Real-World Treatment Patterns, Sequencing, and Outcomes in Patients with Locally Advanced or Metastatic Urothelial Carcinoma Receiving Avelumab First-Line Maintenance in the United States
Abstract
1. Introduction
2. Methods
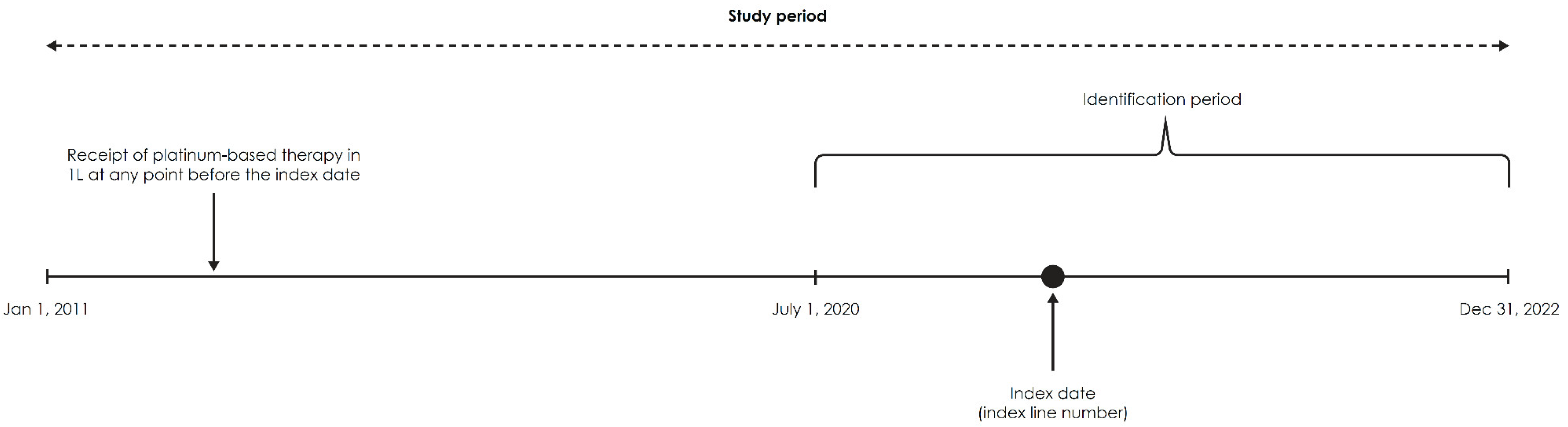
2.1. Data Source and Study Design
2.2. Study Population
2.3. Study Measures
2.4. Statistical Analysis
3. Results
3.1. Baseline Demographics and Clinical Characteristics
3.2. Treatment Patterns/Sequencing
3.3. Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, Based on 2022 Submission Data (1999–2020). Available online: https://www.cdc.gov/cancer/dataviz (accessed on 17 April 2024).
- American Cancer Society. Key Statistics for Bladder Cancer. Available online: https://www.cancer.org/cancer/types/bladder-cancer/about/key-statistics.html (accessed on 3 March 2024).
- National Cancer Institute. Cancer Stat Facts: Bladder Cancer. Available online: https://seer.cancer.gov/statfacts/html/urinb.html (accessed on 17 April 2024).
- National Comprehensive Cancer Network. Bladder Cancer (Version 4.2024). Available online: https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf (accessed on 16 May 2024).
