) | 34 (25.5–44) | 0.184 |
| BMI (kg/m2) | 23.6 (21.1–26.6) | 21.4 (20.4–26.1) | 25.9 (22.1–31.1) | 27.6 (22–28) | 0.089 |
| Pregnancy history, n (%) | 4 (36.4) | 3 (27.3) | 23 (25) | 1 (25) | 0.890 |
| Parity, n(%) | 1 (9.1) | 1 (9.1) | 12 (13) | 1 (25) | 0.882 |
| Waist (cm) | 84 (72–93) | 82.5(71.8–102.5) | 84 (78–97) | 87.5 (71–93.5) | 0.703 |
| Hip (cm) | 96 (89–107) | 96.5 (90.8–112.8) | 100.5 (94.3–111.4) | 100 (94–102.3) | 0.569 |
| Diabetes, n (%) | 1 (9.1) | 2 (18.2) | 11 (12) | 1 (25) | 0.826 |
| IR n (%) | 3 (27.3) | 4 (40) | 45 (51.1) | 1 (25) | 0.331 |
| Hypertension, n (%) | 1 (9.1) | 0 | 12 (13) | 0 | 0.328 |
| Hyperlipidemia, n (%) | 4 (50) | 5 (71.4) | 45 (57) | 1 (25) | 0.487 |
| HDL-C (mmol/L) | 1.3 (1–1.6) | 1.3 (1.2–1.5) | 1.1 (1–1.3) | 1.4 (1.3–1.7) | 0.010 |
| LDL-C (mmol/L) | 2.7 (2.2–3.1) | 3.5 (2.6–4.1) | 2.8 (2.4–3.4) | 2.7 (2.2–3.5) | 0.185 |
| Triacylglycerol (mmol/L) | 1 (0.9–1.1) | 1.5 (0.9–3.7) | 1 (0.8–1.5) | 0.8 (0.7–1.1) | 0.224 |
| Cholesterol (mmol/L) | 4.3 (4.2–4.7) | 4.6 (3.5–5.7) | 4.6 (4.1–5.2) | 4.5 (4.1–5.9) | 0.987 |
| PCOS, n (%) | 2 (18.2) | 1 (9.1) | 21 (23.9) | 1 (25) | 0.658 |
| Thyroid disease, n (%) | 1 (9.1) | 0 | 9 (9.8) | 0 | 0.414 |
| Family history of cancer, n (%) | 4 (36.4) | 6 (54.5) | 22 (23.9) | 0 | 0.068 |
| CA-125 (U/mL) | 11 (6.9–17.8) | 18.1 (9.8–26.8) | 15.4 (10.3–22.4) | 17 (7.6–50.6) | 0.353 |
| Therapy | 0.000 | ||||
| MPA/MA | 46 (60) | 64 (36.4) | 39 (54.3) | 1 (25) | |
| MPA/MA→GnRHa + LNG-IUS | 2 (20) | 2 (18.2) | 36 (40.9) | 0 | |
| GnRHa + LNG-IUS/letrozol | 1 (10) | 0 | 13 (14.8) | 3 (75) | |
| Combined with ICI | 1 (10) | 5 (45.5) | 0 | 0 | |
| Therapy outcomes, n (%) | |||||
| CR | 10 (90.9) | 8 (72.7) | 60 (65.2) | 3 (75) | 0.284 |
| PD | 0 | 3 (27.3) | 1 (1) | 0 | 0.007 |
| Recurrence | 4 (40) | 2 (25) | 13 (21.7) | 1 (33.3) | 0.671 |
| Time to CR (months) | 7 (3.8–10) | 11 (7.3–15.5) | 6.5 (3.3–12) | 9 (3–12) | 0.383 |
| Follow-up period (months) | 8.5 (3.8–13.8) | 5 (0–9) | 10.5 (4–21) | 6 (1–11) | 0.367 |
| Case No. | Diagnosis | BMI kg/m2 | Complications | Deficient MMR Protein | Regimen | Time to CR (Months) | Oncological Outcomes |
|---|---|---|---|---|---|---|---|
| 1 | ECG2 | 21.3 | LS | MLH1/ PMS2- | MPA 250 → 500 mg, 6 m, SD MPA 500 mg, 6 m, PR GnRHa + LNG-IUS, 12 m, CR → SD GnRHa + LNG-IUS, 6 m, SD Chemo TC × 2, AP × 1, 3 m, PD | / | PD Staging surgery Endometrial dedifferentiated cancer IIIC1 |
