Evaluating the Effects of Prostate Radiotherapy Intensified with Pelvic Nodal Radiotherapy and Androgen Deprivation Therapy on Myelosuppression: Single-Institution Experience
Abstract
1. Introduction
2. Materials and Methods
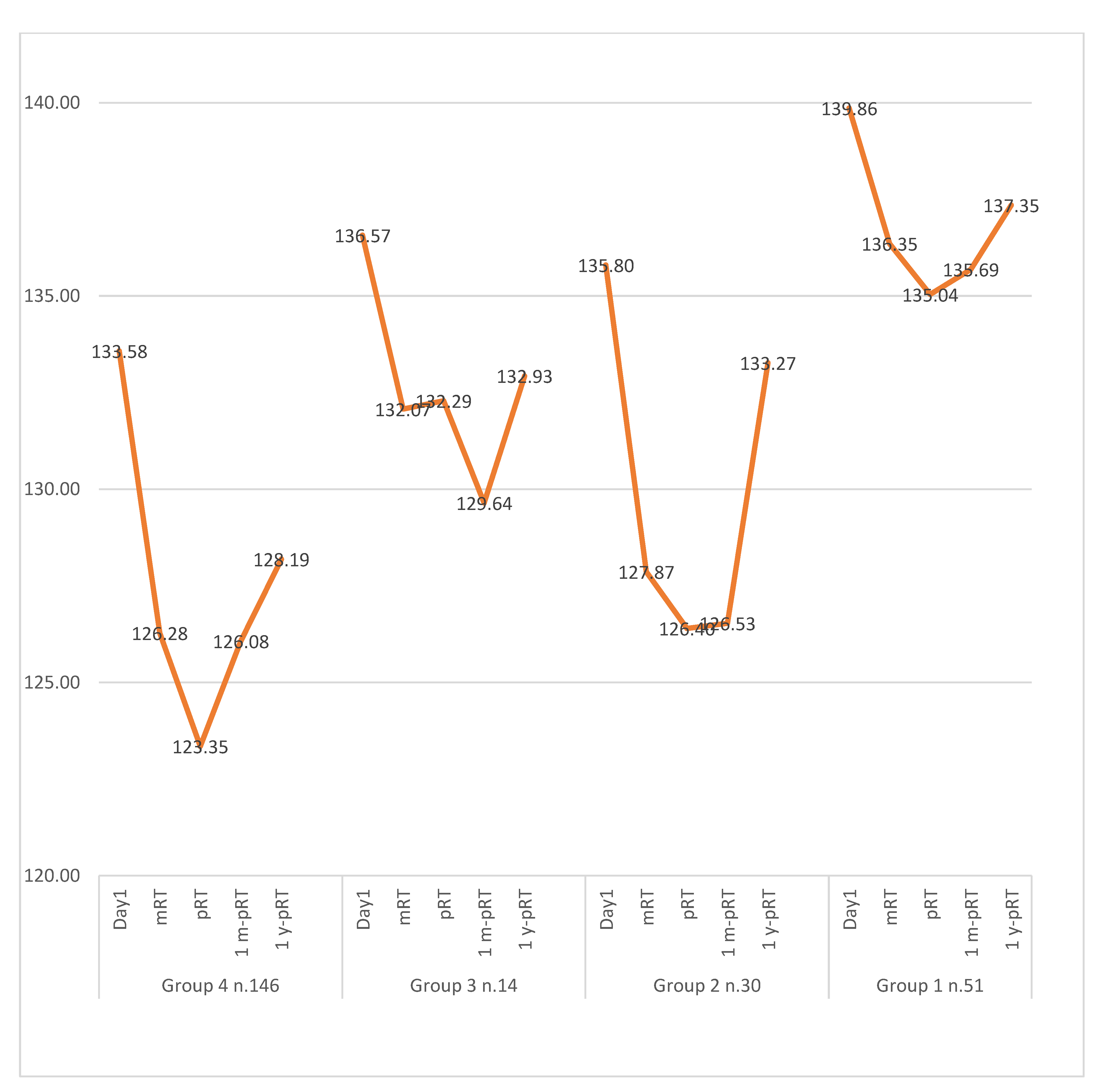
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCa | Prostate cancer |
| PRT | Prostate radiotherapy |
| PNRT | Pelvic nodal radiotherapy |
| RT | Radiotherapy |
| ADT | Androgen deprivation therapy |
| LHRH | Luteinizing hormone-releasing hormone |
| 3D-CRT | 3D conformal radiotherapy |
| IMRT | Intensity-modulated radiotherapy |
| HGB | Hemoglobin |
| WBC | White blood cell |
| PLT | Platelets |
| NEUT | Neutrophils |
| LYMPH | Lymphocytes |
| BM | Bone marrow |
| CTCAE | Common Terminology Criteria for Adverse Effects |
References
- Bray, F.; Vyas, M.; Henderson, A.M.; Leslie, S.W. Nuclear Medicine Applications in Prostate Cancer. In Stat Pearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Islami, F.; Ward, E.M.; Sung, H.; Cronin, K.; Tangka, F.K.L.; Sherman, R.L.; Zhao, J.; Anderson, R.N.; Henley, S.J.; Yabroff, K.R.; et al. Annual report to the nation on the status of cancer, part 1: National cancer statistics. J. Natl. Cancer Inst. 2021, 113, 1648–1669. [Google Scholar] [CrossRef] [PubMed]
- Rasul, S.; Haug, A.R. Clinical Applications of PSMA PET Examination in Patients with Prostate Cancer. Cancers 2022, 14, 3768. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Iagaru, A.; Aparici, C.M. Radiotheranostics—Precision Medicine in Nuclear Medicine and Molecular Imaging. Nanotheranostics 2022, 6, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Gillessen, S.; Heidenreich, A.; Horwich, A. Cancer of the prostate: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. S5), v69–v77. [Google Scholar] [CrossRef] [PubMed]
- Bolla, M.; de Reijke, T.M.; Van Tienhoven, G.; Bergh, A.C.V.D.; Oddens, J.; Poortmans, P.M.; Gez, E.; Kil, P.; Akdas, A.; Soete, G.; et al. Duration of androgen suppression in the treatment of prostate cancer. N. Engl. J. Med. 2009, 360, 2516–2527. [Google Scholar] [CrossRef]
- Erpolat, O.P.; Alco, G.; Caglar, H.B.; Igdem, S.; Saran, A.; Dagoglu, N.; Aslay, I.; Ozsaran, Z.; Demirci, S.; Keven, E.; et al. Comparison of hematologic toxicity between 3DCRT and IMRT planning in cervical cancer patients after concurrent chemoradiotherapy: A national multi-center study. Eur. J. Gynaecol. Oncol. 2014, 35, 62–66. [Google Scholar]
- Hayman, J.A.; Callahan, J.W.; Herschtal, A.; Everitt, S.; Binns, D.S.; Hicks, R.J.; Mac Manus, M. Distribution of proliferating bone marrow in adult cancer patients determined using FLT-PET imaging. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 847–852. [Google Scholar] [CrossRef]
