Oncotype DX Recurrence Score Predicts Survival in Invasive Micropapillary Breast Carcinoma: A National Cancer Database Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
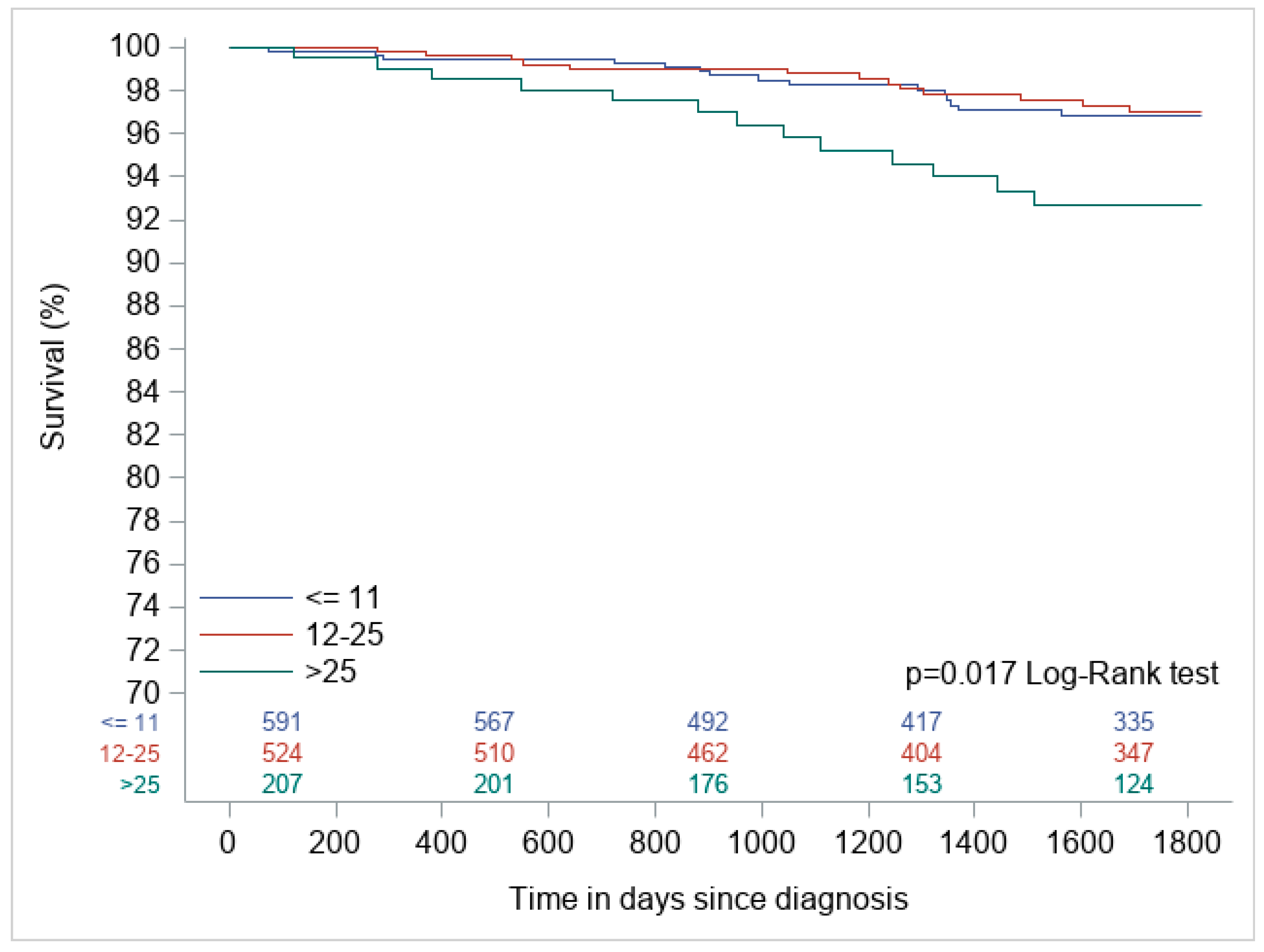
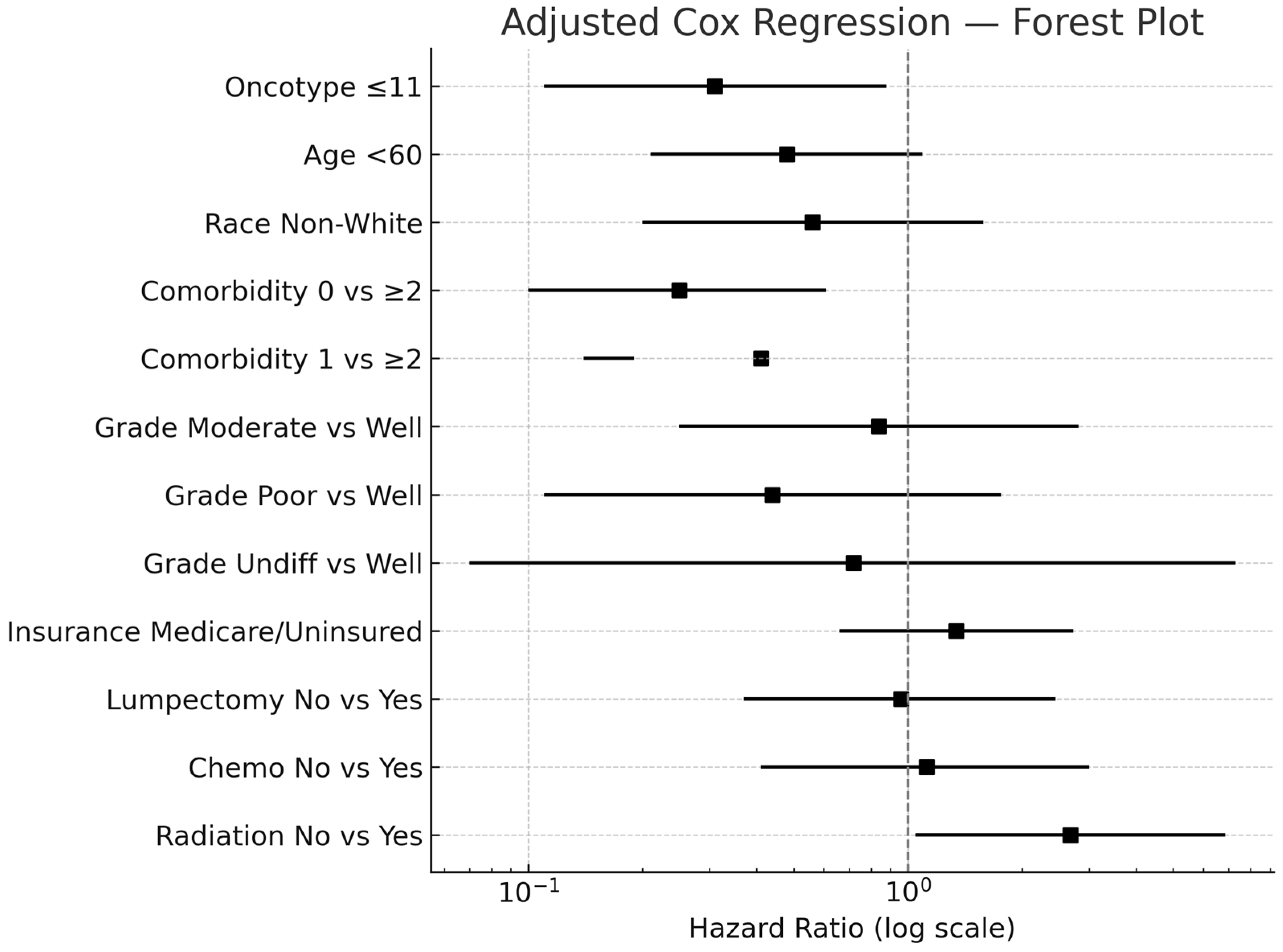
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, P.; Cui, Q.; Huang, S.; Zhang, Y.; Zhang, H.; Luo, H. An overview of invasive micropapillary carcinoma of the breast: Past, present, and future. Front. Oncol. 2024, 14, 1435421. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.H.; Yu, X.J.; Zhang, H.; Zhang, H.J.; Jia, Z.; Wang, X.H. Advances in invasive micropapillary carcinoma of the breast research: A review. Medicine 2024, 103, e36631. [Google Scholar] [CrossRef] [PubMed]
- Zekioglu, O.; Erhan, Y.; Ciris, M.; Bayramoglu, H.; Ozdemir, N. Invasive micropapillary carcinoma of the breast: High incidence of lymph node metastasis with extranodal extension and its immunohistochemical profile compared with invasive ductal carcinoma. Histopathology 2004, 45, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.L.; Yang, J.Q.; Du, Z.G.; Tan, Q.W.; Zhou, Y.T.; Zhang, D.; Lv, Q. Clinicopathologic study of invasive micropapillary carcinoma of the breast. Oncotarget 2017, 8, 42455–42465. [Google Scholar] [CrossRef]