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulovic, S.; Demey, W.; Ullen, A.; et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.H.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Ullen, A.; Loriot, Y.; Sridhar, S.S.; Sternberg, C.N.; Bellmunt, J.; et al. Avelumab first-line maintenance for advanced urothelial carcinoma: Results from the JAVELIN Bladder 100 trial after >/=2 years of follow-up. J. Clin. Oncol. 2023, 41, 3486–3492. [Google Scholar] [CrossRef] [PubMed]
- Dash, A.; Galsky, M.D.; Vickers, A.J.; Serio, A.M.; Koppie, T.M.; Dalbagni, G.; Bochner, B.H. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer 2006, 107, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Hahn, N.M.; Rosenberg, J.; Sonpavde, G.; Hutson, T.; Oh, W.K.; Dreicer, R.; Vogelzang, N.; Sternberg, C.N.; Bajorin, D.F.; et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J. Clin. Oncol. 2011, 29, 2432–2438. [Google Scholar] [CrossRef]
- Gupta, S.; Bellmunt, J.; Plimack, E.R.; Sonpavde, G.P.; Grivas, P.; Apolo, A.B.; Pal, S.K.; Siefker-Radtke, A.O.; Flaig, T.W.; Galsky, M.D.; et al. Defining “platinum-ineligible” patients with metastatic urothelial cancer (mUC). J. Clin. Oncol. 2022, 40, 4577. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Sridhar, S.S.; Zhang, J.; Smith, D.C.; Ruether, J.D.; Flaig, T.W.; Baranda, J.C.; Lang, J.M.; Plimack, E.R.; Sangha, R.S.; et al. Updated results from the enfortumab vedotin phase 1 (EV-101) study in patients with metastatic urothelial cancer (mUC). J. Clin. Oncol. 2018, 36, 4504. [Google Scholar] [CrossRef]
- Siefker-Radtke, A.O.; Necchi, A.; Park, S.H.; GarcÃa-Donas, J.; Huddart, R.A.; Burgess, E.F.; Fleming, M.T.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. First results from the primary analysis population of the phase 2 study of erdafitinib (ERDA; JNJ-42756493) in patients (pts) with metastatic or unresectable urothelial carcinoma (mUC) and FGFR alterations (FGFRalt). J. Clin. Oncol. 2018, 36, 4503. [Google Scholar] [CrossRef]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Balar, A.V.; Petrylak, D.P.; Kalebasty, A.R.; Loriot, Y.; Flechon, A.; Jain, R.K.; Agarwal, N.; Bupathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J. Clin. Oncol. 2021, 39, 2474–2485. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.Y.; Banerjee, S.; Gonzalez-Martin, A.; Jung, K.H.; Lugowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: Primary results from the DESTINY-PanTumor02 phase II trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef]
- Birnbaum, B.; Nussbaum, N.; Seidl-Rathkopf, K.; Agrawal, M.; Estévez, M.; Estola, E.; Haimson, J.; He, L.; Larson, P.; Richardson, P. [Pre-print] Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv 2020. [Google Scholar] [CrossRef]
- Flatiron Health. Quick Facts. Available online: https://flatiron.com/media (accessed on 17 April 2024).