- Strum, S.B.; McDermed, J.E.; Scholz, M.C.; Johnson, H.; Tisman, G. Anaemia associated with androgen deprivation in patients with prostate cancer receiving combined hormone blockade. Br. J. Urol. 1997, 79, 933–941. [Google Scholar] [CrossRef]
- Gruca, D.; Bacher, P.; Tunn, U. Safety and tolerability of intermittent androgen deprivation therapy: A literature review. Int. J. Urol. 2012, 19, 614–625. [Google Scholar] [CrossRef]
- Lehar, T.J.; Kiely, J.M.; Pease, G.L.; Scanlon, P.W. Effect of focal irradiation on human bone marrow. Am. J. Roentgenol. Radium. Ther. Nucl. Med. 1966, 96, 183–190. [Google Scholar] [CrossRef]
- James, N.D.; Spears, M.R.; Clarke, N.W.; Dearnaley, D.P.; De Bono, J.S.; Gale, J.; Hetherington, J.; Hoskin, P.J.; Jones, R.J.; Laing, R.; et al. Survival with newly diagnosed meta- static prostate cancer in the “docetaxel era”: Data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019). Eur. Urol. 2015, 67, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Everitt, S.; Hicks, R.J.; Ball, D.; Kron, T.; Schneider-Kolsky, M.; Walter, T.; Binns, D.; Mac Manus, M. Imaging cellular proliferation during chemo-radiotherapy: A pilot study of serial 18F-FLT positron emission tomography/computed tomography imaging for non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Sykes, M.P.; Chu, F.C.; Savel, H.; Bonadonna, G.; Mathis, H. The effects of varying dosages of irradiation upon sternal-marrow regeneration. Radiology 1964, 83, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Sykes, M.P.; Savel, H.; Chu, F.C.; Bonadonna, G.; Farrow, J.; Mathis, H. Long-term effects of therapeutic irradiation upon bone marrow. Cancer 1964, 17, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- Tubiana, M.; Frindel, E.; Croizat, H.; Parmentier, C. Effects of radiations on bone marrow. Pathol. Biol. 1979, 27, 326–334. [Google Scholar]
- Hui, B.; Zhang, Y.; Shi, F.; Wang, J.; Wang, T.; Wang, J.; Yuan, W.; Li, Y.; Liu, Z. Association between bone marrow dosimetric parameters and acute hematologic toxicity in cervical cancer patients undergoing concurrent chemoradiotherapy: Comparison of three-dimensional conformal radiotherapy and intensity-modulated radiation therapy. Int. J. Gynecol. Cancer 2014, 24, 1648–1652. [Google Scholar] [CrossRef]
- Rose, B.S.; Aydogan, B.; Liang, Y.; Yeginer, M.; Hasselle, M.D.; Dandekar, V.; Bafana, R.; Yashar, C.M.; Mundt, A.J.; Roeske, J.C.; et al. Normal tissue complication probability modeling of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 800–807. [Google Scholar] [CrossRef]