- Elzohery, Y.H.A.M.; Radwan, A.H.; Gareer, S.W.Y.; Mamdouh, M.M.; Moaz, I.; Khalifa, A.M.; Mohen, O.A.; Elithy, M.F.A.A.; Hassaan, M. Micropapillary breast carcinoma in comparison with invasive duct carcinoma. Does it have an aggressive clinical presentation and an unfavorable prognosis? BMC Cancer 2024, 24, 992. [Google Scholar] [CrossRef]
- Verras, G.I.; Tchabashvili, L.; Mulita, F.; Grypari, I.M.; Sourouni, S.; Panagodimou, E.; Argentou, M.I. Micropapillary breast carcinoma: From molecular pathogenesis to prognosis. Breast Cancer 2022, 14, 41–61. [Google Scholar] [CrossRef]
- Shi, W.B.; Yang, L.J.; Hu, X.; Zhou, J.; Zhang, Q.; Shao, Z.M. Clinico-Pathological Features and Prognosis of Invasive Micropapillary Carcinoma Compared to Invasive Ductal Carcinoma: A Population-Based Study from China. PLoS ONE 2014, 9, e101390. [Google Scholar] [CrossRef]
- Paik, S.; Shak, S.; Tang, G.; Kim, C.; Baker, J.; Cronin, M.; Baehner, F.L.; Walker, M.G.; Watson, D.; Park, T.; et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 2004, 351, 2817–2826. [Google Scholar] [CrossRef]
- Paik, S.; Tang, G.; Shak, S.; Kim, C.; Baker, J.; Kim, W.; Cronin, M.; Baehner, F.; Watson, D.; Bryant, J.; et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J. Clin. Oncol. 2006, 24, 3726–3734. [Google Scholar] [CrossRef]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E., Jr.; Dees, E.C.; Goetz, M.P.; Olson, J.A., Jr.; et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N. Engl. J. Med. 2018, 379, 111–121. [Google Scholar] [CrossRef]
- Kalinsky, K.; Barlow, W.E.; Gralow, J.R.; Meric-Bernstam, F.; Albain, K.S.; Hayes, D.F.; Lin, N.U.; Perez, E.A.; Goldstein, L.J.; Chia, S.K.L.; et al. 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. N. Engl. J. Med. 2021, 385, 2336–2347. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group. Polychemotherapy for early breast cancer: An overview of the randomised trials. Lancet 1998, 352, 930–942. [Google Scholar] [CrossRef]
- Albain, K.S.; Barlow, W.E.; Shak, S.; Hortobagyi, G.N.; Livingston, R.B.; Yeh, I.T.; Ravdin, P.; Bugarini, R.; Baehner, F.L.; Davidson, N.E.; et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol. 2010, 11, 55–65. [Google Scholar] [CrossRef]
- Jacobs, F.; Gaudio, M.; Benvenuti, C.; De Sanctis, R.; Santoro, A.; Zambelli, A. Controversies and opportunities in the clinical daily use of the 21-gene assay for prognostication and prediction of chemotherapy benefit in HR+/HER2− early breast cancer. Cancers 2023, 15, 148. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer; Version 4.2025; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2025; Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 9 May 2025).