- Ma, X.; Long, L.; Moon, S.; Adamson, B.J.S.; Baxi, S.S. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. medRXiv 2020. [Google Scholar] [CrossRef]
- Public Policy Committee; International Society of Pharmacoepidemiology. Guidelines for good pharmacoepidemiology practice (GPP). Pharmacoepidemiol. Drug Saf. 2016, 25, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.L.; Sox, H.; Willke, R.J.; Brixner, D.L.; Eichler, H.G.; Goettsch, W.; Madigan, D.; Makady, A.; Schneeweiss, S.; Tarricone, R.; et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: Recommendations from the joint ISPOR-ISPE Special Task Force on real-world evidence in health care decision making. Pharmacoepidemiol. Drug Saf. 2017, 26, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, N.A.; Bryant, A.; Velentgas, P. The GRACE checklist: A validated assessment tool for high quality observational studies of comparative effectiveness. J. Manag. Care Spec. Pharm. 2016, 22, 1107–1113. [Google Scholar] [CrossRef]
- Moon, H.H.; Aragon-Ching, J.B.; Thompson, A.; Abraham, A.; Vlahiotis, A.; Ike, C.; Benjumea, D.; Shao, A.; Sun, H.; Kearney, M.; et al. Real-world response rates and clinical outcomes of patients treated with first-line (1L) platinum-based chemotherapy (PBC) in advanced urothelial cancer (aUC). J. Clin. Oncol. 2023, 41, 4567. [Google Scholar] [CrossRef]
- Carson, K.; Mahmoudpour, H.; Ike, C.; Monzon, S.; Fragkogianni, S.; Ferrer, J.; Kearney, M. 2387P Avelumab first-line maintenance (1LM) for locally advanced or metastatic urothelial carcinoma (la/mUC): Treatment (tx) patterns and real-world (rw) outcomes in the US. Ann. Oncol. 2023, 34, S1215–S1216. [Google Scholar] [CrossRef]
- Powles, T.; Valderrama, B.P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J.; et al. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N. Engl. J. Med. 2024, 390, 875–888. [Google Scholar] [CrossRef]
- van der Heijden, M.S.; Sonpavde, G.; Powles, T.; Necchi, A.; Burotto, M.; Schenker, M.; Sade, J.P.; Bamias, A.; Beuzeboc, P.; Bedke, J.; et al. Nivolumab plus gemcitabine-cisplatin in advanced urothelial carcinoma. N. Engl. J. Med. 2023, 389, 1778–1789. [Google Scholar] [CrossRef]
- Grivas, P.; Barata, P.C.; Moon, H.H.; Gupta, S.; Hutson, T.E.; Sternberg, C.N.; Brown, J.; Dave, V.; Downey, C.; Shillington, A.C.; et al. Avelumab first-line maintenance therapy for locally advanced/metastatic urothelial carcinoma: Results from the real-world US PATRIOT-II study. J. Clin. Oncol. 2024, 42, 697. [Google Scholar] [CrossRef]
- Bracarda, S.; Antonuzzo, L.; Maruzzo, M.; Santini, D.; Tambaro, R.; Buti, S.; Carrozza, F.; Calabrò, F.; Di Lorenzo, G.; Fornarini, G.; et al. Subgroup analyses from READY: Real-world data from an Italian compassionate use program (CUP) of avelumab first-line maintenance (1LM) treatment for locally advanced or metastatic urothelial carcinoma (la/mUC). J. Clin. Oncol. 2024, 42, 558. [Google Scholar] [CrossRef]