- Blomlie, V.; Rofstad, E.K.; Skjønsberg, A.; Tverå, K.; Lien, H.H. Female pelvic bone marrow: Serial MR imaging before, during, and after radiation therapy. Radiology 1995, 194, 537–543. [Google Scholar] [CrossRef]
- Sacks, E.L.; Goris, M.L.; Glatstein, E.; Gilbert, E.; Kaplan, H.S. Bone marrow regeneration following large field radiation: Influence of volume, age, dose, and time. Cancer 1978, 42, 1057–1065. [Google Scholar] [CrossRef]
- Mauch, P.; Constine, L.; Greenberger, J.; Knospe, W.; Sullivan, J.; Liesveld, J.L.; Deeg, H. Hematopoietic stem cell compartment: Acute and late effects of radiation therapy and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1319–1339. [Google Scholar] [CrossRef]
- Zelefsky, M.J.; Fuks, Z.; Happersett, L.; Lee, H.J.; Ling, C.; Burman, C.M.; Hunt, M.; Wolfe, T.; Venkatraman, E.; Jackson, A.; et al. Clinical experience with intensity modulated radiation therapy (IMRT) in prostate cancer. Radiother. Oncol. 2000, 55, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Hummel, S.; Simpson, E.L.; Hemingway, P.; Stevenson, M.D.; Rees, A. Intensity-modulated radiotherapy for the treatment of prostate cancer: A systematic review and economic evaluation. Health Technol. Assess. 2010, 14, 1–108, iii–iv. [Google Scholar] [CrossRef] [PubMed]
- Miszczyk, M.; Majewski, W. Hematologic Toxicity of Conformal Radiotherapy and Intensity Modulated Radiotherapy in Prostate and Bladder Cancer Patients. Asian Pac. J. Cancer Prev. 2018, 19, 2803–2806. [Google Scholar]
- Mell, L.K.; Kochanski, J.D.; Roeske, J.C.; Haslam, J.J.; Mehta, N.; Yamada, S.D.; Hurteau, J.A.; Collins, Y.C.; Lengyel, E.; Mundt, A.J. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 1356–1365. [Google Scholar] [CrossRef]
- Mell, L.K.; Schomas, D.A.; Salama, J.K.; Devisetty, K.; Aydogan, B.; Miller, R.C.; Jani, A.B.; Kindler, L.H.; Mundt, A.J.; Roeske, J.C.; et al. Association between bone marrow dosimetric parameters and acute hematologic toxicity in anal cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.S.; Kim, R.Y.; Duan, J.; Meleth, S.; Jennifer, F.; Fiveash, J.B. IMRT dose escalation for positive para-aortic lymph nodes in patients with locally advanced cervical cancer while reducing dose to bone marrow and other organs at risk. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 505–512. [Google Scholar] [CrossRef]
- Brixey, C.J.; Roeske, J.C.; Lujan, A.E.; Yamada, S.D.; Rotmensch, J.; Mundt, A.J. Impact of intensity-modulated radiotherapy on acute hematologic toxicity in women with gynecologic malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 1388–1396. [Google Scholar] [CrossRef]