- Yang, Y.L.; Liu, B.B.; Zhang, X.; Fu, L. Invasive micropapillary carcinoma of the breast: An update. Arch. Pathol. Lab. Med. 2016, 140, 799–805. [Google Scholar] [CrossRef]
- Kim, K.; Jung, J.; Shin, K.H.; Kim, J.H.; Chang, J.H.; Kim, S.S.; Kim, H.; Park, W.; Kim, Y.B.; Chang, J.S. Impact of Oncotype DX recurrence score on the patterns of locoregional recurrence in breast cancer (Korean Radiation Oncology Group 19-06). J. Breast Cancer 2020, 23, 314–319. [Google Scholar] [CrossRef]
- Mamounas, E.P.; Tang, G.; Fisher, B.; Paik, S.; Shak, S.; Constantino, J.P.; Watson, D.; Geyer, C.E., Jr.; Wickerham, D.L.; Wolmark, N. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor–positive breast cancer: Results from NSABP B-14 and NSABP B-20. J. Clin. Oncol. 2010, 28, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Buzdar, A.U.; Suman, V.J.; Meric-Bernstam, F.; Leitch, A.M.; Ellis, M.J.; Boughey, J.C.; Unzeitig, G.W.; Royce, M.E.; Hunt, K.K. Disease-free and overall survival among patients with operable HER2− positive breast cancer treated with sequential vs. concurrent chemotherapy: The ACOSOG Z1041 (Alliance) randomized clinical trial. JAMA Oncol. 2018, 5, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Chevli, N.; Haque, W.; Tran, K.T.; Farach, A.M.; Schwartz, M.R.; Hatch, S.S.; Butler, E.B.; Teh, B.S. 21-gene recurrence score predictive for prognostic benefit of radiotherapy in patients age ≥70 with T1N0 ER/PR+ HER2− breast cancer treated with breast conserving surgery and endocrine therapy. Radiother. Oncol. 2022, 174, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, E.; Zhang, B.; Schaafsma, M.; Tong, C.Y.; Zhang, L.; Cheng, C. Impact of Oncotype DX testing on ER+ breast cancer treatment and survival in the first decade of use. Breast Cancer Res. 2021, 23, 74. [Google Scholar] [CrossRef]
- Commission on Cancer. National Cancer Data Base Participant User File (PUF) Data Dictionary; American College of Surgeons: Chicago, IL, USA, 2015. [Google Scholar]
- Tadros, A.B.; Wen, H.Y.; Morrow, M. Breast cancers of special histologic subtypes are biologically diverse. Ann. Surg. Oncol. 2018, 25, 3158–3164. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Xu, G.; Shi, A.; Zou, Y.; Zhan, Y.; Fan, Z.; Dong, Y. Comparison of clinicopathological characteristics and prognosis among patients with pure invasive ductal carcinoma, invasive ductal carcinoma coexisted with invasive micropapillary carcinoma, and invasive ductal carcinoma coexisted with ductal carcinoma in situ: A retrospective cohort study. Medicine 2020, 99, e23487. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Paulino, A.C.; Schwartz, M.R.; Rodriguez, A.A.; Bass, B.L.; Chang, J.C.; Teh, B.S. Population-based comparison of prognostic factors in invasive micropapillary and invasive ductal carcinoma of the breast. Br. J. Cancer 2014, 111, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Shao, K.; Jia, H.; Cao, B.; Li, W.; Dong, S.; Liu, J.; Wu, K.; Liu, M.; Liu, F.; et al. Genomic alterations and evolution of cell clusters in metastatic invasive micropapillary carcinoma of the breast. Nat. Commun. 2022, 13, 111. [Google Scholar] [CrossRef]
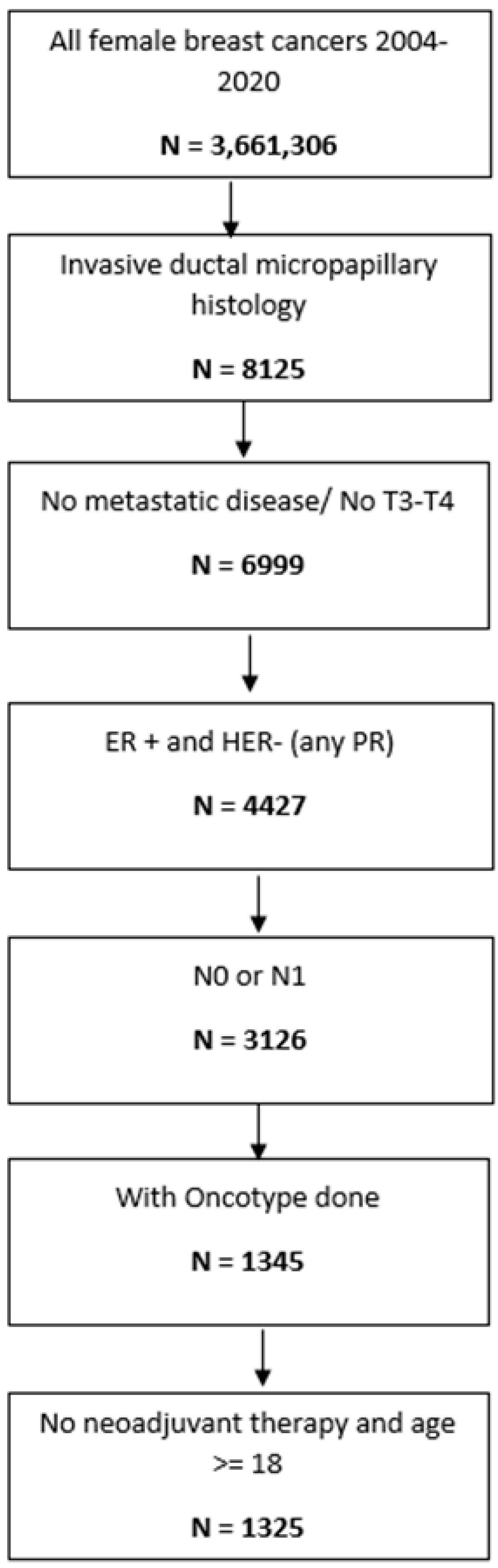
| Characteristics | Category | Total | ≤11 N (%) | 12–25 N (%) | >25 N (%) | Chi-Square p-Value |
|---|---|---|---|---|---|---|
| Age | <60 | 508 | 192 (37.8) | 223 (43.9) | 93 (18.31) | 0.0003 |
| ≥60 | 817 | 401 (49.08) | 302 (36.96) | 114 (13.95) | ||
| Comorbidity score | 0 | 1100 | 499 (45.36) | 433 (39.36) | 168 (15.27) | 0.4171 |
| 1 | 164 | 73 (44.51) | 66 (40.24) | 25 (15.24) | ||
| ≥2 | 61 | 21 (34.43) | 26 (42.62) | 14 (22.95) | ||
| Race | Non-White | 221 | 88 (39.82) | 91 (41.18) | 42 (19) | 0.1697 |
| White | 1104 | 505 (45.74) | 434 (39.31) | 165 (14.95) | ||
| Insurance | Medicare/Uninsured | 634 | 303 (47.79) | 246 (38.8) | 85 (13.41) | 0.0381 |
| Private | 691 | 290 (41.97) | 279 (40.38) | 122 (17.66) | ||
| Grade | Well differentiated | 77 | 42 (54.55) | 34 (44.16) | 1 (1.3) | <0.0001 |
| Moderately differentiated | 853 | 438 (51.35) | 333 (39.04) | 82 (9.61) | ||
| Poorly differentiated | 374 | 106(28.34) | 147 (39.3) | 121 (32.35) | ||
| Undifferentiated | 21 | 7 (33.33) | 11 (52.38) | 3 (14.29) | ||
| Lumpectomy | No | 400 | 157 (39.25) | 178 (44.5) | 65 (16.25) | 0.0239 |
| Yes | 925 | 436 (47.14) | 347 (37.51) | 142 (15.35) | ||
| Radiation | No | 442 | 185 (41.86) | 185 (41.86) | 72 (16.29) | 0.3216 |
| Yes | 883 | 408 (46.21) | 340 (38.51) | 135 (15.29) | ||
| Chemotherapy | No | 1057 | 578 (54.68) | 434 (41.06) | 45 (4.26) | <0.0001 |
| Yes | 262 | 13 (4.96) | 87 (33.21) | 162 (61.83) |
| Oncotype | Alive at 5 Years | Dead at 5 Years | Total |
|---|---|---|---|
| ≤11 | 578 (97.5) | 15 (2.5) | 593 |
| 12–25 | 512 (97.5) | 13 (2.4) | 525 |
| >25 | 194 (93.7) | 13 (6.2) | 207 |
| Variables | Univariable Hazard Ratio (95% CI) | Multivariable Hazard Ratio (95% CI) | |
|---|---|---|---|
| Oncotype | ≤11 | 0.41 (0.20, 0.87) | 0.31 (0.11, 0.88) |