- Barthelemy, P.; Loriot, Y.; Thibault, C.; Gross-Goupil, M.; Eymard, J.C.; Voog, E.; Abraham Jaillon, C.; Le Moulec, S.; Chasseray, M.; Gobert, A.; et al. Updated results from AVENANCE: Real-world effectiveness of avelumab first-line maintenance (1LM) in patients (pts) with advanced urothelial carcinoma (aUC) and analysis of subsequent treatment. J. Clin. Oncol. 2024, 42, 561. [Google Scholar] [CrossRef]
- Thomas, V.M.; Jo, Y.; Tripathi, N.; Roy, S.; Chigarira, B.; Narang, A.; Gebrael, G.; Hage Chehade, C.; Sayegh, N.; Galarza Fortuna, G.; et al. Treatment patterns and attrition with lines of therapy for advanced urothelial carcinoma in the US. JAMA Netw. Open 2024, 7, e249417. [Google Scholar] [CrossRef]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Duran, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef]
- Nizam, A.; Jindal, T.; Jiang, C.Y.; Alhalabi, O.; Bakaloudi, D.R.; Talukder, R.; Davidsohn, M.P.; Nguyen, C.B.; Oh, E.; Taylor, A.K.; et al. Outcomes in patients (pts) with advanced urothelial carcinoma (aUC) treated with enfortumab vedotin (EV) after switch maintenance avelumab (MAv) in the UNITE study. J. Clin. Oncol. 2024, 42, 537. [Google Scholar] [CrossRef]
- Tucker, T.C.; Charlton, M.E.; Schroeder, M.C.; Jacob, J.; Tolle, C.L.; Evers, B.M.; Mullett, T.W. Improving the quality of cancer care in community hospitals. Ann. Surg. Oncol. 2021, 28, 632–638. [Google Scholar] [CrossRef]
- Schlack, K.; Sultanbaev, A.; Lorente, D.; Zihler, D.; Kostkova, L.; Cremer, L.; Gschwend, J. Baseline characteristics from AVENUE, a real-world observational study of avelumab first-line maintenance in patients with locally advanced/metastatic urothelial carcinoma. In Proceedings of the DGU 2023, Leipzig, Germany, 20–23 September 2023. [Google Scholar]
- Su, P.-J.; Park, S.H.; Tsai, Y.C.; Kim, M.; Wu, W.-J.; Gao, S.; Shin, S.J. SPADE: Design of a real-world observational study of avelumab first-line (1L) maintenance in advanced urothelial carcinoma (UC) in the Asia-Pacific (APAC) region. J. Clin. Oncol. 2023, 41, TPS577. [Google Scholar] [CrossRef]
- Ike, C.; Kongnakorn, T.; Tichy, E.; Sanchez Alvarez, J.; Kearney, M. Costs associated with novel first-line (1L) treatments in patients (pts) with locally advanced or metastatic urothelial carcinoma (la/mUC) from a United States (US) Medicare and commercial payer perspective. J. Clin. Oncol. 2024, 42, e16552. [Google Scholar] [CrossRef]
- Climent, M.A.; Alvarez, C.; Morales, R.; Maroto, P.; Rodriguez-Vida, A.; Mendez-Vidal, M.J.; Del Muro, X.G.; Puente, J.; Lainez, N.; Vazquez, S.; et al. Exploratory analyses of treatment subgroup interaction by PD-L1 status and according to PD-L1 expression in the JAVELIN Bladder 100 trial. Clin. Transl. Oncol. 2024, 26, 1532–1538. [Google Scholar] [CrossRef]
