Baseline Characteristics of Individuals with Metastatic Cancer Enrolled in the Alberta Cancer Exercise Study and 12-Week Findings for Symptom-Related and Physical Fitness Measures
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Screening Process
- (1)
- Pre-screening to identify participants with high-risk cancers (e.g., lung, neurological, pancreatic) and/or metastatic disease spread.
- (2)
- Cancer intake form and Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) to identify specific cancer-related concerns and other health conditions potentially impacting physical activity participation.
- (3)
- In-person or telephone interview with a clinical exercise physiologist to review screening findings and to determine need for medical clearance and/or specialized support.
- (4)
- Physical fitness assessment to evaluate physical function and mobility and to identify participants with underlying issues requiring medical clearance and/or specialized support.
2.3. Program Characteristics
2.4. Data Collection
2.5. Participant Characteristics
- (1)
- Sociodemographic factors: Age, biological sex, gender identity, ethnicity, marital status, education level, income, employment status, smoking status, drinking status
- (2)
- Anthropometric measures: Body mass index
- (3)
- Medical and cancer history: Number of comorbidities, primary cancer type, number and location of metastases, treatment status, current and completed treatments
- (4)
- Exercise-related outcomes: Physical Activity Stages of Change, Godin-Shephard Leisure-Time Physical Activity Questionnaire (to calculate physical activity minutes per week [30])
- (5)
- Symptom-related and quality of life measures: Functional Assessment of Cancer Therapy-Fatigue (FACT-F; also referred to as FACIT-F) scale (higher FACT scores indicate better quality of life), Edmonton Symptom Assessment Scale (ESAS; higher ESAS scores indicate higher symptom burden), 5-level EQ-5D (EQ-5D-5L; higher levels on EQ-5D-5L dimensions indicate more severe problems), EQ visual analogue scale (EQ VAS; higher EQ VAS scores indicate better health)
- (6)
- Physical fitness measures: 30 s timed sit-to-stand (in-person and virtual assessments), one-legged stance (in-person and virtual assessments), 6-Minute Walk Test (6MWT) (in-person assessments only; discontinued during the COVID-19 pandemic), 2 min step test (included in virtual assessments to replace the 6MWT during the COVID-19 pandemic), active shoulder flexion range of motion (in-person and virtual assessments)
2.6. Data Analysis
3. Results
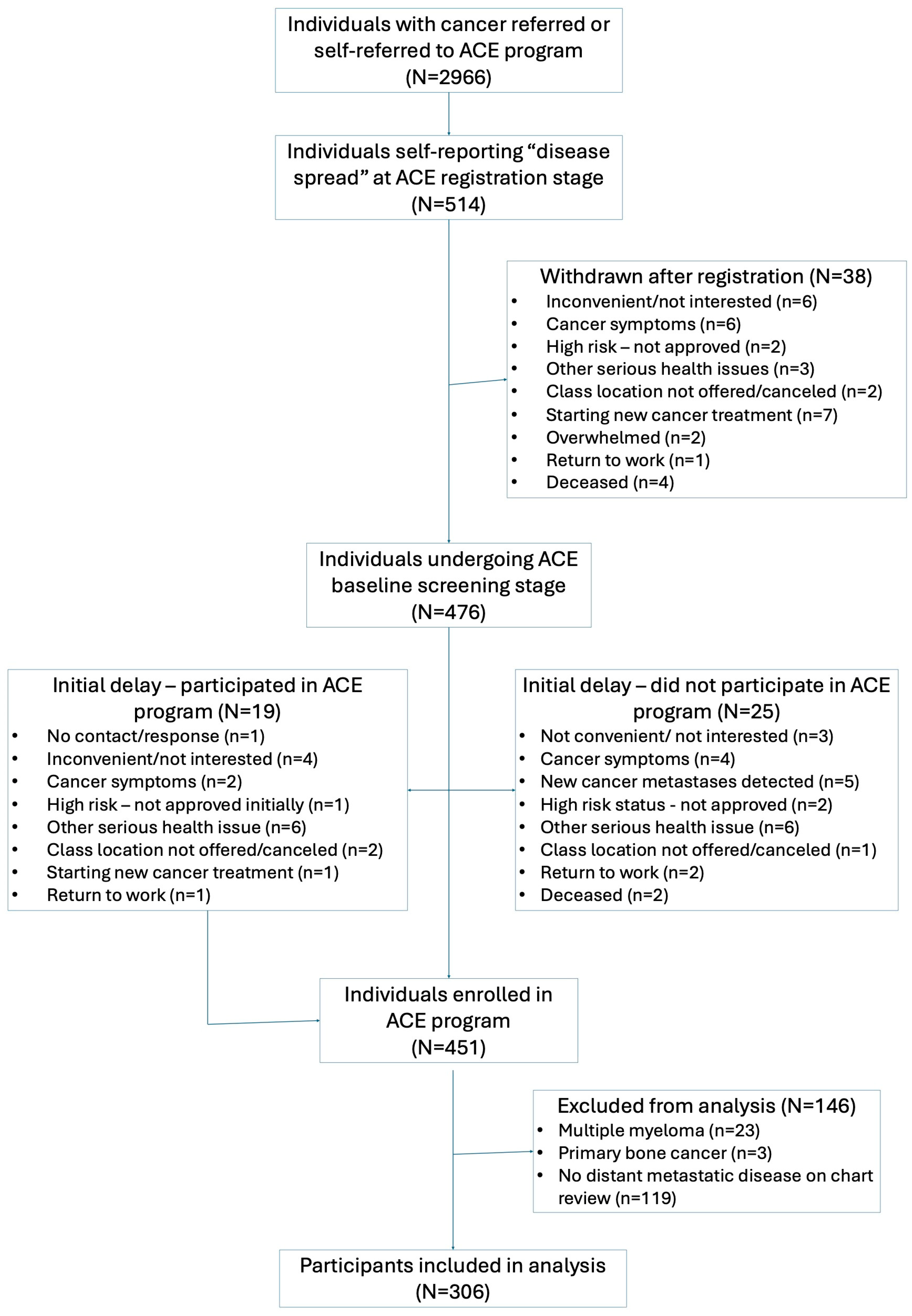
3.1. Participant Flow
3.2. Baseline Characteristics
3.3. Program Feasibility and Safety
3.4. Changes in Symptom-Related, Quality of Life, and Physical Fitness Measures
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hart, N.H.; Nekhlyudov, L.; Smith, T.J.; Yee, J.; Fitch, M.I.; Crawford, G.B.; Koczwara, B.; Ashbury, F.D.; Lustberg, M.B.; Mollica, M.; et al. Survivorship care for people affected by advanced or metastatic cancer: MASCC-ASCO standards and practice recommendations. Support Care Cancer 2024, 20, 1160–1172. [Google Scholar] [CrossRef]
- Cheville, A.L.; Troxel, A.B.; Basford, J.R.; Kornblith, A.B. Prevalence and treatment patterns of physical impairments in patients with metastatic breast cancer. J. Clin. Oncol. 2008, 26, 2621–2629. [Google Scholar] [CrossRef]
- Dunn, J.; Watson, M.; Aitken, J.F.; Hyde, M.K. Systematic review of psychosocial outcomes for patients with advanced melanoma. Psycho-Oncology 2017, 26, 1722–1731. [Google Scholar] [CrossRef]
- Hart, N.H.; Crawford-Williams, F.; Crichton, M.; Yee, J.; Smith, T.J.; Koczwara, B.; Fitch, M.I.; Crawford, G.B.; Mukhopadhyay, S.; Mahony, J.; et al. Unmet supportive care needs of people with advanced cancer and their caregivers: A systematic scoping review. Crit. Rev. Oncol. Hematol. 2022, 176, 103728. [Google Scholar] [CrossRef]
