Long-Term Risk of Pancreatic Cancer After Acute Acetylcholinesterase Inhibitor Insecticide Exposure: A Nationwide Cohort Study
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Source and Ethics
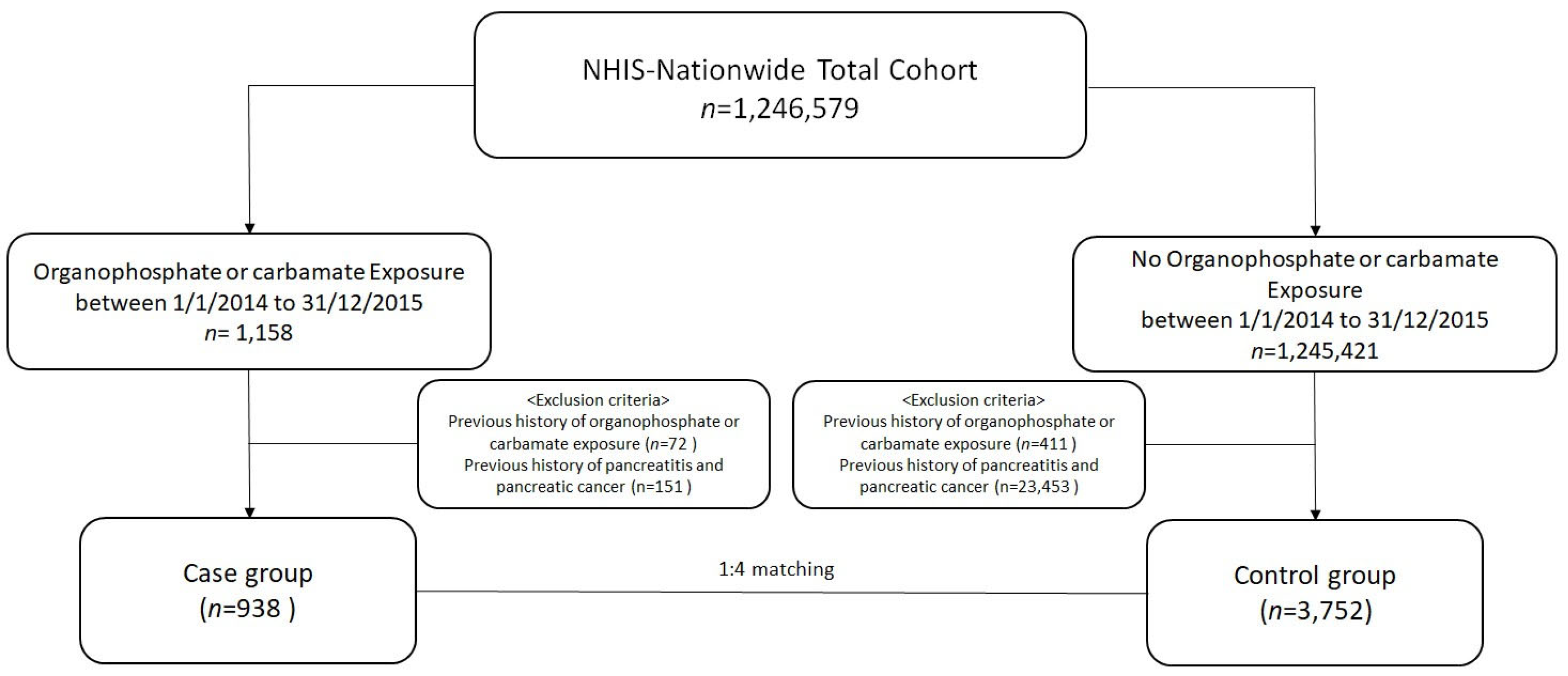
2.2. Study Population
2.3. Primary Outcome and Definitions
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Study Population
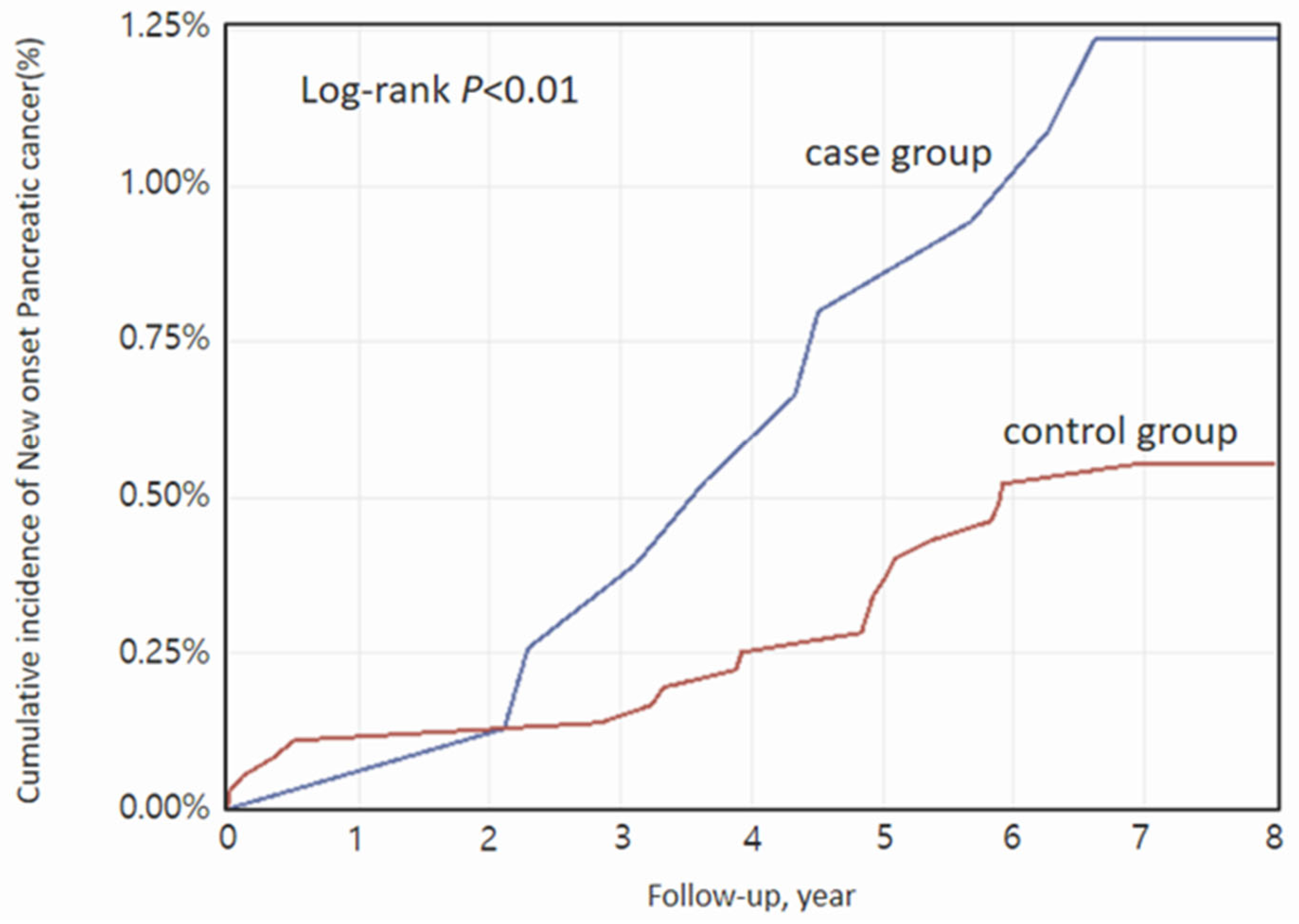
3.2. Main Outcomes
3.3. Subgroup Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, J.X.; Zhao, C.F.; Chen, W.B.; Liu, Q.C.; Li, Q.W.; Lin, Y.Y.; Gao, F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J. Gastroenterol. 2021, 27, 4298–4321. [Google Scholar] [CrossRef]
- Park, H.M.; Won, Y.J.; Kang, M.M.; Park, S.J.; Kim, S.W.; Jung, K.W.; Han, S.S. Trend analysis and prediction of hepatobiliary pancreatic cancer incidence and mortality in Korea. J. Korean Med. Sci. 2022, 37, e216. [Google Scholar] [CrossRef]
- National Cancer Information Center. A Statistical Perspective on Cancer. Natl. Cancer. Inf. Cent. 2025. Available online: http://www.cancer.go.kr (accessed on 1 April 2025).