| 2 | ECG1 | 28.2 | LS | MSH2/ MSH6- | MA 320 mg, 3 m, SD MPA 500 mg + GnRHa + metformin, 9 m, SD MPA 500 + GnRHa + metformin, 2 m | / | NR Staging surgery EC G1 Ia Ovary endometrioid cancer G1 Ia |
| 3 | ECG1 | 22.2 | Diabetes LS | MLH1/ PMS2- | LNG-IUS, 3 m, SD MA160 mg + LNG-IUS + metformin, 5 m, SD GnRHa + letrozole, 6 m, CR Recurrence 8 m after CR | 13 | Recurrence Staging surgery Pathology unknown |
| 4 | ECG2 | 22.3 | Breast cancerLS | MSH2/ MSH6- | MPA250 mg, 3 m, SD MPA250 mg + GnRHa, 9 m, CR Recurrence 13 m after CR, ECG1 | 12 | Recurrence Staging surgery EC G2 Ia |
| 5 | ECG2 | 21.4 | None | MSH2/ MSH6- | MPA500 mg, 1 m, MPA500 mg + chemo TC × 2, 2 m, PD MPA500 mg + IAP×6 + PD-1, 6 m, SD Refused following treatment | / | PD Survival at 12-month follow-up |
| 6 | AH | 18.4 | LS | MSH2/ MSH6- | MPA 500 mg, 5 m, SD MPA 500 mg, 3 m, CR | 8 | CR |
| 7 | ECG2 | 34.5 | DM, PCOS | PMS2- | MPA 500 mg + metformin, 3 m, PR Chemo AP × 2 + MPA 500 mg + metformin, 4 m, PR GnRHa + LNG-IUS, 6 m, PR GnRHa + LNG-IUS + letrozole + PD-1i + metformin, 6 m, PR GnRHa + LNG-IUS + letrozole + PD-1i + metformin + statin, 6 m, CR | 25 | CR |
| 8 | ECG1 | 26.0 | None | MSH6- | MA 320 mg, 4 m, PR MPA 500 mg + GnRHa + LNG-IUS, 2 m, MPA 500 mg + GnRHa + LNG-IUS + PD-1i, 2 m CR, 3 m CR | 8 | CR |
| 9 | ECG2 | 19.83 | None | MSH2/ MSH6- | MPA 500 mg, 11 m, PR GnRHa + LNG-IUS + PD-1i, 3 m, CR | 14 | CR |
| 10 | ECG1 | 16.6 | None | MSH2/ MSH6- | MPA 250 mg + PD-1i, 3 m, CR | 3 | CR |
| 11 | ECG1 | 20.4 | None | MSH6- | MPA 250 mg + GnRH + PD-1i, 4 m, PR GnRH + LNG-IUS + PD-1i, 3 m, CR | 7 | CR |
| Case No. | Diagnosis | MI | BMI kg/m2 | POLE Mutation Sites | Regimen | Time to CR (Months) | Oncological Outcomes |
|---|---|---|---|---|---|---|---|
| 12 | ECG2 | Yes, Superficial, Intraperitoneal metastasis | 19.7 | S459F | MPA 500 mg + chemo, 6 m CR AH recurrence 25 m after CR | 6 | CR IVF-ET Live birth Recurrence and CR |
| 13 | AH | No | 25.5 | P286R | MPA 250 → 500 mg + metformin, 14 m CR | 14 | CR IVF-ET ongoing |
| 14 | ECG1 | No | 28.6 | P286R | GnRHa + LNG-IUS, 3 m PR GnRHa + LNG-IUS + MPA 250 mg, 5 m CR | 8 | CR No fertility plan |
| 15 | ECG1 | No | 32.1 | V411L | MPA 250 mg + metformin 4 m CR Recurrence after 6 m MPA 250 mg + metformin + GnRHa + LNG-IUS, 10 m CR | 4 | CR Recurrence and CR IVF-ET ongoing |
| 16 | ECG1 | No | 26.6 | P286R | MPA 500 mg + metformin, 5 m, CR Ovarian tumor 5 m after CR, chemo TC × 4 | 6 | CR Ovary endometrioid cancer G1 Ic1 IVF-ET ongoing |
| 17 | ECG1 | No | 23.6 | P286R | MPA 250 mg 3 m CR | 3 | CR |