- Rojas-Concha, L.; Hansen, M.B.; Petersen, M.A.; Groenvold, M. Symptoms of advanced cancer in palliative medicine: A longitudinal study. BMJ Support. Palliat. Care 2023, 13, e415–e427. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Harbeck, N.; Mertz, S.; Fenech, D. Evolving psychosocial, emotional, functional, and support needs of women with advanced breast cancer: Results from the Count Us, Know Us, Join Us and Here & Now surveys. Breast 2016, 28, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Cheville, A.L.; Beck, L.A.; Petersen, T.L.; Marks, R.S.; Gamble, G.L. The detection and treatment of cancer-related functional problems in an outpatient setting. Support. Care Cancer 2009, 17, 61–67. [Google Scholar] [CrossRef]
- Di Lascio, S.; Pagani, O. Is it time to address survivorship in advanced breast cancer? A review article. Breast 2017, 31, 167–172. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Ma, H.; Zhang, X.; Wang, B.; Guo, T.; Xiao, Y.; Bing, Z.; Ge, L.; Yang, K.; et al. Exercise training for improving patient-reported outcomes in patients with advanced-stage cancer: A systematic review and meta-analysis. J. Pain Symptom Manag. 2020, 59, 734–749.e10. [Google Scholar] [CrossRef]
- De Lazzari, N.; Niels, T.; Tewes, M.; Götte, M. A Systematic review of the safety, feasibility and benefits of exercise for patients with advanced cancer. Cancers 2021, 13, 4478. [Google Scholar] [CrossRef]
- Heywood, R.; McCarthy, A.L.; Skinner, T.L. Safety and feasibility of exercise interventions in patients with advanced cancer: A systematic review. Support. Care Cancer 2017, 25, 3031–3050. [Google Scholar] [CrossRef] [PubMed]
- Heywood, R.; McCarthy, A.L.; Skinner, T.L. Efficacy of exercise interventions in patients with advanced cancer: A systematic review. Arch. Phys. Med. Rehabil. 2018, 99, 2595–2620. [Google Scholar] [CrossRef]
- Nadler, M.B.; Desnoyers, A.; Langelier, D.M.; Amir, E. The effect of exercise on quality of life, fatigue, physical function, and safety in advanced solid tumor cancers: A meta-analysis of randomized control trials. J. Pain Symptom Manag. 2019, 58, 899–908.e7. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.; Tookman, A.; Bristowe, K.; Maddocks, M. ‘I am actually doing something to keep well. That feels really good’: Experiences of exercise within hospice care. Prog. Palliat. Care 2016, 24, 204–212. [Google Scholar] [CrossRef]
- Rodríguez-Cañamero, S.; Cobo-Cuenca, A.I.; Carmona-Torres, J.M.; Pozuelo-Carrascosa, D.P.; Santacruz-Salas, E.; Rabanales-Sotos, J.A.; Cuesta-Mateos, T.; Laredo-Aguilera, J.A. Impact of physical exercise in advanced-stage cancer patients: Systematic review and meta-analysis. Cancer Med. 2022, 11, 3714–3727. [Google Scholar] [CrossRef]
- Burke, S.; Utley, A.; Belchamber, C.; McDowall, L. Physical activity in hospice care: A social ecological perspective to inform policy and practice. Res. Q. Exerc. Sport 2020, 91, 500–513. [Google Scholar] [CrossRef]
- Cheville, A.L.; Dose, A.M.; Basford, J.R.; Rhudy, L.M. Insights into the reluctance of patients with late-stage cancer to adopt exercise as a means to reduce their symptoms and improve their function. J. Pain Symptom Manag. 2012, 44, 84–94. [Google Scholar] [CrossRef]
- Frikkel, J.; Götte, M.; Beckmann, M.; Kasper, S.; Hense, J.; Teufel, M.; Schuler, M.; Tewes, M. Fatigue, barriers to physical activity and predictors for motivation to exercise in advanced cancer patients. BMC Palliat Care 2020, 19, 43. [Google Scholar] [CrossRef]
- Mikkelsen, M.K.; Nielsen, D.L.; Vinther, A.; Lund, C.M.; Jarden, M. Attitudes towards physical activity and exercise in older patients with advanced cancer during oncological treatment—A qualitative interview study. Eur. J. Oncol. Nurs. 2019, 41, 16–23. [Google Scholar] [CrossRef]
