Role of Systematic Biopsy in the Era of Targeted Biopsy: A Review
Abstract
1. Introduction
2. Systematic Biopsy
2.1. Impact of Systematic Biopsy Result on Treatment
2.2. Advantages of Systematic Biopsy in Detecting Prostate Cancer
2.3. Limitations and Drawbacks of Systematic Biopsy
3. Emergence and Advancement of Targeted Biopsy Techniques
3.1. Types of MRI-Targeted Biopsy
3.1.1. Perilesional Biopsy (Figure 1)
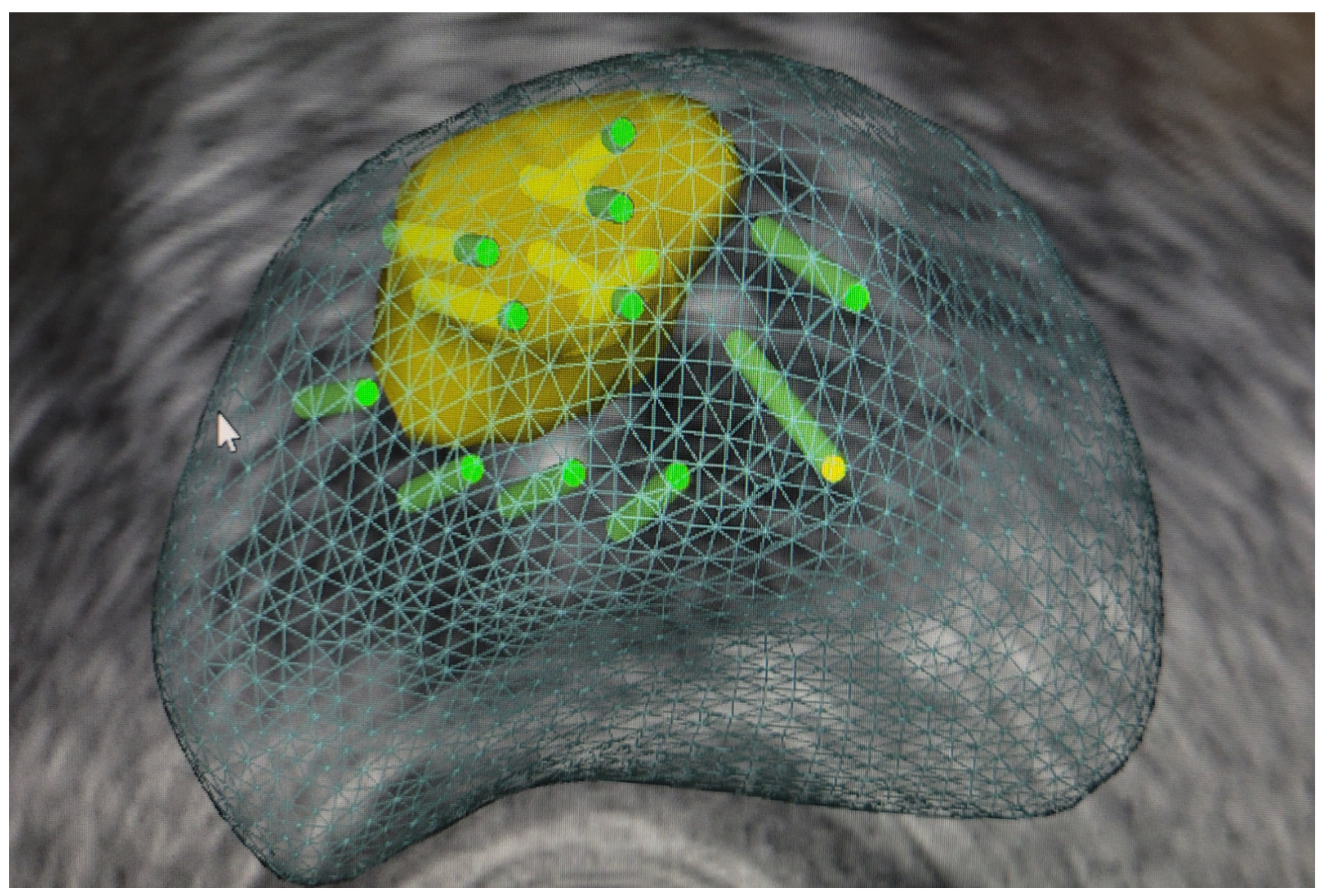
3.1.2. Cognitive Biopsy
3.1.3. Fusion Targeted Biopsy (Fus-TB) (Figure 2)
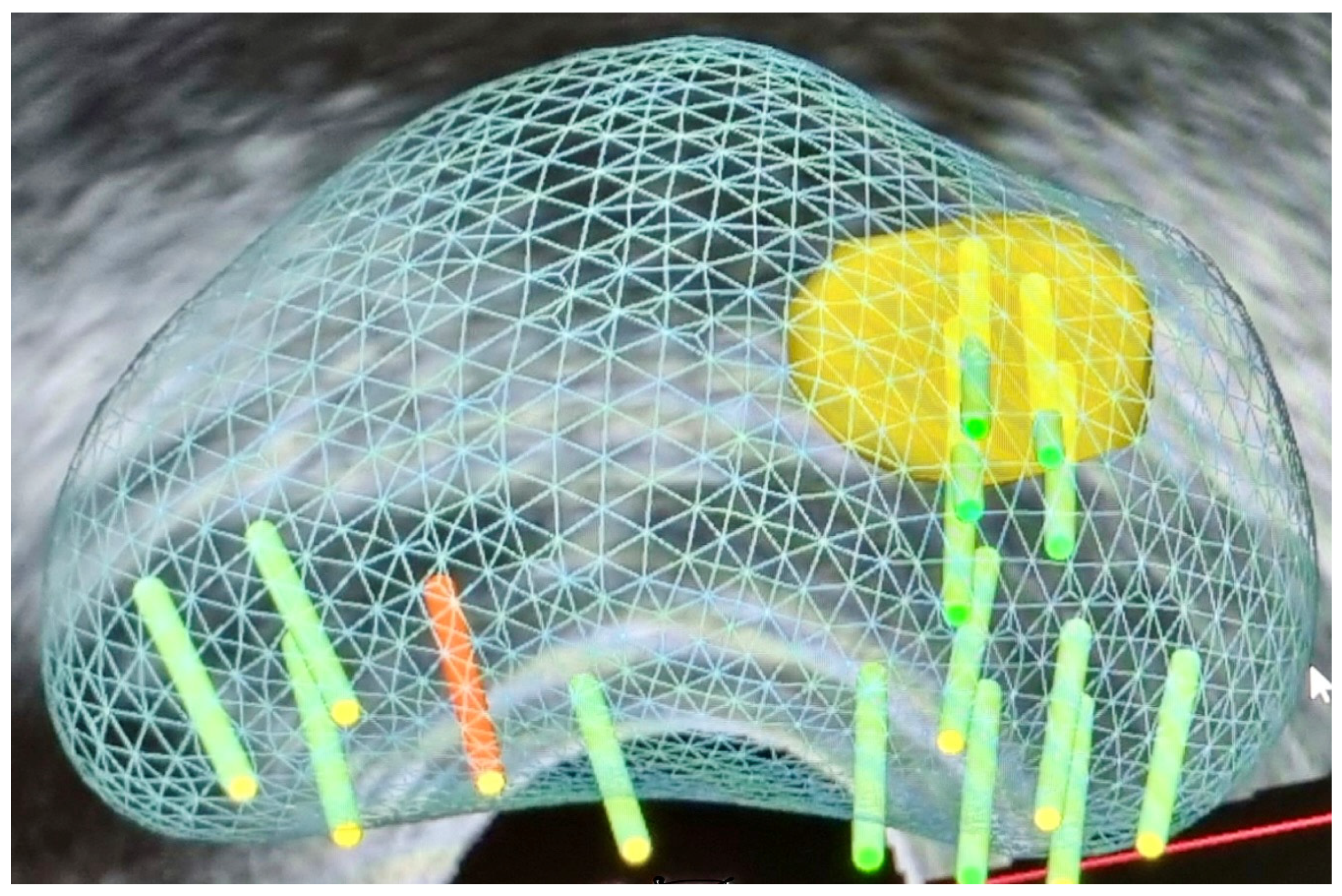
3.1.4. MRI In-Bore Biopsy
3.2. Transrectal Versus Trans-Perineal Fusion Biopsy
3.3. Number of Cores Per Target
4. Comparative Analysis of Systematic Biopsy vs. Other Diagnostic Techniques (Table 1)
| Study | Systematic Biopsy | Targeted Biopsy |
|---|---|---|
| Abd Ali, F., et al. [5] | 75% | 77% |
| Rouvière, O., et al. [69] | 14% | 20% |
| van der Leest, M., et al. [77] | 25% | 23% |
| Drost, F.-J.H., et al. [81] | 21,16% | 18.9% |
| Ahmed, H.U., et al. [101] | 51% | 49% |
| Klotz, L., et al. [144] | 30% | 35% |
| Kasivisvanathan, V., et al. [137] | 43% | 41% |
| Kasivisvanathan, V., et al. [112] | 26% | 38% |
| Porpiglia, F., et al. [146] | 44% | 18% |
| Tonttila, P.P., et al. [147] | 64% | 67% |
| Valerio, M., et al. [148] | 24% | 33% |
5. Integration Strategies: Harmonizing Systematic and Targeted Biopsy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations
| PCa | Prostate cancer |
| csPCA | clinically significant prostate carcinoma |
| inPCA | insignificant prostate cancer |
| MRI | magnetic resonance imaging |
| ISUP | International Society of Urological Pathology |
| PSA | Prostate-specific antigen |
| DRE | digital rectal examination |
| TRUS | transrectal ultrasound |
| CDR | cancer detection rates |
| GG | Grade group |
| SB | systematic biopsy |
| ROI | region of interest |
| GS | Gleason score |
| mpMRI | multiparametric magnetic resonance imaging |
| PI-RADS | Prostate Imaging Reporting and Data System |
| PSAD | prostate-specific antigen density |
| TR | transrectal |
| TP | trans-perineal |
| DWI | diffusion-weighted images |
| DCE | dynamic contrast-enhanced |
| TB | targeted biopsy |
| fus-TB | fusion targeted biopsy |
| comPB | combined prostate biopsy |
| RCT | randomized clinical trials |
| DR | detection ratio |
| CI | confidence interval |
| RR | relative risk |
| 3D GSB | three-dimensional-guided systematic prostate biopsy (3D-GSB) |
| FSB | focal perilesional saturation biopsy |
| AS | active surveillance |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Bell, K.J.; Del Mar, C.; Wright, G.; Dickinson, J.; Glasziou, P. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int. J. Cancer 2015, 137, 1749–1757. [Google Scholar] [CrossRef]