- Mell, L.K.; Tiryaki, H.; Ahn, K.H.; Mundt, A.J.; Roeske, J.C.; Aydogan, B. Dosimetric comparison of bone marrow-sparing intensity-modulated radiotherapy versus conventional techniques for treatment of cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1504A–1510A. [Google Scholar] [CrossRef]
- Viani, G.A.; Viana, B.S.; Martin, J.E.; Rossi, B.T.; Zuliani, G.; Stefano, E.J. Intensity-modulated radiotherapy reduces toxicity with similar biochemical control compared with 3-dimensional conformal radiotherapy for prostate cancer: A randomized clinical trial. Cancer 2016, 122, 2004–2011. [Google Scholar] [CrossRef]
- Moreno, A.; Clemente, J.; Crespo, C.; Martínez, A.; Navarro, M.; Fernández, L.; Minguell, J.; Vázquez, G.; Andreu, F.J. Pelvic insufficiency fractures in patients with pelvic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 61–66. [Google Scholar] [CrossRef]
- Ikushima, H.; Osaki, K.; Furutani, S.; Yamashita, K.; Kishida, Y.; Kudoh, T.; Nishitani, H. Pelvic bone complications following radiation therapy of gynecologic malignancies: Clinical evaluation of radiation-induced pelvic insufficiency fractures. Gynecol. Oncol. 2006, 103, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Okonogi, N.; Saitoh, J.-I.; Suzuki, Y.; Noda, S.-E.; Ohno, T.; Oike, T.; Ohkubo, Y.; Ando, K.; Sato, H.; Nakano, T. Changes in bone mineral density in uterine cervical cancer patients after radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Avinash, H.U.; Arul Ponni, T.R.; Janaki, M.G.; Kirthi Koushik, A.S.; Kumar, S.M. A prospective dosimetric and clinical comparison of acute hematological toxicities in three-dimensional conformal radiation therapy and intensity modulated radiation therapy with concurrent chemotherapy in carcinoma cervix. J. Cancer Res. Ther. 2015, 11, 83–87. [Google Scholar] [CrossRef] [PubMed]

| Frequency n/(%) | |
|---|---|
| Age | |
| Mean | 71.5 |
| Range | 52–86 |
| Associated Chronic Diseases | |
| DM | |
| Yes | 132 (22.3%) |
| No | 460 (77.7%) |
| HTN | |
| Yes | 303 (51.2% |
| No | 289 (48.8%) |
| Cholesterol | |
| Yes | 221 (37.3%) |
| No | 371 (62.7%) |
| AJCC Tumor Stage (n = 600) | |
| 194 (32.3%) |
| 212 (35.3%) |
| 167 (27.8%) |
| 004 (0.7%) |
| 021 (3.5%) |
| 598 (99.7%) |
| 2 (0.3%) |
| Global stage | |
| 2 (0.3%) |
| Gleason score | |
| 041 (6.9%) |
| 281 (47.1%) |
| 275 (46.1%) |
| NCCN risk group | |
| 015 (2.5%) |
| 220 (36.6%) |
| . Favorable | 129 (21.5%) |
| . Unfavorable | 91 (15.1%) |