- Sheill, G.; Guinan, E.; Neill, L.O.; Hevey, D.; Hussey, J. The views of patients with metastatic prostate cancer towards physical activity: A qualitative exploration. Support. Care Cancer 2018, 26, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Shallwani, S.M.; Thomas, R.; King, J.; Toupin-April, K.; Poitras, S. Perspectives and experiences of leisure-time physical activity in adults with stage 4 cancer: A qualitative interpretive-description study. Disabil. Rehabil. 2024, 46, 1515–1526. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Shallwani, S.M.; King, J.; Thomas, R.; Thevenot, O.; De Angelis, G.; Aburub, A.S.; Brosseau, L. Methodological quality of clinical practice guidelines with physical activity recommendations for people diagnosed with cancer: A systematic critical appraisal using the AGREE II tool. PLoS ONE 2019, 14, e0214846. [Google Scholar] [CrossRef] [PubMed]
- Shallwani, S.M.; Ranger, M.-C.; Thomas, R.; Brosseau, L.; Poitras, S.; Sikora, L.; King, J. A scoping review of studies exploring leisure-time physical activity in adults diagnosed with advanced cancer. Palliat. Support. Care 2021, 19, 615–630. [Google Scholar] [CrossRef]
- McNeely, M.L.; Sellar, C.; Williamson, T.; Shea-Budgell, M.; Joy, A.A.; Lau, H.Y.; Easaw, J.C.; Murtha, A.D.; Vallance, J.; Courneya, K.; et al. Community-based exercise for health promotion and secondary cancer prevention in Canada: Protocol for a hybrid effectiveness-implementation study. BMJ Open 2019, 9, e029975. [Google Scholar] [CrossRef]
- McNeely, M.L.; Shallwani, S.M.; Williamson, T.; Sellar, C.; Gobeil, E.; Joy, A.A.; Lau, H.; Easaw, J.; Sexsmith, J.; Courneya, K.S.; et al. Baseline characteristics of participants in the Alberta Cancer Exercise hybrid effectiveness–implementation study: A wake-up call for action. Cancers 2025, 17, 772. [Google Scholar] [CrossRef]
- Lowe, S.S.; Watanabe, S.M.; Baracos, V.E.; Courneya, K.S. Home-based functional walking program for advanced cancer patients receiving palliative care: A case series. BMC Palliat. Care 2013, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Tsianakas, V.; Harris, J.; Ream, E.; Van Hemelrijck, M.; Purushotham, A.; Mucci, L.; Green, J.S.A.; Fewster, J.; Armes, J. CanWalk: A feasibility study with embedded randomised controlled trial pilot of a walking intervention for people with recurrent or metastatic cancer. BMJ Open 2017, 7, e013719. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Molassiotis, A.; Chung, B.P.M.; Tan, J.-Y. Unmet care needs of advanced cancer patients and their informal caregivers: A systematic review. BMC Palliat. Care 2018, 17, 96. [Google Scholar] [CrossRef]
- Trinh, L.; Plotnikoff, R.C.; Rhodes, R.E.; North, S.; Courneya, K.S. Associations between physical activity and quality of life in a population-based sample of kidney cancer survivors. Cancer Epidemiol. Biomark. Prev. 2011, 20, 859–868. [Google Scholar] [CrossRef]
- Mishra, P.; Pandey, C.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.; Eton, D.T.; Lai, J.-S.; Peterman, A.H.; Merkel, D.E. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J. Pain Symptom Manag. 2002, 24, 547–561. [Google Scholar] [CrossRef]
- Hui, D.; Darke, A.K.; Guthrie, K.A.; Subbiah, I.M.; Unger, J.M.; Hershman, D.L.; Krouse, R.S.; Bakitas, M.; O’Rourke, M.A. Association between health-related quality of life and progression-free survival in patients with advanced cancer: A secondary analysis of SWOG clinical trials. JCO Oncol. Pract. 2022, 18, e442–e451. [Google Scholar] [CrossRef]
- Hui, D.; Bruera, E. The Edmonton Symptom Assessment System 25 years later: Past, present, and future developments. J. Pain Symptom Manag. 2017, 53, 630–643. [Google Scholar] [CrossRef]
- Selby, D.; Cascella, A.; Gardiner, K.; Do, R.; Moravan, V.; Myers, J.; Chow, E. A single set of numerical cutpoints to define moderate and severe symptoms for the Edmonton Symptom Assessment System. J. Pain Symptom Manag. 2010, 39, 241–249. [Google Scholar] [CrossRef]
- EuroQol Research Foundation. EQ-5D-5L User Guide; EuroQol Research Foundation, 2019; Available online: https://euroqol.org/publications/user-guides (accessed on 28 May 2025).