- Powles, T.; Bellmunt, J.; Comperat, E.; De Santis, M.; Huddart, R.; Loriot, Y.; Necchi, A.; Valderrama, B.P.; Ravaud, A.; Shariat, S.F.; et al. ESMO Clinical Practice Guideline interim update on first-line therapy in advanced urothelial carcinoma. Ann. Oncol. 2024, 35, 485–490. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Carrión, A.; Cathomas, R.; Compérat, E.M.; Efstathiou, J.A.; Fietkau, R.; Gakis, G.; van der Heijden, A.G.; Lorch, A.; et al. EAU Guidelines on Muscle-Invasive and Metastatic Bladder Cancer; EAU Guidelines Office: Arnhem, The Netherlands, 2024. [Google Scholar]



| Characteristic | Avelumab 1LM (n = 214) |
|---|---|
| Age at la/mUC diagnosis, years | |
| Mean (Std Dev) | 69.0 (9.2) |
| Median (Q1, Q3) | 70.0 (64.0, 76.0) |
| Year of index date, n (%) | |
| 2020 | 43 (20.1) |
| 2021 | 75 (35.0) |
| 2022 | 96 (44.9) |
| Sex, n (%) | |
| Male | 164 (76.6) |
| Female | 50 (23.4) |
| Race, n (%) | |
| White | 142 (66.4) |
| Other | 31 (14.5) |
| Black or African American | 6 (2.8) |
| Asian | 2 (0.9) |
| Unknown | 33 (15.4) |
| Region of residence, n (%) | |
| South | 99 (46.3) |
| Northeast | 37 (17.3) |
| Midwest | 30 (14.0) |
| West | 23 (10.7) |
| Other | 1 (0.5) |
| Unknown | 24 (11.2) |
| Practice type, n (%) | |
| Community | 191 (89.3) |
| Academic | 18 (8.4) |
| Both | 5 (2.3) |
| Site of disease, n (%) | |
| Bladder | 158 (73.8) |
| Renal pelvis | 30 (14.0) |
| Ureter | 24 (11.2) |
| Urethra | 2 (0.9) |
| Disease grade, n (%) | |
| High grade (G2/G3/G4) | 174 (81.3) |
| Low grade (G1) | 12 (5.6) |
| Unknown/not documented | 28 (13.1) |
| Stage at initial diagnosis, n (%) | |
| 0 | 2 (0.9) |
| I | 3 (1.4) |
| II | 9 (4.2) |
| III | 7 (3.3) |
| IV | 99 (46.3) |
| Unknown/not documented | 94 (43.9) |
| ECOG PS at la/mUC diagnosis date, n (%) | |
| 0 | 69 (32.2) |
| 1 | 96 (44.9) |
| 2+ | 21 (9.8) |
| Unknown/not documented | 28 (13.1) |
| Body mass index, kg/m2, n (%) | |
| Underweight (<18.5) | 9 (4.2) |
| Normal (18.5–24.9) | 69 (32.2) |
| Overweight (25–29.9) | 59 (27.6) |
| Obese (≥30) | 60 (28.0) |
| Unknown | 17 (7.9) |
| Sites of metastases, n (%) | |
| Distant lymph node | 122 (57.0) |
| Bone | 63 (29.4) |
| Lung | 58 (27.1) |
| Liver | 37 (17.3) |
| Soft tissue | 21 (9.8) |
| Peritoneum | 8 (3.7) |
| Other | 6 (2.8) |
| Pleura | 5 (2.3) |
| Adrenal | 5 (2.3) |
| Brain | 2 (0.9) |
| Skin | 1 (0.5) |
| Kidney | 1 (0.5) |
| PD-L1 status, n (%) | |
| Positive | 25 (11.7) |
| Negative | 39 (18.2) |
| Unknown/not documented | 150 (70.1) |
| Follow-up time, months | |
| Median (Q1, Q3) | 8.7 (4.5, 15.7) |
| 1L carboplatin-based regimens (n = 99, 46.3%), n (%) | |
| Carboplatin plus gemcitabine | 98 (99.0) |
| Carboplatin plus paclitaxel | 1 (1.0) |
| 1L cisplatin-based regimens (n = 115, 53.7%), n (%) | |
| Cisplatin plus gemcitabine | 101 (87.8) |