- Aslanian, H.R.; Lee, J.H.; Canto, M.I. AGA clinical practice update on pancreas cancer screening in high-risk individuals: Expert review. Gastroenterology 2020, 159, 358–362. [Google Scholar] [CrossRef]
- Dbouk, M.; Katona, B.W.; Brand, R.E.; Amitabh, C.; Syngal, S.; Farrell, J.J.; Kastrinos, F.; Goggins, M.; Lennon, A.M.; Petersen, G.M.; et al. The multicenter cancer of pancreas screening study: Impact on stage and survival. J. Clin. Oncol. 2022, 40, 3257–3266. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.V.; Sebastian, E.M.; Iqbal, T.; Tom, A.A. Pesticide applicators and cancer: A systemic review. Rev. Environ. Health 2020, 36, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.C.; Huff, J.; Haseman, J.K.; Alison, R.; Prejean, J.D. Carcinogenesis studies of dichlorvos in Fischer rats and B6C3F1 mice. Jpn. J. Cancer Res. 1991, 82, 157–164. [Google Scholar] [CrossRef]
- Coral, M.N.U.; Ucman, S.; Yildiz, H.; Oztas, H.; Dalkilic, S. Potential neoplastic effects of parathion-methyl on rat liver. J. Environ. Sci. 2009, 21, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, K.; Wakakura, M.; Ishikawa, S. Light microscopic study of endocrine organs of rats treated by carbamate insecticide. J. Toxicol. Sci. 1977, 2, 53–60. [Google Scholar] [CrossRef]
- Rodriguez, V.I.; Mammadova, J.; Permuth, J.B.; Luthra, A.; Pena, L.; Friedman, M.; Dam, A.; Cappelle, S.; Malafa, M.P.; Hallmon, C.; et al. Elevated urinary levels of fungal and environmental toxins in patients with pancreatic ductal adenocarcinoma. J. Gastrointest. Cancer 2024, 56, 4. [Google Scholar] [CrossRef]
- Jung, E.J.; Lee, S.H.; Ryu, H.H. Clinical outcomes according to intentionality of acute pesticide poisoning in South Korea: Retrospective observation study. J. Pak. Med. Assoc. 2022, 72, 1474–1478. [Google Scholar] [CrossRef]
- Park, J.; Kim, Y.W.; Oh, S.H.; Cha, Y.S.; Cha, K.C.; Kim, O.H.; Lee, J.H.; Kim, H.J.; Lee, Y.S.; Kim, T.H.; et al. Acute pancreatitis after carbamate poisoning. J. Korean Soc. Clin. Toxicol. 2014, 12, 77–84. [Google Scholar] [CrossRef]
- Liu, S.; Oguchi, Y.; Borner, J.W.; Runge, W.; Dressel, T.D.; Goodale, R.L. Increased canine pancreatic acinar cell damage after organophosphate and acetylcholine or cholecystokinin. Pancreas 1990, 5, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Okada, H.; Nakano, S.; Shirai, K.; Yuhara, T.; Kojima, H.; Doi, T.; Kato, H.; Suzuki, K.; Morishita, K.; et al. Much caution does no harm! Organophosphate poisoning often causes pancreatitis. J. Intensive Care 2015, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Cha, E.S.; Choi, Y.C.; Kim, J.Y.; Kim, J.H.; Lee, W.J. The burden of acute pesticide poisoning and pesticide regulation in Korea. J. Korean Med. Sci. 2018, 33, e208. [Google Scholar] [CrossRef]
- Zhao, L.Z.Y.; Liu, W. Pancreatic cancer: A review of risk factors, diagnosis, and treatment. Technol. Cancer Res. Treat. 2020, 19, 1533033820962117. [Google Scholar] [CrossRef]
- Cohen, S.M.; Arnold, L.L. Chemical carcinogenesis. Toxicol. Sci. 2011, 120 (Suppl. 1), S76–S92. [Google Scholar] [CrossRef]
- Satar, S.; Kayraldiz, A.; Rencuzogullari, E.; Karakoc, E.; Sebe, A.; Avci, A.; Yesilagac, H.; Topaktas, M. The genotoxicity and cytotoxicity among patients diagnosed with organophosphate poisoning. Bratisl. Lek. Listy 2009, 110, 476–479. [Google Scholar] [PubMed]