- McKay, M.J.; Baldwin, J.N.; Ferreira, P.; Simic, M.; Vanicek, N.; Burns, J.; For the 1000 Norms Project Consortium; 1000 Norms Project Consortium; Baldwin, J.; McKay, M.; et al. Reference values for developing responsive functional outcome measures across the lifespan. Neurology 2017, 88, 1512–1519. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. Functional fitness normative scores for community-residing older adults, ages 60–94. J. Aging Phys. Act. 1999, 7, 162–181. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist 2013, 53, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Springer, B.A.; Marin, R.; Cyhan, T.; Roberts, H.; Gill, N.W. Normative values for the unipedal stance test with eyes open and closed. J. Geriatr. Phys. Ther. 2007, 30, 8–15. [Google Scholar] [CrossRef]
- Hawley-Hague, H.; Horne, M.; Skelton, D.A.; Todd, C. Review of how we should define (and measure) adherence in studies examining older adults’ participation in exercise classes. BMJ Open 2016, 6, e011560. [Google Scholar] [CrossRef] [PubMed]
- Bullard, T.; Ji, M.; An, R.; Trinh, L.; Mackenzie, M.; Mullen, S.P. A systematic review and meta-analysis of adherence to physical activity interventions among three chronic conditions: Cancer, cardiovascular disease, and diabetes. BMC Public Health 2019, 19, 636. [Google Scholar] [CrossRef]
- Rivera-Torres, S.; Fahey, T.D.; Rivera, M.A. Adherence to exercise programs in older adults: Informative report. Gerontol. Geriatr. Med. 2019, 5, 2333721418823604. [Google Scholar] [CrossRef]
- Sheill, G.; Guinan, E.; Brady, L.; Hevey, D.; Hussey, J. Exercise interventions for patients with advanced cancer: A systematic review of recruitment, attrition, and exercise adherence rates. Palliat. Support. Care 2019, 17, 686–696. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE); Version 5.0; National Cancer Institute, 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 17 June 2025).
- Jayadevappa, R.; Cook, R.; Chhatre, S. Minimal important difference to infer changes in health-related quality of life-a systematic review. J. Clin. Epidemiol. 2017, 89, 188–198. [Google Scholar] [CrossRef]
- Webster, K.; Cella, D.; Yost, K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: Properties, applications, and interpretation. Health Qual. Life Outcomes 2003, 1, 79. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.; Shamieh, O.; Paiva, C.E.; Perez-Cruz, P.E.; Kwon, J.H.; Muckaden, M.A.; Park, M.; Yennu, S.; Kang, J.H.; Bruera, E. Minimal clinically important differences in the Edmonton Symptom Assessment Scale in cancer patients: A prospective, multicenter study. Cancer 2015, 121, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.; Shamieh, O.; Paiva, C.E.; Khamash, O.; Perez-Cruz, P.E.; Kwon, J.H.; Muckaden, M.A.; Park, M.; Arthur, J.; Bruera, E. Minimal clinically important difference in the physical, emotional, and total symptom distress scores of the Edmonton Symptom Assessment System. J. Pain Symptom Manag. 2016, 51, 262–269. [Google Scholar] [CrossRef]
- Bourke, S.; Bennett, B.; Oluboyede, Y.; Li, T.; Longworth, L.; O’Sullivan, S.B.; Braverman, J.; Soare, I.-A.; Shaw, J.W. Estimating the minimally important difference for the EQ-5D-5L and EORTC QLQ-C30 in cancer. Health Qual. Life Outcomes 2024, 22, 81. [Google Scholar] [CrossRef]
- Wright, A.A.; Cook, C.E.; Baxter, G.D.; Dockerty, J.D.; Abbott, J.H. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J. Orthop. Sports Phys. Ther. 2011, 41, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Zanini, A.; Crisafulli, E.; D’Andria, M.; Gregorini, C.; Cherubino, F.; Zampogna, E.; Azzola, A.; Spanevello, A.; Schiavone, N.; Chetta, A. Minimum clinically important difference in 30-s sit-to-stand test after pulmonary rehabilitation in subjects with COPD. Respir. Care 2019, 64, 1261–1269. [Google Scholar] [CrossRef]
- Goldberg, A.; Casby, A.; Wasielewski, M. Minimum detectable change for single-leg-stance-time in older adults. Gait Posture 2011, 33, 737–739. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Crouch, R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: A systematic review. J. Eval. Clin. Pract. 2017, 23, 377–381. [Google Scholar] [CrossRef]
- Perera, S.; Mody, S.H.; Woodman, R.C.; Studenski, S.A. Meaningful change and responsiveness in common physical performance measures in older adults. J Am. Geriatr. Soc. 2006, 54, 743–749. [Google Scholar] [CrossRef]
- Kwon, S.; Perera, S.; Pahor, M.; Katula, J.A.; King, A.C.; Groessl, E.J.; Studenski, S.A. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study). J. Nutr. Health Aging 2009, 13, 538–544. [Google Scholar] [CrossRef]
- Muir, S.W.; Corea, C.L.; Beaupre, L. Evaluating change in clinical status: Reliability and measures of agreement for the assessment of glenohumeral range of motion. N. Am. J. Sports Phys. Ther. 2010, 5, 98–110. [Google Scholar] [PubMed]