| 365 (60.8%) |
| Treatment techniques | |
| 171 (28.5%) |
| 429 (71.5%) |
| Treatment site | |
| 360 (60%) |
| 240 (40%) |
| LHRH | |
| 412 (68.7%) |
| 188 (31.3%) |
| Type of treatment | |
| Cohort 1: Prostate radiation only | 149 (24.8%) |
| Cohort 2: Prostate radiation and ADT | 091 (24.8%) |
| Cohort 3: Prostate and pelvic radiation | 039 (6.5%) |
| Cohort 4: prostate, pelvic radiation, and ADT | 321 (53.5%) |
| Hematological Counts (median/range) | |
| Hemoglobin | 138.5 (82–176) |
| Neutrophils | 4.2 (0–16.9) |
| Lymphocytes | 1.8 (0–30.4) |
| Platelets | 207 (0–612) |
| HGB (<140 g/L) * | HGB (≥140 g/L) | PLT (<150 × 109/L) | PLT (≥150 × 109/L) | WBC (<4 × 109/L) | WBC (≥4 × 109/L) | NEUT (<1.8 × 109/L) | NEUT (≥1.8 × 109/L) | LYMPH (<1.2 × 109/L) | LYMPH (≥1.2 × 109/L) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N. | % | N. | % | N. | % | N. | % | N. | % | N. | % | N. | % | N. | % | N. | % | N. | % | |
| Cohort 1 | 56 | 37.6 | 93 | 62.4 | 18 | 12.1 | 131 | 87.9 | 4 | 2.7 | 145 | 97.3 | 5 | 3.4 | 144 | 96.6 | 17 | 11.4 | 132 | 88.6 |
| Cohort 2 | 49 | 53.8 | 42 | 46.2 | 11 | 12.1 | 80 | 87.9 | 3 | 3.3 | 88 | 96.7 | 2 | 2.2 | 89 | 97.8 | 11 | 12.1 | 80 | 87.9 |
| Cohort 3 | 13 | 33.3 | 26 | 66.7 | 3 | 7.7 | 36 | 92.3 | 2 | 5.1 | 37 | 94.9 | 2 | 5.1 | 37 | 94.9 | 5 | 12.8 | 34 | 87.2 |
| Cohort 4 * | 203 | 63.2 | 118 | 36.8 | 31 | 9.7 | 290 | 90.3 | 10 | 3.1 | 311 | 96.9 | 5 | 1.6 | 316 | 98.4 | 31 | 9.7 | 290 | 90.3 |
| Total | 321 | 53.5 | 279 | 46.5 | 63 | 10.5 | 537 | 89.5 | 19 | 3.2 | 581 | 96.8 | 14 | 2.3 | 586 | 97.7 | 64 | 10.7 | 536 | 89.3 |
| Normal–Abnormal | Abnormal–Normal | Normal–Normal | Abnormal–Abnormal | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n. | % | n. | % | n. | % | n. | % | ||
| Cohort 1 | 3dCRT | 29 | 54.7 | 0 | 0 | 8 | 15.1 | 16 | 30.2 |
| IMRT | 43 | 44.8 | 2 | 2.1 | 13 | 13.5 | 38 | 39.6 | |
| Cohort 2 | 3dCRT | 7 | 17.5 | 2 | 5.0 | 8 | 20.0 | 23 | 57.5 |
| IMRT | 12 | 23.5 | 1 | 2.0 | 15 | 29.4 | 23 | 45.1 | |
| Cohort 3 | 3dCRT | 3 | 37.5 | 0 | 0 | 1 | 12.5 | 4 | 50.0 |
| IMRT | 6 | 19.4 | 1 | 3.2 | 16 | 51.6 | 8 | 25.8 | |
| Cohort 4 * | 3dCRT | 5 | 7.1 | 1 | 1.4 | 29 | 41.4 | 35 | 50 |
| IMRT | 28 | 11.2 | 7 | 2.8 | 56 | 22.3 | 160 | 63.7 | |
| HGB (<140 g/L) | PLT (<150 × 109/L) | LYMPH (<1.2 × 109/L) | WBC (<4 × 109/L) | NEUT (<1.8 × 109/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N. | % | N. | % | N. | % | N. | % | N. | % | |
| Cohort 1 | 54 | 36.2 | 14 | 9.4 | 13 | 8.7 | 2 | 1.3 | 3 | 2 |
| Cohort 2 | 46 | 50.2 | 9 | 9.9 | 10 | 11 | 2 | 2.2 | 1 | 1.1 |
| Cohort 3 | 12 | 30.8 | 2 | 5.1 | 5 | 12.8 | 2 | 5.1 | 2 | 5.1 |
