Neoadjuvant Strategies for Patients with Resectable Biliary Tract Cancers: A Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Current Data on the Role of Neoadjuvant Therapy
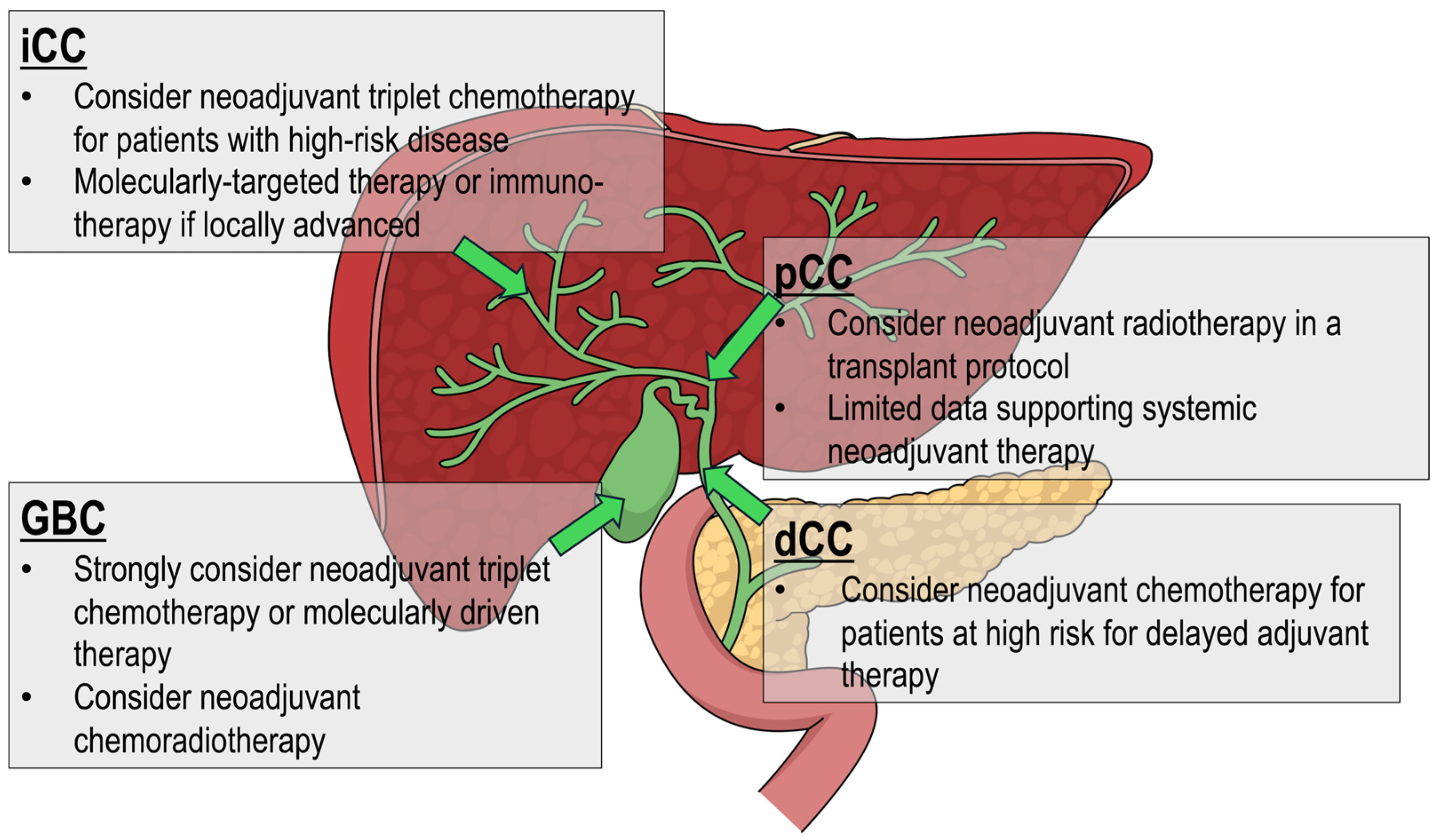
3.2. Intrahepatic Cholangiocarcinoma
3.3. Perihilar Cholangiocarcinoma
3.4. Distal Cholangiocarcinoma
3.5. Gallbladder Carcinoma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Anaya, D.A.; Anders, R.; Are, C.; Bachini, M.; Borad, M.; Brown, D.; Burgoyne, A.; et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021, 19, 541–565. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; D’Angelica, M.I.; Abrams, T.; Abbott, D.E.; Ahmed, A.; Anaya, D.A.; Anders, R.; Are, C.; Bachini, M.; Binder, D.; et al. NCCN Guidelines(R) Insights: Biliary Tract Cancers, Version 2.2023. J. Natl. Compr. Canc. Netw. 2023, 21, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, G.; Giordano, M.; Paladina, I.; Rando, A.; Uccello, M.; Basile, F.; Biondi, A.; Carnazzo, S.; Alessandria, I.; Mazzarino, C. Markers of bile duct tumors. World J. Gastrointest Oncol. 2011, 3, 49–59. [Google Scholar] [CrossRef]
- Akateh, C.; Ejaz, A.M.; Pawlik, T.M.; Cloyd, J.M. Neoadjuvant treatment strategies for intrahepatic cholangiocarcinoma. World J. Hepatol. 2020, 12, 693–708. [Google Scholar] [CrossRef]
- Cools, K.S.; Glazer, E.S. A Tool for Patient-Focused Care Regarding Neoadjuvant Chemotherapy for Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2021, 28, 1874–1875. [Google Scholar] [CrossRef]
- Maithel, S.K.; Keilson, J.M.; Cao, H.S.T.; Rupji, M.; Mahipal, A.; Lin, B.S.; Javle, M.M.; Cleary, S.P.; Akce, M.; Switchenko, J.M.; et al. NEO-GAP: A Single-Arm, Phase II Feasibility Trial of Neoadjuvant Gemcitabine, Cisplatin, and Nab-Paclitaxel for Resectable, High-Risk Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2023, 30, 6558–6566. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Prakash, L.; Vauthey, J.N.; Aloia, T.A.; Chun, Y.S.; Tzeng, C.W.; Kim, M.P.; Lee, J.E.; Katz, M.H.G. The role of preoperative therapy prior to pancreatoduodenectomy for distal cholangiocarcinoma. Am. J. Surg. 2019, 218, 145–150. [Google Scholar] [CrossRef]
- Elias, C.; Rahman, A.; Mial-Anthony, J.; Packiaraj, G.; Crane, A.; Alshamery, S.; Ganoza, A.; Gunabushanam, V.; Powers, C.; Dharmayan, S.; et al. Advancements in cholangiocarcinoma: Evolving strategies for diagnosis, treatment, and palliation over three decades. Chin. Clin. Oncol. 2024, 13, 70. [Google Scholar] [CrossRef]