- Gallicchio, L.; Devasia, T.P.; Tonorezos, E.; Mollica, M.A.; Mariotto, A. Estimation of the number of individuals living with metastatic cancer in the United States. JNCI J. Natl. Cancer Inst. 2022, 114, 1476–1483. [Google Scholar] [CrossRef]
- Lai-Kwon, J.; Heynemann, S.; Hart, N.H.; Chan, R.J.; Smith, T.J.; Nekhlyudov, L.; Jefford, M. Evolving landscape of metastatic cancer survivorship: Reconsidering clinical care, policy, and research priorities for the modern era. J. Clin. Oncol. 2023, 41, 3304–3310. [Google Scholar] [CrossRef]
- Cheung, W.Y.; Le, L.W.; Zimmermann, C. Symptom clusters in patients with advanced cancers. Support. Care Cancer 2009, 17, 1223–1230. [Google Scholar] [CrossRef]
- Shallwani, S.M.; Simmonds, M.J.; Kasymjanova, G.; Spahija, J. Quality of life, symptom status and physical performance in patients with advanced non-small cell lung cancer undergoing chemotherapy: An exploratory analysis of secondary data. Lung Cancer 2016, 99, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Vistad, I.; Cvancarova, M.; Astrup, G.L.; Rustøen, T.; Liavaag, A.H. Symptom experience and self-rated physical functioning in patients with ovarian cancer receiving chemotherapy. Int. J. Gynecol. Cancer 2018, 28, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Silver, H.J.; Dietrich, M.S.; Murphy, B.A. Changes in body mass, energy balance, physical function, and inflammatory state in patients with locally advanced head and neck cancer treated with concurrent chemoradiation after low-dose induction chemotherapy. Head Neck 2007, 29, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Van Abbema, D.L.; Van Den Akker, M.; Janssen-Heijnen, M.L.; Van Den Berkmortel, F.; Hoeben, A.; De Vos-Geelen, J.; Buntinx, F.; Kleijnen, J.; Tjan-Heijnen, V.C.G. Patient- and tumor-related predictors of chemotherapy intolerance in older patients with cancer: A systematic review. J. Geriatr. Oncol. 2019, 10, 31–41. [Google Scholar] [CrossRef]
- Groen, W.G.; Naaktgeboren, W.R.; van Harten, W.H.; van Vulpen, J.K.; Kool, N.; Sonke, G.S.; Van Der Wall, E.; Velthuis, M.J.; Aaronson, N.K.; May, A.M.; et al. Physical fitness and chemotherapy tolerance in patients with early-stage breast cancer. Med. Sci. Sports Exerc. 2022, 54, 537–542. Available online: https://journals.lww.com/acsm-msse/fulltext/2022/04000/physical_fitness_and_chemotherapy_tolerance_in.1.aspx (accessed on 16 July 2025). [CrossRef]
- Barbera, L.; Seow, H.; Howell, D.; Sutradhar, R.; Earle, C.; Liu, Y.; Stitt, A.; Husain, A.; Sussman, J.; Dudgeon, D. Symptom burden and performance status in a population-based cohort of ambulatory cancer patients. Cancer 2010, 116, 5767–5776. [Google Scholar] [CrossRef]
- Stout, N.L.; Brown, J.C.; Schwartz, A.L.; Marshall, T.F.; Campbell, A.M.; Nekhlyudov, L.; Zucker, D.S.; Basen-Engquist, K.M.; Campbell, G.; Meyerhardt, J.; et al. An exercise oncology clinical pathway: Screening and referral for personalized interventions. Cancer 2020, 126, 2750–2758. [Google Scholar] [CrossRef]
- Weller, S.; Hart, N.H.; Bolam, K.A.; Mansfield, S.; Santa Mina, D.; Winters-Stone, K.M.; Campbell, A.; Rosenberger, F.; Wiskemann, J.; Quist, M.; et al. Exercise for individuals with bone metastases: A systematic review. Crit. Rev. Oncol. Hematol. 2021, 166, 103433. [Google Scholar] [CrossRef] [PubMed]
- Dunn, R.M.; Hayes, S.C.; Sandler, C.X.; Spence, R.R. Adverse event assessment and reporting in exercise oncology: A review. Exerc. Sport Mov. 2023, 1, 1–7. Available online: https://journals.lww.com/acsm-esm/fulltext/2023/09000/adverse_event_assessment_and_reporting_in_exercise.2.aspx (accessed on 9 June 2025). [CrossRef]
- Thomsen, S.N.; Lahart, I.M.; Thomsen, L.M.; Fridh, M.K.; Larsen, A.; Mau-Sørensen, M.; Bolam, K.A.; Fairman, C.M.; Christensen, J.F.; Simonsen, C. Harms of exercise training in patients with cancer undergoing systemic treatment: A systematic review and meta-analysis of published and unpublished controlled trials. eClinicalMedicine 2023, 59, 101937. [Google Scholar] [CrossRef]
- Campbell, K.L.; Cormie, P.; Weller, S.; Alibhai, S.M.H.; Bolam, K.A.; Campbell, A.; Cheville, A.L.; Dalzell, M.-A.; Hart, N.H.; Higano, C.S.; et al. Exercise recommendation for people with bone metastases: Expert consensus for health care providers and exercise professionals. JCO Oncol. Pract. 2022, 18, e697–e709. [Google Scholar] [CrossRef]
- Jones, L.W. Evidence-based risk assessment and recommendations for physical activity clearance. Appl. Physiol. Nutr. Metab. 2011, 36, S101–S112. [Google Scholar] [CrossRef] [PubMed]
- Spence, R.R.; Sandler, C.X.; Jones, T.L.; McDonald, N.; Dunn, R.M.; Hayes, S.C. Practical suggestions for harms reporting in exercise oncology: The Exercise Harms Reporting Method (ExHaRM). BMJ Open 2022, 12, e067998. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada. 2021 Census of Population. 2023. Available online: https://www12.statcan.gc.ca/census-recensement/2021/dp-pd/prof/index.cfm?Lang=E. (accessed on 1 July 2025).