- Dandapani, M.; Zachariah, A.; Kavitha, M.R.; Jeyaseelan, L.; Oommen, A. Oxidative damage in intermediate syndrome of acute organophosphorous poisoning. Indian J. Med. Res. 2003, 117, 253–259. [Google Scholar]
- Hundekari, I.A.; Suryakar, A.N.; Rathi, D.B. Acute organophosphorus pesticide poisoning in North Karnataka, India: Oxidative damage, haemoglobin level and total leukocyte. Afr. Health Sci. 2013, 13, 129–136. [Google Scholar] [CrossRef]
- El Rahman, H.A.A.; Salama, M.; El-Hak, S.A.G.; El-Harouny, M.A.; ElKafrawy, P.; Abou-Donia, M.B. A panel of autoantibodies against neural proteins as peripheral biomarker for pesticide-induced neurotoxicity. Neurotox. Res. 2018, 33, 316–336. [Google Scholar] [CrossRef]
- Liang, L.P.; Pearson-Smith, J.N.; Huang, J.; McElroy, P.; Day, B.J.; Patel, M. Neuroprotective effects of AEOL10150 in a rat organophosphate model. Toxicol. Sci. 2018, 162, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Gundogan, K.; Donmez-Altuntas, H.; Hamurcu, Z.; Akbudak, I.H.; Sungur, M.; Bitgen, N.; Baskol, G.; Bayram, F. Evaluation of chromosomal DNA damage, cytotoxicity, cytostasis, oxidative DNA damage and their relationship with endocrine hormones in patients with acute organophosphate poisoning. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 825, 1–7. [Google Scholar] [CrossRef]
- Medina-Buelvas, D.; Estrada-Muñiz, E.; Flores-Valadez, M.; Vega, L. Genotoxic and immunotoxic effects of the organophosphate metabolite diethyldithiophosphate (DEDTP) in vivo. Toxicol. Appl. Pharmacol. 2019, 366, 96–103. [Google Scholar] [CrossRef]
- El-Bini Dhouib, I.; Lasram, M.M.; Abdeladhim, M.; Gharbi, N.; Ben Ahmed, M.; El-Fazaa, S. Immunosuppression and oxidative stress induced by subchronic exposure to carbosulfan in rat spleen: Immunomodulatory and antioxidant role of N-acetylcysteine. Toxicol. Mech. Methods 2014, 24, 417–427. [Google Scholar] [CrossRef]
- Bemis, J.C.; Labash, C.; Avlasevich, S.L.; Carlson, K.; Berg, A.; Torous, D.K.; Barragato, M.; MacGregor, J.T.; Dertinger, S.D. Rat Pig-a mutation assay responds to the genotoxic carcinogen ethyl carbamate but not the non-genotoxic carcinogen methyl carbamate. Mutagenesis 2015, 30, 343–347. [Google Scholar] [CrossRef]
- Thayer, K.A.; Heindel, J.J.; Bucher, J.R.; Gallo, M.A. Role of environmental chemicals in diabetes and obesity: A National Toxicology Program workshop review. Environ. Health Perspect. 2012, 120, 779–789. [Google Scholar] [CrossRef]
- Gifford, R.M.; Chathuranga, U.; Lamb, T.; Verma, V.; Sattar, M.A.; Thompson, A.; Siribaddana, S.; Ghose, A.; Forbes, S.; Reynolds, R.M.; et al. Short-term glucose dysregulation following acute poisoning with organophosphorus insecticides but not herbicides, carbamate or pyrethroid insecticides in South Asia. Clin. Toxicol. 2019, 57, 254–264. [Google Scholar] [CrossRef]
- Lam, B.Q.; Shrivastava, S.K.; Shrivastava, A.; Shankar, S.; Srivastava, R.K. The impact of obesity and diabetes mellitus on pancreatic cancer: Molecular mechanisms and clinical perspectives. J. Cell. Mol. Med. 2020, 24, 7706–7716. [Google Scholar] [CrossRef] [PubMed]