- Hakeem, A.R.; Papoulas, M.; Menon, K.V. The role of neoadjuvant chemotherapy or chemoradiotherapy for advanced gallbladder cancer—A systematic review. Eur. J. Surg. Oncol. 2019, 45, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Goetze, T.O.; Bechstein, W.O.; Bankstahl, U.S.; Keck, T.; Konigsrainer, A.; Lang, S.A.; Pauligk, C.; Piso, P.; Vogel, A.; Al-Batran, S.E. Neoadjuvant chemotherapy with gemcitabine plus cisplatin followed by radical liver resection versus immediate radical liver resection alone with or without adjuvant chemotherapy in incidentally detected gallbladder carcinoma after simple cholecystectomy or in front of radical resection of BTC (ICC/ECC)—A phase III study of the German registry of incidental gallbladder carcinoma platform (GR)- the AIO/ CALGP/ ACO-GAIN-trial. BMC Cancer 2020, 20, 122. [Google Scholar] [CrossRef]
- Harrison, J.M.; Visser, B.C. Cholangiocarcinoma. Surg. Clin. N. Am. 2024, 104, 1281–1293. [Google Scholar] [CrossRef]
- Ilyas, S.I.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef]
- Roth, G.S.; Verlingue, L.; Sarabi, M.; Blanc, J.F.; Boleslawski, E.; Boudjema, K.; Bretagne-Bignon, A.L.; Camus-Duboc, M.; Coriat, R.; Crehange, G.; et al. Biliary tract cancers: French national clinical practice guidelines for diagnosis, treatments and follow-up (TNCD, SNFGE, FFCD, UNICANCER, GERCOR, SFCD, SFED, AFEF, SFRO, SFP, SFR, ACABi, ACHBPT). Eur. J. Cancer 2024, 202, 114000. [Google Scholar] [CrossRef]
- Ercolani, G.; Vetrone, G.; Grazi, G.L.; Aramaki, O.; Cescon, M.; Ravaioli, M.; Serra, C.; Brandi, G.; Pinna, A.D. Intrahepatic cholangiocarcinoma: Primary liver resection and aggressive multimodal treatment of recurrence significantly prolong survival. Ann. Surg. 2010, 252, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Schunemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef]
- Medin, C.R.; Maithel, S.K. Neoadjuvant therapy trials in biliary tract malignancies. J. Surg. Oncol. 2022, 125, 84–88. [Google Scholar] [CrossRef]
- Saffo, S.; Peng, C.; Salem, R.; Taddei, T.; Nagar, A. Impact of Neoadjuvant Chemotherapy and Pretreatment Biliary Drainage for Pancreatic Head Ductal Adenocarcinoma. Dig. Dis. Sci. 2021, 67, 1409–1416. [Google Scholar] [CrossRef]
- Parente, A.; Kamarajah, S.K.; Baia, M.; Tirotta, F.; Manzia, T.M.; Hilal, M.A.; Pawlik, T.M.; White, S.A.; Dahdaleh, F.S. Neoadjuvant Chemotherapy for Intrahepatic, Perihilar, and Distal Cholangiocarcinoma: A National Population-Based Comparative Cohort Study. J. Gastrointest Surg. 2023, 27, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Grindrod, N.; Breadner, D.; Lock, M. The Current Role of Radiation in the Management of Cholangiocarcinoma-A Narrative Review. Cancers 2024, 16, 1776. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.L.; Pitt, H.A.; Zinner, M.J.; Kaufman, S.L.; Coleman, J. Management of proximal cholangiocarcinomas by surgical resection and radiotherapy. Am. J. Surg. 1990, 159, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Ji, J.; Li, S.; Lai, J.; Wei, G.; Wu, J.; Chen, W.; Xie, W.; Wang, S.; Qiao, L.; et al. Adjuvant Chemoradiation and Immunotherapy for Extrahepatic Cholangiocarcinoma and Gallbladder Cancer: A Randomized Clinical Trial. JAMA Oncol. 2025, 1, 1021–1029. [Google Scholar] [CrossRef]
- Yun, W.G.; Chae, Y.S.; Han, Y.; Lee, I.; Choi, G.W.; Seo, Y.; Cho, Y.J.; Jung, H.S.; Park, J.S.; Jang, J.Y.; et al. Impact of Resection Margin Status on Recurrence and Possible Candidates for Adjuvant Radiotherapy in Resected Distal Cholangiocarcinoma. Ann. Surg. 2025; ahead of print. [Google Scholar] [CrossRef]
- Utuama, O.; Permuth, J.B.; Dagne, G.; Sanchez-Anguiano, A.; Alman, A.; Kumar, A.; Denbo, J.; Kim, R.; Fleming, J.B.; Anaya, D.A. Neoadjuvant Chemotherapy for Intrahepatic Cholangiocarcinoma: A Propensity Score Survival Analysis Supporting Use in Patients with High-Risk Disease. Ann. Surg. Oncol. 2021, 28, 1939–1949. [Google Scholar] [CrossRef]
- Shroff, R.T.; Javle, M.M.; Xiao, L.; Kaseb, A.O.; Varadhachary, G.R.; Wolff, R.A.; Raghav, K.P.S.; Iwasaki, M.; Masci, P.; Ramanathan, R.K.; et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 824–830. [Google Scholar] [CrossRef]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klumpen, H.J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef]
- Oh, D.Y.; Ruth He, A.; Qin, S.; Chen, L.T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Ah Lee, M.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef] [PubMed]
- Gujarathi, R.; Peshin, S.; Zhang, X.; Bachini, M.; Meeks, M.N.; Shroff, R.T.; Pillai, A. Intrahepatic cholangiocarcinoma: Insights on molecular testing, targeted therapies, and future directions from a multidisciplinary panel. Hepatol. Commun. 2025, 9, e0743. [Google Scholar] [CrossRef]
- Liu, J.J.; Zhou, M.; Yuan, T.; Huang, Z.Y.; Zhang, Z.Y. Conversion treatment for advanced intrahepatic cholangiocarcinoma: Opportunities and challenges. World J. Gastroenterol. 2025, 31, 104901. [Google Scholar] [CrossRef] [PubMed]
- Schleimer, L.E.; Liu, A.; Kalvin, H.L.; Barekzai, A.B.; Choubey, A.P.; Jung, J.; Haque, R.; Jarnagin, W.R.; Balachandran, V.P.; Geevarghese, R.; et al. Robotic Versus Open Placement of Hepatic Artery Infusion Pumps. Ann. Surg. Oncol. 2025, 32, 3488–3498. [Google Scholar] [CrossRef]
- Rea, D.J.; Heimbach, J.K.; Rosen, C.B.; Haddock, M.G.; Alberts, S.R.; Kremers, W.K.; Gores, G.J.; Nagorney, D.M. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann. Surg. 2005, 242, 451–458. [Google Scholar] [CrossRef]