| 12–25 | 0.38 (0.18, 0.83) | 0.32(0.12, 0.86) | |
| >25 | ref | ref | |
| Age | <60 | 0.43 (0.20, 0.90) | 0.48 (0.21, 1.09) |
| ≥60 | ref | ref | |
| Race | Non-White | 0.58 (0.21–1.63) | 0.56 (0.20, 1.58) |
| White | ref | ref | |
| Number of comorbidities | 0 vs. ≥2 | 0.21 (0.1, 0.52) | 0.25 (0.10, 0.61) |
| 1 vs. ≥2 | 0.43 (0.15, 1.25) | 0.41 (0.14, 1.19) | |
| ≥2 | ref | ref | |
| Grade | Moderate vs. Well | 0.88 (0.27, 2.9) | 0.84 (0.25, 2.82) |
| Poor vs. Well | 0.62 (0.17, 2.29) | 0.44 (0.11, 1.76) | |
| Undifferentiated vs. Well | 1.14 (0.12, 10.9) | 0.72 (0.07, 7.30) | |
| Well | ref | ref | |
| Insurance | Medicare/Uninsured | 1.98 (1.05, 3.73) | 1.34 (0.66, 2.73) |
| Commercial | ref | ref | |
| Lumpectomy | No | 2.00 (1.08, 3.69) | 0.96 (0.37, 2.45) |
| Yes | ref | ref | |
| Chemotherapy | No | 0.71 (0.34, 1.41) | 1.12 (0.41, 3.00) |
| Yes | ref | ref | |
| Radiation | No | 2.61 (1.41, 4.84) | 2.68 (1.05, 6.86) |
| Yes | ref | ref | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haider, A.J.; Kazmi, M.; Chang, K.; Haque, W.M.; Polychronopoulou, E.; Cummock, J.S.; Hatch, S.S.; Farach, A.M.; Parvathaneni, U.; Butler, E.B.; et al. Oncotype DX Recurrence Score Predicts Survival in Invasive Micropapillary Breast Carcinoma: A National Cancer Database Analysis. Curr. Oncol. 2025, 32, 559. https://doi.org/10.3390/curroncol32100559
Haider AJ, Kazmi M, Chang K, Haque WM, Polychronopoulou E, Cummock JS, Hatch SS, Farach AM, Parvathaneni U, Butler EB, et al. Oncotype DX Recurrence Score Predicts Survival in Invasive Micropapillary Breast Carcinoma: A National Cancer Database Analysis. Current Oncology. 2025; 32(10):559. https://doi.org/10.3390/curroncol32100559
Chicago/Turabian StyleHaider, Ali J., Mohummad Kazmi, Kyle Chang, Waqar M. Haque, Efstathia Polychronopoulou, Jonathon S. Cummock, Sandra S. Hatch, Andrew M. Farach, Upendra Parvathaneni, E. Brian Butler, and et al. 2025. "Oncotype DX Recurrence Score Predicts Survival in Invasive Micropapillary Breast Carcinoma: A National Cancer Database Analysis" Current Oncology 32, no. 10: 559. https://doi.org/10.3390/curroncol32100559
APA StyleHaider, A. J., Kazmi, M., Chang, K., Haque, W. M., Polychronopoulou, E., Cummock, J. S., Hatch, S. S., Farach, A. M., Parvathaneni, U., Butler, E. B., & Teh, B. S. (2025). Oncotype DX Recurrence Score Predicts Survival in Invasive Micropapillary Breast Carcinoma: A National Cancer Database Analysis. Current Oncology, 32(10), 559. https://doi.org/10.3390/curroncol32100559