| MVAC | 8 (7.0) |
| Cisplatin monotherapy | 2 (1.7) |
| Cisplatin, bicalutamide, gemcitabine, and leuprolide | 1 (0.9) |
| Cisplatin, gemcitabine, and hydroxyurea | 1 (0.9) |
| Cisplatin plus paclitaxel | 1 (0.9) |
| MVAC plus leuprolide | 1 (0.9) |
| Best response to 1L PBC *, n (%) | |
| rwCR | 14 (6.9) |
| rwPR | 159 (78.7) |
| rwSD | 29 (14.4) |
| Patients Treated with Avelumab 1LM (n = 214) | |
| Duration (months) of avelumab 1LM treatment, median (Q1, Q3) | 3.9 (1.9, 7.2) |
| Median follow-up, months (Q1, Q3) | 8.7 (4.5, 15.7) |
| Best response to 1LM *, n (%) | |
| rwCR | 16 (9.4) |
| rwPR | 44 (25.7) |
| rwSD | 44 (25.7) |
| ORR, % (95% CI) | 35.1 (28.0–42.7) |
| Outcomes from 1LM initiation, median (95% CI), months | |
| rwOS | 23.8 (18.2—NE) |
| rwPFS | 5.1 (4.1–7.0) |
| TTD | 4.9 (4.2–6.5) |
| TTNT | 7.0 (5.6–8.6) |
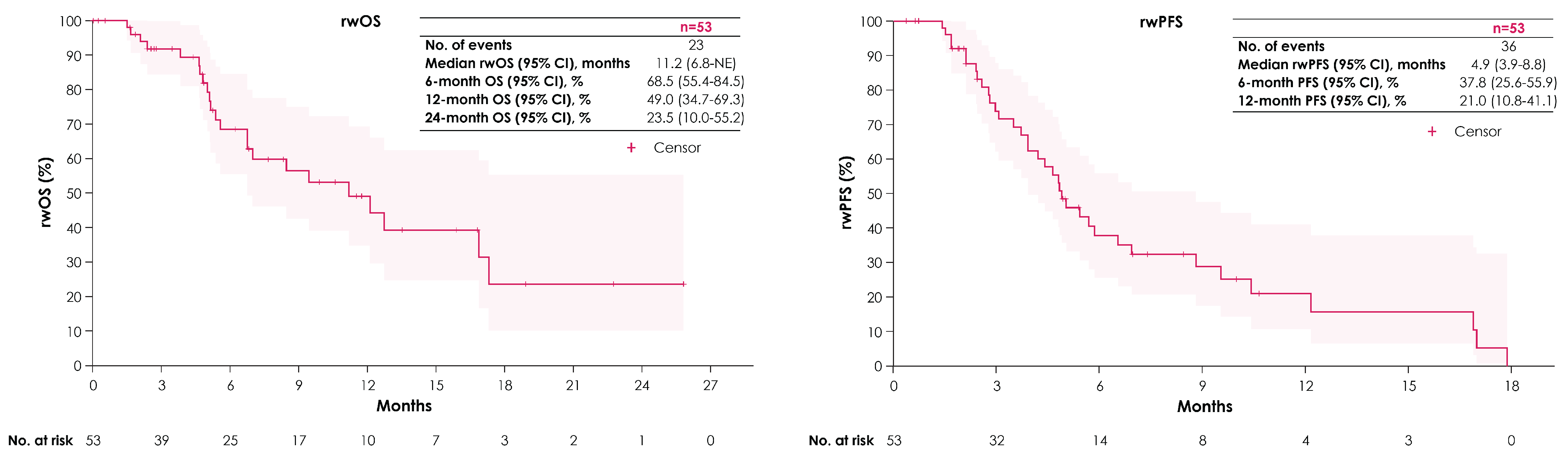
| Patients Treated with Subsequent 2L EV (n = 53) | |
| Duration (months) of 2L EV treatment, median (Q1, Q3) | 4.2 (1.6, 6.3) |
| Outcomes from 2L EV initiation, median (95% CI), months | |
| rwOS | 11.2 (6.8—NE) |
| rwPFS | 4.9 (3.9–8.8) |
| TTD | 4.7 (4.2–8.5) |
| TTNT | 5.8 (5.2–9.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, H.H.; Kearney, M.; Mahmoudpour, S.H.; Ike, C.; Morris, V.; Rava, A.; Kim, S.; Sun, H.; Boyd, M.; Gomez Rey, G. Real-World Treatment Patterns, Sequencing, and Outcomes in Patients with Locally Advanced or Metastatic Urothelial Carcinoma Receiving Avelumab First-Line Maintenance in the United States. Curr. Oncol. 2024, 31, 5662-5676. https://doi.org/10.3390/curroncol31090420
Moon HH, Kearney M, Mahmoudpour SH, Ike C, Morris V, Rava A, Kim S, Sun H, Boyd M, Gomez Rey G. Real-World Treatment Patterns, Sequencing, and Outcomes in Patients with Locally Advanced or Metastatic Urothelial Carcinoma Receiving Avelumab First-Line Maintenance in the United States. Current Oncology. 2024; 31(9):5662-5676. https://doi.org/10.3390/curroncol31090420
Chicago/Turabian StyleMoon, Helen H., Mairead Kearney, Seyed Hamidreza Mahmoudpour, Chiemeka Ike, Valerie Morris, Andrew Rava, Sonia Kim, Haiyan Sun, Marley Boyd, and Gabriel Gomez Rey. 2024. "Real-World Treatment Patterns, Sequencing, and Outcomes in Patients with Locally Advanced or Metastatic Urothelial Carcinoma Receiving Avelumab First-Line Maintenance in the United States" Current Oncology 31, no. 9: 5662-5676. https://doi.org/10.3390/curroncol31090420
APA StyleMoon, H. H., Kearney, M., Mahmoudpour, S. H., Ike, C., Morris, V., Rava, A., Kim, S., Sun, H., Boyd, M., & Gomez Rey, G. (2024). Real-World Treatment Patterns, Sequencing, and Outcomes in Patients with Locally Advanced or Metastatic Urothelial Carcinoma Receiving Avelumab First-Line Maintenance in the United States. Current Oncology, 31(9), 5662-5676. https://doi.org/10.3390/curroncol31090420