- Sudan, D.; DeRoover, A.; Chinnakotla, S.; Fox, I.; Shaw, B., Jr.; McCashland, T.; Sorrell, M.; Tempero, M.; Langnas, A. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am. J. Transpl. 2002, 2, 774–779. [Google Scholar] [CrossRef]
- Darwish Murad, S.; Kim, W.R.; Harnois, D.M.; Douglas, D.D.; Burton, J.; Kulik, L.M.; Botha, J.F.; Mezrich, J.D.; Chapman, W.C.; Schwartz, J.J.; et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012, 143, 88–98.e3. [Google Scholar] [CrossRef] [PubMed]
- Croome, K.P.; Rosen, C.B.; Heimbach, J.K.; Nagorney, D.M. Is Liver Transplantation Appropriate for Patients with Potentially Resectable De Novo Hilar Cholangiocarcinoma? J. Am. Coll. Surg. 2015, 221, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Zaborowski, A.; Heneghan, H.M.; Fiore, B.; Stafford, A.; Gallagher, T.; Geoghegan, J.; Maguire, D.; Hoti, E. Neoadjuvant Chemoradiotherapy and Liver Transplantation for Unresectable Hilar Cholangiocarcinoma: The Irish Experience of the Mayo Protocol. Transplantation 2020, 104, 2097–2104. [Google Scholar] [CrossRef]
- McMasters, K.M.; Tuttle, T.M.; Leach, S.D.; Rich, T.; Cleary, K.R.; Evans, D.B.; Curley, S.A. Neoadjuvant chemoradiation for extrahepatic cholangiocarcinoma. Am. J. Surg. 1997, 174, 605–608. [Google Scholar] [CrossRef]
- Nelson, J.W.; Ghafoori, A.P.; Willett, C.G.; Tyler, D.S.; Pappas, T.N.; Clary, B.M.; Hurwitz, H.I.; Bendell, J.C.; Morse, M.A.; Clough, R.W.; et al. Concurrent chemoradiotherapy in resected extrahepatic cholangiocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 148–153. [Google Scholar] [CrossRef]
- Jung, J.H.; Lee, H.J.; Lee, H.S.; Jo, J.H.; Cho, I.R.; Chung, M.J.; Park, J.Y.; Park, S.W.; Song, S.Y.; Bang, S. Benefit of neoadjuvant concurrent chemoradiotherapy for locally advanced perihilar cholangiocarcinoma. World J. Gastroenterol. 2017, 23, 3301–3308. [Google Scholar] [CrossRef]
- Chaudhari, V.A.; Ostwal, V.; Patkar, S.; Sahu, A.; Toshniwal, A.; Ramaswamy, A.; Shetty, N.S.; Shrikhande, S.V.; Goel, M. Outcome of neoadjuvant chemotherapy in “locally advanced/borderline resectable” gallbladder cancer: The need to define indications. HPB 2018, 20, 841–847. [Google Scholar] [CrossRef]
- Engineer, R.; Goel, M.; Chopra, S.; Patil, P.; Purandare, N.; Rangarajan, V.; Ph, R.; Bal, M.; Shrikhande, S.; Shrivastava, S.K.; et al. Neoadjuvant Chemoradiation Followed by Surgery for Locally Advanced Gallbladder Cancers: A New Paradigm. Ann. Surg. Oncol. 2016, 23, 3009–3015. [Google Scholar] [CrossRef] [PubMed]
- Engineer, R.; Patkar, S.; Lewis, S.C.; Sharma, A.D.; Shetty, N.; Ostwal, V.; Ramaswamy, A.; Chopra, S.; Agrawal, A.; Patil, P.; et al. A phase III randomised clinical trial of perioperative therapy (neoadjuvant chemotherapy versus chemoradiotherapy) in locally advanced gallbladder cancers (POLCAGB): Study protocol. BMJ Open 2019, 9, e028147. [Google Scholar] [CrossRef]