- Majumder, K.; Gupta, A.; Arora, N.; Singh, P.P.; Singh, S. Premorbid obesity and mortality in patients with pancreatic cancer: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 355–368.e2. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Li, N.; Ma, M.; Rao, K.; Yang, R.; Wang, Z. Tricresyl phosphate isomers exert estrogenic effects via G protein-coupled estrogen receptor-mediated pathways. Environ. Pollut. 2020, 264, 114747. [Google Scholar] [CrossRef] [PubMed]
- Gea, M.; Zhang, C.; Tota, R.; Gilardi, G.; Di Nardo, G.; Schilirò, T. Assessment of five pesticides as endocrine disrupting chemicals: Effects on estrogen receptors and aromatase. Int. J. Environ. Res. Public Health 2022, 19, 1959. [Google Scholar] [CrossRef]
- Longnecker, D.S.; Sumi, C. Effects of sex steroid hormones on pancreatic cancer in the rat. Int. J. Pancreatol. 1990, 7, 159–165. [Google Scholar] [CrossRef]
- Qie, Y.; Qin, W.; Zhao, K.; Liu, C.; Zhao, L.; Guo, L.H. Environmental estrogens and their biological effects through GPER mediated signal pathways. Environ. Pollut. 2021, 278, 116826. [Google Scholar] [CrossRef] [PubMed]
- Lumpe, M.; Schurr, J.; Rabe, C.; Ott, A.; Zellner, T.; Rentrop, M.; Eyer, F.; Geith, S. Socio-demographic and psychiatric profiles of patients hospitalized due to self-poisoning with suicidal intension. Ann. Gen. Psychiatry 2022, 21, 16. [Google Scholar] [CrossRef]
- Han, X.; Lin, X.; Li, G.; Wang, J.; Meng, X.; Chen, T.; Zhang, Y.; Fu, X. Association of cancer and schizophrenia, major depression and bipolar disorder: A Mendelian randomization study. J. Psychosom. Res. 2024, 183, 111806. [Google Scholar] [CrossRef]
- Parker, G.; Brotchie, H. Pancreatic Cancer and Depression: A Narrative Review. J. Nerv. Ment. Dis. 2017, 205, 487–490. [Google Scholar] [CrossRef]
- Moessinger, H.; Jacob, L.; Smith, L.; Koyanagi, A.; Kostev, K. Psychiatric disorder and its association with gastrointestinal cancer: A retrospective cohort study with 45,842 patients in Germany. J. Cancer Res. Clin. Oncol. 2023, 149, 14509–14518. [Google Scholar] [CrossRef]
- Alguacil, J.; Kauppinen, T.; Porta, M.; Partanen, T.; Malats, N.; Kogevinas, M.; Benavides, F.G.; Obiol, J.; García-Pérez, J.; Pérez-Gómez, B.; et al. Risk of pancreatic cancer and occupational exposures in Spain. Ann. Occup. Hyg. 2000, 44, 391–403. [Google Scholar] [CrossRef]
- Andreotti, G.; Freeman, L.E.; Hou, L.; Coble, J.; Rusiecki, J.; Hoppin, J.A.; Alavanja, M.C.R.; Beane Freeman, L.E.; Sandler, D.P.; Blair, A.; et al. Agricultural pesticide use and pancreatic cancer risk in the Agricultural Health Study Cohort. Int. J. Cancer 2009, 124, 2495–2500. [Google Scholar] [CrossRef] [PubMed]
- Fritschi, L.; Benke, G.; Risch, H.A.; Schulte, A.; Webb, P.M.; Whiteman, D.C.; Fawcett, J.; Neale, R.E.; McDonald, S.; Armstrong, B.K.; et al. Occupational exposure to N-nitrosamines and pesticides and risk of pancreatic cancer. Occup. Environ. Med. 2015, 72, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.M.; Chun, B.J.; Cho, Y.S. The characteristics of emergency department presentations related to acute herbicide or insecticide poisoning in South Korea between 2011 and 2014. J. Toxicol. Environ. Health A 2016, 79, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Cha, E.S.; Khang, Y.H.; Lee, W.J. Mortality from and Incidence of Pesticide Poisoning in South Korea: Findings from National Death and Health Utilization Data between 2006 and 2010. PLoS ONE 2014, 9, e95299. [Google Scholar] [CrossRef] [PubMed]