| Cohort 4 | 195 | 60.7 | 26 | 8.1 | 27 | 8.4 | 9 | 2.8 | 3 | 0.9 |
| Cohort | OR (95% Confidence Interval) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HGB | WBT | PLT | LYMPH | |||||||||||||
| mRT | pRT | 1m-pRT | 1y-pRT | mRT | pRT | 1m-pRT | 1y-pRT | mRT | pRT | 1m-pRT | 1y-pRT | mRT | pRT | 1m-pRT | 1y-pRT | |
| Cohort 2 | 6.71 (2.84–15.83) | 8.80 (3.36–23.03) | 4.08 (1.87–8.91) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Cohort 3 | 5.11 (1.94–13.44) | 5.15 (1.8–13.67) | 6.7 (2.6–17.2) | N/A | 4.02 (1.20–13.41) | N/A | N/A | 6.83 (1.02–45.82), | N/A | N/A | N/A | N/A | 8.37 (2.76–25.46) | 3.52 (1.24–9.97) | 3.03 (1.34–6.8) | N/A |
| Cohort 4 | 9.52 (4.7–19.04) | 12.20 (5.95–25.01) | 9.1 (4.8–17.1) | 2.84 (1.14–7.08) | 3.91 (1.62–9.43) | 2.39 (1.16–4.93). | 2.42 (1.18–99) | N/A | 2.57 (1.42–4.66). | N/A | N/A | N/A | 8.24 (4.91–13.82 | 6.42 (3.73–11.05) | 5.12 (3.25–8.06) | 3.54 (1.79–7.04) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katib, Y.; Tisseverasinghe, S.; Gerard, I.J.; Royal-Preyra, B.; Chaddad, A.; Sasson, T.; Bahoric, B.; Roncarolo, F.; Niazi, T. Evaluating the Effects of Prostate Radiotherapy Intensified with Pelvic Nodal Radiotherapy and Androgen Deprivation Therapy on Myelosuppression: Single-Institution Experience. Curr. Oncol. 2024, 31, 5439-5451. https://doi.org/10.3390/curroncol31090402
Katib Y, Tisseverasinghe S, Gerard IJ, Royal-Preyra B, Chaddad A, Sasson T, Bahoric B, Roncarolo F, Niazi T. Evaluating the Effects of Prostate Radiotherapy Intensified with Pelvic Nodal Radiotherapy and Androgen Deprivation Therapy on Myelosuppression: Single-Institution Experience. Current Oncology. 2024; 31(9):5439-5451. https://doi.org/10.3390/curroncol31090402
Chicago/Turabian StyleKatib, Yousef, Steven Tisseverasinghe, Ian J. Gerard, Benjamin Royal-Preyra, Ahmad Chaddad, Tania Sasson, Boris Bahoric, Federico Roncarolo, and Tamim Niazi. 2024. "Evaluating the Effects of Prostate Radiotherapy Intensified with Pelvic Nodal Radiotherapy and Androgen Deprivation Therapy on Myelosuppression: Single-Institution Experience" Current Oncology 31, no. 9: 5439-5451. https://doi.org/10.3390/curroncol31090402
APA StyleKatib, Y., Tisseverasinghe, S., Gerard, I. J., Royal-Preyra, B., Chaddad, A., Sasson, T., Bahoric, B., Roncarolo, F., & Niazi, T. (2024). Evaluating the Effects of Prostate Radiotherapy Intensified with Pelvic Nodal Radiotherapy and Androgen Deprivation Therapy on Myelosuppression: Single-Institution Experience. Current Oncology, 31(9), 5439-5451. https://doi.org/10.3390/curroncol31090402