- de Scordilli, M.; Bortolot, M.; Torresan, S.; Noto, C.; Rota, S.; Di Nardo, P.; Fumagalli, A.; Guardascione, M.; Ongaro, E.; Foltran, L.; et al. Precision oncology in biliary tract cancer: The emerging role of liquid biopsy. ESMO Open 2025, 10, 105079. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N. Biomarkers and Management of Cholangiocarcinoma: Unveiling New Horizons for Precision Therapy. Cancers 2025, 17, 1243. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, A.; Balsano, R.; Pressiani, T.; Bozzarelli, S.; Rimassa, L.; Lleo, A. The Immune-Genomics of Cholangiocarcinoma: A Biological Footprint to Develop Novel Immunotherapies. Cancers 2025, 17, 272. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, M.; Di Martino, M.; Polidoro, M.A.; Forti, L.; Tober, N.; Gennari, A.; Pagano, N.; Donadon, M. Management of intrahepatic cholangiocarcinoma: A review for clinicians. Gastroenterol. Rep. 2025, 13, goaf005. [Google Scholar] [CrossRef]
- Ethun, C.G.; Lopez-Aguiar, A.G.; Anderson, D.J.; Adams, A.B.; Fields, R.C.; Doyle, M.B.; Chapman, W.C.; Krasnick, B.A.; Weber, S.M.; Mezrich, J.D.; et al. Transplantation Versus Resection for Hilar Cholangiocarcinoma: An Argument for Shifting Treatment Paradigms for Resectable Disease. Ann. Surg. 2018, 267, 797–805. [Google Scholar] [CrossRef]
| Stage | Intrahepatic | Perihilar | Extrahepatic | Gallbladder |
|---|---|---|---|---|
| 0 | 0 Tis: In situ (intraductal) | 0 Tis: In situ | 0 Tis: In situ/High Grade Dysplasia | 0 Tis: In situ |
| I | IA T1aN0M0: solitary tumor ≤ 5 cm without vascular invasion | I T1N0M0: invasion into muscle layer but confined to bile ducts. | I T1N0M0: Invades bile duct wall with depth of invasion < 5 mm | I T1N0M0: Invades lamina propria or muscle layer of gallbladder |
| IB T1bN0M0: solitary tumor > 5 cm without vascular invasion | ||||
| II | II T2N0M0: solitary tumor of any size with intrahepatic vascular invasion or multiple tumors with or without vascular invasion | II T2aN0M0: tumor invades beyond wall of the duct to surrounding adipose tissue T2bN0M0: tumor invades beyond wall of the duct to surrounding hepatic parenchyma | IIA T1N1M0: tumor invades bile duct wall < 5 mm with 1–3 regional lymph nodes positive T2N0M0: tumor invades bile duct 5–12 mm with no nodes positive | IIA T2aN0M0: tumor invades perimuscular connective tissue on peritoneal side without involvement of serosa (visceral peritoneum) |