- Norris, M.K.; Fox, F.S.; Lee, C.; Wang, E.; Green, K.; Yan, H.; Dieli-Conwright, C.M. Narrowing the Gap for Minority Cancer Survivors: Exercise Oncology in the Past, Present, and Future. J. Clin. Exerc. Physiol. 2020, 9, 155–170. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Demanelis, K.; Crisafio, M.E.; Kennedy, M.A.; Schwartz, A.L.; Campbell, A.; Gorzelitz, J.; Wood, K.C.; Wilson, C.M.; Scalise, R.L.; et al. Proximity to cancer rehabilitation and exercise oncology by geography, race, and socioeconomic status. Cancer 2025, 131, e35515. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, K.M.; Wilson, O.W.A.; Shiels, M.S.; Sheppard, V.B.; Jayasekera, J. Racial, ethnic, and socioeconomic disparities in meeting physical activity guidelines among female breast cancer survivors in the United States. Cancer Epidemiol. Biomark. Prev. 2024, 33, 1610–1622. [Google Scholar] [CrossRef]
| Sociodemographic Characteristic | ACE-Met Participants | ACE-Met Participants Completing Study | ACE-Met Participants Not Completing Study | |||
|---|---|---|---|---|---|---|
| N = 306 | N = 274 | N = 32 | ||||
| Mean/n | SD/% | Mean/n | SD/% | Mean/n | SD/% | |
| Age at diagnosis, mean, SD | 57.6 | 11.9 | 58.0 | 12.0 | 54.1 | 11.0 |
| Age category at diagnosis | ||||||
| Young adult (18–39) | 23 | 7.5 | 20 | 7.3 | 3 | 9.4 |
| Middle-aged (40–64) | 191 | 62.4 | 169 | 61.7 | 22 | 68.8 |
| Older adult (65+) | 92 | 30.1 | 85 | 31.0 | 7 | 21.9 |
| Biological sex | ||||||
| Female | 200 | 65.4 | 181 | 66.1 | 19 | 59.4 |
| Male | 106 | 34.6 | 93 | 33.9 | 13 | 40.6 |
| Ethnicity | ||||||
| North American—Indigenous | 2 | 0.7 | 2 | 0.7 | 0 | 0.0 |
| North American—Other | 4 | 1.3 | 4 | 1.5 | 0 | 0.0 |
| European | 232 | 75.8 | 209 | 76.3 | 23 | 71.9 |
| Latin, Central, or South American or Caribbean | 4 | 1.3 | 4 | 1.5 | 0 | 0.0 |
| African | 2 | 0.7 | 2 | 0.7 | 0 | 0.0 |
| Asian or Oceanian | 28 | 9.2 | 23 | 8.4 | 5 | 15.6 |
| Multiple or other | 18 | 5.9 | 18 | 6.6 | 0 | 0.0 |
| Missing or unknown | 16 | 5.2 | 12 | 4.4 | 4 | 12.5 |
| Marital status | ||||||
| Married or common-law | 226 | 73.9 | 201 | 73.4 | 25 | 78.1 |
| Divorced, separated, widowed | 53 | 17.3 | 48 | 17.5 | 5 | 15.6 |
| Single (never married) | 27 | 8.8 | 25 | 9.1 | 2 | 6.3 |
| Highest level of education | ||||||
| Did not complete college/university | 102 | 33.3 | 86 | 31.4 | 16 | 50.0 |
| Completed college/university or higher | 204 | 66.7 | 188 | 68.6 | 16 | 50.0 |
| Income | ||||||
| ≤$60,000 | 95 | 31.0 | 89 | 32.5 | 6 | 18.8 |
| $60,000 to $99,999 | 82 | 26.8 | 70 | 25.5 | 12 | 37.5 |
| >$100,000 | 98 | 32.0 | 89 | 32.5 | 9 | 28.1 |
| Missing | 31 | 10.1 | 26 | 9.5 | 5 | 15.6 |
| Employment | ||||||
| Disability or temporarily unemployed | 139 | 45.4 | 124 | 45.3 | 15 | 46.9 |
| Retired | 100 | 32.7 | 92 | 33.6 | 8 | 25.0 |
| Homemaker | 12 | 3.9 | 10 | 3.6 | 2 | 6.3 |
| Part-time or full-time | 55 | 18.0 | 48 | 17.5 | 7 | 21.9 |
| Smoking | ||||||
| Non-smoker | 174 | 56.9 | 152 | 55.5 | 22 | 68.8 |
| Ex-smoker | 118 | 38.6 | 109 | 39.8 | 9 | 28.1 |
| Occasional or regular smoker | 14 | 4.6 | 13 | 4.7 | 1 | 3.1 |
| Drinking | ||||||
| Non- or ex-drinker | 77 | 25.2 | 71 | 25.9 | 6 | 18.8 |
| Occasional or social drinker | 219 | 71.6 | 193 | 70.4 | 26 | 81.3 |
| Daily drinker | 10 | 3.3 | 10 | 3.6 | 0 | 0.0 |
| BMI, mean, SD | 28.0 | 6.2 | 28.0 | 6.0 | 27.9 | 7.2 |
| BMI category | ||||||
| Normal/underweight | 98 | 32.0 | 86 | 31.4 | 12 | 37.5 |
| Overweight | 109 | 35.6 | 101 | 36.9 | 8 | 25.0 |
| Obese | 99 | 32.4 | 87 | 31.8 | 12 | 37.5 |
| Number of comorbidities | ||||||
| None | 86 | 28.1 | 75 | 27.4 | 11 | 34.4 |
| One | 113 | 36.9 | 102 | 37.2 | 11 | 34.4 |