| 18 | ECG2 | Yes, Superficial | 26.0 | L424I | MPA 500 mg + chemo TC + GnRHa + PD-1i, 3 m CR | 3 | CR |
| 19 | ECG1 | No | 23.0 | Unknown | MA 160 → 320 mg + LNG-IUS, 13 m SD, GnRHa + letrozole, 6 m CR AH recurrence 5 m after CR GnRHa + letrozole + LNG-IUS, 3 m CR | 19 | CR Recurrence and CR Pregnant, 11-week gestation |
| 20 | ECG2 | Yes, Superficial | 18.9 | V411L | MPA 500 mg + LNG-IUS 3 m, PR GnRHa + LNG-IUS + metformin | / | In treatment |
| 21 | ECG1 | No | 21.5 | MPA 250 mg, 6 m, CR | 6 | CR IVF-ET ongoing | |
| 22 | ECG1 | No | 21.1 | V411L | MPA 250 mg, 3 m, PR MPA 250 mg + metformin, 3 m, PR+ Ovarian endometrioid adenocarcinoma G1 MPA 250 mg + metformin, 9 m, CR ECG1 recurrence 12 m after CR | 9 | Ovary endometrioid cancer G1 Ia CR Recurrence and staging surgery |
| Case No. | Diagnosis | MI | BMI | Regimen | Time to CR (Months) | Oncological Outcomes |
|---|---|---|---|---|---|---|
| 23 | ECG2 | <1/2 MI | 20.2 | GnRHa + LNG-IUS, 3 m CR | 3 | CR IVF-ET Cesarean section Ovarian borderline tumor |
| 24 | ECG1 | None | 27.5 | MPA250 9 m PR GnRHa + LNG-IUS, 3m CR | 12 | CR Focal hyperplasia 6 m after CR |
| 25 | ECG2 | None | 28.4 | GnRHa + LNG-IUS, 3 m PR GnRHa + LNG-IUS + MPA 250 mg, 6 m CR | 9 | CR |
| 26 | ECG1 | None | GnRHa + LNG-IUS, 3 m NR Chemo TC × 4, PR | / | Treatment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Bo, L.; Fan, X.; Kang, N.; Zhang, X.; Tian, L.; Zhou, R.; Wang, J. Molecular Classification Guides Fertility-Sparing Treatment for Endometrial Cancer and Atypical Hyperplasia Patients. Curr. Oncol. 2025, 32, 317. https://doi.org/10.3390/curroncol32060317
Wang Y, Bo L, Fan X, Kang N, Zhang X, Tian L, Zhou R, Wang J. Molecular Classification Guides Fertility-Sparing Treatment for Endometrial Cancer and Atypical Hyperplasia Patients. Current Oncology. 2025; 32(6):317. https://doi.org/10.3390/curroncol32060317
Chicago/Turabian StyleWang, Yiqin, Linlin Bo, Xiaowei Fan, Nan Kang, Xiaobo Zhang, Li Tian, Rong Zhou, and Jianliu Wang. 2025. "Molecular Classification Guides Fertility-Sparing Treatment for Endometrial Cancer and Atypical Hyperplasia Patients" Current Oncology 32, no. 6: 317. https://doi.org/10.3390/curroncol32060317
APA StyleWang, Y., Bo, L., Fan, X., Kang, N., Zhang, X., Tian, L., Zhou, R., & Wang, J. (2025). Molecular Classification Guides Fertility-Sparing Treatment for Endometrial Cancer and Atypical Hyperplasia Patients. Current Oncology, 32(6), 317. https://doi.org/10.3390/curroncol32060317