| IIB T2N1M0: tumor invades 5–12 mm with 1–3 regional lymph nodes positive T3N0-1M0: tumor invades > 12 mm with 0–3 regional lymph nodes positive | IIB T2bN0M0: tumor invades the perimuscular connective tissue on hepatic side with no extension into the liver | |||
| III | IIIA T3N0M0: tumor perforates visceral peritoneum | IIIA T3N0M0: tumor invades unilateral branches of portal vein or hepatic artery | IIIA T1-3N2M0: any tumor invasion of the bile duct with ≥4 regional lymph nodes positive | IIIA T3N0M0: tumor perforates serosa and/or directly invades liver, and/or one other adjacent organ or structure (stomach, duodenum, colon, pancreas, omentum, extrahepatic bile ducts) |
| IIIB T4N0M0: tumor involving local extrahepatic structures by direct invasion T1-4N1M0: Any T stage with any regional lymph metastases present. | IIIB T4N0M0: tumor invades main portal vein or its branches bilaterally, or the CHA, or unilateral 2nd order biliary radicals bilaterally with contralateral portal vein/hepatic artery involvement. | IIIB T4N0-2M0: tumor involves the celiac axis, SMA, and or CHA with any number of nodes (including 0) positive | IIIB T1-3 N1M0: T stage 1–3 with 1–3 regional lymph nodes positive | |
| IIIC T1-4N1M0: Any T stage with 1–3 regional lymph nodes positive. | ||||
| IV | IV T1-4N0-1M1: distant metastasis present | IVA T1-4N2M0: Any T stage with 4 or more positive regional lymph nodes | IV T1-4N0-2M1: distant metastasis present | IVA T4N0-1M0: tumor invades main portal vein, or hepatic artery, or invades 2 or more extrahepatic organs/structures with 0–3 regional lymph nodes positive |
| IVB T1-4N0-2M1: distant metastasis present | IVB T1-4N2M0: Any T stage with 4 or more regional lymph nodes positive. T1-4N0-2M1: distant metastasis present |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olson, C.R.; Aitken, G.L.; Spinrad, M.W.; Glazer, E.S. Neoadjuvant Strategies for Patients with Resectable Biliary Tract Cancers: A Review. Curr. Oncol. 2025, 32, 584. https://doi.org/10.3390/curroncol32100584
Olson CR, Aitken GL, Spinrad MW, Glazer ES. Neoadjuvant Strategies for Patients with Resectable Biliary Tract Cancers: A Review. Current Oncology. 2025; 32(10):584. https://doi.org/10.3390/curroncol32100584
Chicago/Turabian StyleOlson, Chelsea R., Gabriela L. Aitken, Michael W. Spinrad, and Evan S. Glazer. 2025. "Neoadjuvant Strategies for Patients with Resectable Biliary Tract Cancers: A Review" Current Oncology 32, no. 10: 584. https://doi.org/10.3390/curroncol32100584
APA StyleOlson, C. R., Aitken, G. L., Spinrad, M. W., & Glazer, E. S. (2025). Neoadjuvant Strategies for Patients with Resectable Biliary Tract Cancers: A Review. Current Oncology, 32(10), 584. https://doi.org/10.3390/curroncol32100584