| Variables | All | Case Group | Control Group | p-Value |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| All | 4690 (100.0) | 938 (100.0) | 3752 (100.0) | |
| Age (years) | 1.00 | |||
| 18–40 | 300 (6.4) | 60 (6.4) | 240 (6.4) | |
| 41–60 | 2020 (43.1) | 404 (43.1) | 1616 (43.1) | |
| 61–80 | 2000 (42.6) | 400 (42.6) | 1600 (42.6) | |
| 80–120 | 370 (7.9) | 74 (7.9) | 296 (7.9) | |
| Sex | 1.00 | |||
| Male | 3190 (68.0) | 638 (68.0) | 2552 (68.0) | |
| Female | 1500 (32.0) | 300 (32.0) | 1200 (32.0) | |
| Socioeconomic status | 1.00 | |||
| Q1 (Lowest) | 940 (20.0) | 188 (20.0) | 752 (20.0) | |
| Q2 | 710 (15.1) | 142 (15.1) | 568 (15.1) | |
| Q3 | 845 (18.0) | 169 (18.0) | 676 (18.0) | |
| Q4 | 1135 (24.2) | 227 (24.2) | 908 (24.2) | |
| Q5 (Highest) | 1060 (22.6) | 212 (22.6) | 848 (22.6) | |
| Comorbidities | ||||
| Hypertension, yes | 1478 (31.5) | 262 (27.9) | 1216 (32.4) | 0.01 |
| Diabetes, yes | 797 (17.0) | 139 (14.8) | 658 (17.5) | 0.05 |
| CAD, yes | 339 (7.2) | 57 (6.1) | 282 (7.5) | 0.13 |
| CKD, yes | 209 (4.5) | 29 (3.1) | 180 (4.8) | 0.02 |
| Mental disorder, yes | 694 (14.8) | 188 (20.0) | 506 (13.5) | <0.01 |
| Number at Risk | Cancer Events | Person-Years | Incidence Rate per 1000 PYs | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|---|---|---|
| cHR (95% CI) | aHR (95% CI) | aHR (95% CI) | |||||
| Exposure | |||||||
| No | 3752 | 19 | 27,331.5 | 0.70 | ref. | ref. | ref. |
| Yes | 938 | 9 | 5888.3 | 1.53 | 2.41 (1.09–5.34) | 2.61 (1.17–5.81) | 2.57 (1.15–5.75) |
| Number at Risk | Cancer Events | HR (95% CI) | p-Value | |
|---|---|---|---|---|
| Age group (years) | 0.23 | |||
| 18–60 | 464 | 6 | 4.03 (0.65–25.1) | |
| 61–120 | 474 | 3 | 2.39 (0.97–5.89) | |
| Sex | <0.01 | |||
| male | 638 | 4 | 0.99 (0.29–3.41) | |
| female | 300 | 5 | 5.85 (3.49–13.01) | |
| Hypertension | 0.07 | |||
| No | 676 | 6 | 2.02 (0.65–6.27) | |
| Yes | 262 | 3 | 3.48 (1.03–11.68) | |
| Diabetes | <0.01 | |||
| No | 799 | 7 | 1.52 (0.17–13.22) | |
| Yes | 139 | 2 | 2.75 (1.14–6.63) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, J.; Jung, E.; Chun, B.; Kim, D.; Seong, Y. Long-Term Risk of Pancreatic Cancer After Acute Acetylcholinesterase Inhibitor Insecticide Exposure: A Nationwide Cohort Study. Curr. Oncol. 2025, 32, 528. https://doi.org/10.3390/curroncol32100528
Moon J, Jung E, Chun B, Kim D, Seong Y. Long-Term Risk of Pancreatic Cancer After Acute Acetylcholinesterase Inhibitor Insecticide Exposure: A Nationwide Cohort Study. Current Oncology. 2025; 32(10):528. https://doi.org/10.3390/curroncol32100528
Chicago/Turabian StyleMoon, JeongMi, EuJene Jung, ByeongJo Chun, DongKi Kim, and YeonJi Seong. 2025. "Long-Term Risk of Pancreatic Cancer After Acute Acetylcholinesterase Inhibitor Insecticide Exposure: A Nationwide Cohort Study" Current Oncology 32, no. 10: 528. https://doi.org/10.3390/curroncol32100528
APA StyleMoon, J., Jung, E., Chun, B., Kim, D., & Seong, Y. (2025). Long-Term Risk of Pancreatic Cancer After Acute Acetylcholinesterase Inhibitor Insecticide Exposure: A Nationwide Cohort Study. Current Oncology, 32(10), 528. https://doi.org/10.3390/curroncol32100528