| Two or more | 107 | 35.0 | 97 | 35.4 | 10 | 31.3 |
| Type of comorbidity | ||||||
| Arthritis | 146 | 47.7 | 133 | 48.5 | 13 | 40.6 |
| Cardiovascular | 75 | 24.5 | 65 | 23.7 | 10 | 31.3 |
| Mental health | 42 | 13.7 | 38 | 13.9 | 4 | 12.5 |
| Metabolic | 32 | 10.5 | 28 | 10.2 | 4 | 12.5 |
| Other | 76 | 24.8 | 73 | 26.6 | 3 | 9.4 |
| Cancer-Related Characteristic | ACE-Met Participants | ACE-Met Participants Completing Study | ACE-Met Participants Not Completing Study | |||
|---|---|---|---|---|---|---|
| N = 306 | N = 274 | N = 32 | ||||
| Mean/n | SD/% | Mean/n | SD/% | Mean/n | SD/% | |
| Primary cancer type | ||||||
| Breast | 103 | 33.7 | 89 | 32.5 | 14 | 43.8 |
| Lung | 33 | 10.8 | 31 | 11.3 | 2 | 6.3 |
| Digestive | 46 | 15.0 | 36 | 13.1 | 10 | 31.3 |
| Blood | 19 | 6.2 | 19 | 6.9 | 0 | 0.0 |
| Gynecologic | 30 | 9.8 | 28 | 10.2 | 2 | 6.3 |
| Genitourinary | 51 | 16.7 | 48 | 17.5 | 3 | 9.4 |
| Head and neck | 10 | 3.3 | 10 | 3.6 | 0 | 0.0 |
| Skin/melanoma | 7 | 2.3 | 7 | 2.6 | 0 | 0.0 |
| Other | 7 | 2.3 | 6 | 2.2 | 1 | 3.1 |
| Number of organ sites affected by metastases, mean, SD | 1.4 | 0.7 | 1.4 | 0.7 | 1.6 | 0.8 |
| Number of organ sites affected by metastases category | ||||||
| One | 204 | 66.7 | 185 | 67.5 | 19 | 59.4 |
| Two or more | 102 | 33.3 | 89 | 32.5 | 13 | 40.6 |
| Location of metastases | ||||||
| Bone | 137 | 44.8 | 122 | 44.5 | 15 | 46.9 |
| Brain | 31 | 10.1 | 25 | 9.1 | 6 | 18.8 |
| Liver | 88 | 28.8 | 77 | 28.1 | 11 | 34.4 |
| Lung | 79 | 25.8 | 66 | 24.1 | 13 | 40.6 |
| Other distant site | 62 | 20.3 | 58 | 21.2 | 4 | 12.5 |
| Regional spread | 12 | 3.9 | 12 | 4.4 | 0 | 0.0 |
| Treatment status | ||||||
| On | 229 | 74.8 | 204 | 74.5 | 25 | 78.1 |
| Off | 77 | 25.2 | 70 | 25.5 | 7 | 21.9 |
| Current treatment(s) | ||||||
| Chemotherapy | 110 | 35.9 | 92 | 33.6 | 18 | 56.3 |
| Radiation | 8 | 2.6 | 6 | 2.2 | 2 | 6.3 |
| Hormone therapy | 87 | 28.4 | 83 | 30.3 | 4 | 12.5 |
| Biological/targeted/immune therapy | 69 | 22.5 | 65 | 23.7 | 4 | 12.5 |
| Other | 4 | 1.3 | 4 | 1.5 | 0 | 0.0 |
| Completed treatment(s) | ||||||
| Surgery | 165 | 53.9 | 147 | 53.6 | 18 | 56.3 |
| Chemotherapy | 181 | 59.2 | 159 | 58.0 | 22 | 68.8 |
| Radiation | 145 | 47.4 | 128 | 46.7 | 17 | 53.1 |
| Hormone therapy | 58 | 19.0 | 50 | 18.2 | 8 | 25.0 |
| Biological/targeted/immune therapy | 27 | 8.8 | 25 | 9.1 | 2 | 6.3 |
| Stem cell transplant | 9 | 2.9 | 9 | 3.3 | 0 | 0.0 |
| Other | 7 | 2.3 | 7 | 2.6 | 0 | 0.0 |
| No prior therapy | 7 | 2.3 | 5 | 1.8 | 2 | 6.3 |
| Exercise-Related Characteristic | ACE-Met Participants | ACE-Met Participants Completing Study | ACE-Met Participants Not Completing Study | |||
|---|---|---|---|---|---|---|
| N = 306 | N = 274 | N = 32 | ||||
| Mean/n | SD/% | Mean/n | SD/% | Mean/n | SD/% | |
| PA minutes per week at baseline, mean, SD | 90.2 | 157.9 | 93.6 | 160.3 | 60.9 | 134.8 |
| PA classification at baseline | ||||||
| Sedentary (0 min) | 151 | 49.3 | 137 | 50.0 | 14 | 43.8 |
| Insufficiently active (<150 min) | 88 | 28.8 | 73 | 26.6 | 15 | 46.9 |
| Active (150 min or higher) | 66 | 21.6 | 63 | 23.0 | 3 | 9.4 |
| Missing | 1 | 0.3 | 1 | 0.4 | 0 | 0.0 |
| PA stages of change at baseline | ||||||
| Precontemplation | 5 | 1.6 | 4 | 1.5 | 1 | 3.1 |
| Contemplation | 71 | 23.2 | 61 | 22.3 | 10 | 31.3 |
| Preparation | 96 | 31.4 | 85 | 31.0 | 11 | 34.4 |
| Decision/Action | 11 | 3.6 | 11 | 4.0 | 0 | 0.0 |
| Maintenance | 37 | 12.1 | 36 | 13.1 | 1 | 3.1 |
| Missing | 86 | 28.1 | 77 | 28.1 | 9 | 28.1 |
| Exercise location | ||||||
| Calgary | 140 | 45.8 | 129 | 47.1 | 11 | 34.4 |
| Edmonton | 130 | 42.5 | 114 | 41.6 | 16 | 50.0 |
| North Zone (Fort McMurray, Grand Prairie, Red Deer) | 21 | 6.9 | 18 | 6.6 | 3 | 9.4 |
| South Zone (Lethbridge, Medicine Hat) | 15 | 4.9 | 13 | 4.7 | 2 | 6.3 |
| Type of exercise class | ||||||
| In-person circuit training | 133 | 43.5 | 116 | 42.3 | 17 | 53.1 |
| In-person personal training | 100 | 32.7 | 89 | 32.5 | 11 | 34.4 |
| Virtual circuit training | 73 | 23.9 | 69 | 25.2 | 4 | 12.5 |
| Outcome Measures | ACE-Met Participants Completing Study | ||||
|---|---|---|---|---|---|
| n | Baseline Score Mean (sd) | 12-Week Score Mean (sd) | 12-Week Change Mean (95% CI) | p-Value | |
| FACT | |||||
| FACT-G total scale (0–108) | 268 | 74.5 (14.2) | 75.5 (15.3) | 1.0 (−0.3, 2.2) | 0.010 * |
| FACT Trial Outcome Index (0–108) | 269 | 72.4 (17.9) | 75.7 (19.0) | 3.4 (1.6, 5.1) * | <0.001 |
| FACT Fatigue subscale (0–52) | 269 | 34.8 (10.4) | 37.1 (10.5) | 2.4 (1.4, 3.3) * | <0.001 |
| FACT-F total scale (0–160) | 268 | 109.3 (23.0) | 112.7 (24.3) | 3.4 (1.3, 5.4) * | <0.001 |
| ESAS | |||||
| Physical (0–60) | 268 | 10.0 (8.9) | 10.1 (8.9) | 0.1 (−0.9, 1.1) | 0.870 |
| Emotional (0–20) | 268 | 3.5 (4.2) | 3.5 (4.2) | 0.0 (−0.4, 0.4) | 0.873 |
| Wellbeing (0–10) | 268 | 3.4 (2.5) | 3.3 (2.5) | −0.1 (−0.4, 0.3) | 0.659 |
| Total symptom distress (0–90) | 268 | 16.9 (13.1) | 17.0 (13.0) | 0.1 (−1.3, 1.4) | 0.873 |
| EQ VAS (0–100) | 270 | 64.1 (18.2) | 69.4 (18.1) | 5.3 (3.2, 7.3) *† | <0.001 |
| Physical activity minutes per week | 269 | 94.2 (161.3) | 161.9 (183.8) | 67.7 (48.7, 86.8) *† | <0.001 |
| Physical fitness | |||||
| 30 s sit-to-stand | 227 | 13.5 (5.2) | 16.5 (5.8) | 2.9 (2.4, 3.4) *† | <0.001 |
| One-legged stance | 222 | 25.2 (15.9) | 29.6 (15.7) | 4.4 (2.9, 5.9) * | <0.001 |
| 6MWT | 173 | 525.4 (113.3) | 558.7 (113.6) | 33.2 (22.2, 44.3) *† | <0.001 |
| 2 min step test | 50 | 74.0 (24.4) | 84.1 (25.3) | 10.1 (5.7, 14.6) *† | <0.001 |
| Right shoulder AROM | 235 | 148.3 (11.8) | 150.4 (11.7) | 2.1 (1.0, 3.3) * | <0.001 |
| Left shoulder AROM | 235 | 146.3 (12.9) | 148.7 (12.2) | 2.4 (1.2, 3.7) * | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shallwani, S.M.; Culos-Reed, S.N.; Courneya, K.S.; Williamson, T.; Sellar, C.; Lau, H.; Joy, A.A.; Easaw, J.C.; Audoin, M.; Pituskin, E.; et al. Baseline Characteristics of Individuals with Metastatic Cancer Enrolled in the Alberta Cancer Exercise Study and 12-Week Findings for Symptom-Related and Physical Fitness Measures. Curr. Oncol. 2025, 32, 560. https://doi.org/10.3390/curroncol32100560
Shallwani SM, Culos-Reed SN, Courneya KS, Williamson T, Sellar C, Lau H, Joy AA, Easaw JC, Audoin M, Pituskin E, et al. Baseline Characteristics of Individuals with Metastatic Cancer Enrolled in the Alberta Cancer Exercise Study and 12-Week Findings for Symptom-Related and Physical Fitness Measures. Current Oncology. 2025; 32(10):560. https://doi.org/10.3390/curroncol32100560
Chicago/Turabian StyleShallwani, Shirin M., S. Nicole Culos-Reed, Kerry S. Courneya, Tanya Williamson, Christopher Sellar, Harold Lau, Anil Abraham Joy, Jacob C. Easaw, Michelle Audoin, Edith Pituskin, and et al. 2025. "Baseline Characteristics of Individuals with Metastatic Cancer Enrolled in the Alberta Cancer Exercise Study and 12-Week Findings for Symptom-Related and Physical Fitness Measures" Current Oncology 32, no. 10: 560. https://doi.org/10.3390/curroncol32100560
APA StyleShallwani, S. M., Culos-Reed, S. N., Courneya, K. S., Williamson, T., Sellar, C., Lau, H., Joy, A. A., Easaw, J. C., Audoin, M., Pituskin, E., & McNeely, M. L. (2025). Baseline Characteristics of Individuals with Metastatic Cancer Enrolled in the Alberta Cancer Exercise Study and 12-Week Findings for Symptom-Related and Physical Fitness Measures. Current Oncology, 32(10), 560. https://doi.org/10.3390/curroncol32100560