- Drost, F.J.; Osses, D.F.; Nieboer, D.; Steyerberg, E.W.; Bangma, C.H.; Roobol, M.J.; Schoots, I.G. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst. Rev. 2019, 4, Cd012663. [Google Scholar] [CrossRef] [PubMed]
- Abd Ali, F.; Sievert, K.-D.; Eisenblaetter, M.; Titze, B.; Hansen, T.; Barth, P.J.; Titze, U. MRI-Guided Targeted and Systematic Prostate Biopsies as Prognostic Indicators for Prostate Cancer Treatment Decisions. Cancers 2023, 15, 3915. [Google Scholar] [CrossRef]
- Epstein, J.I. An update of the Gleason grading system. J. Urol. 2010, 183, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Committee, G. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: Definition of grading patterns and proposal for a new grading system. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Wolters, T.; Roobol, M.J.; van Leeuwen, P.J.; van den Bergh, R.C.; Hoedemaeker, R.F.; van Leenders, G.J.; Schröder, F.H.; van der Kwast, T.H. A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. J. Urol. 2011, 185, 121–125. [Google Scholar] [CrossRef]
- Harnden, P.; Naylor, B.; Shelley, M.D.; Clements, H.; Coles, B.; Mason, M.D. The clinical management of patients with a small volume of prostatic cancer on biopsy: What are the risks of progression? A systematic review and meta-analysis. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2008, 112, 971–981. [Google Scholar] [CrossRef]
- Gulati, R.; Psutka, S.P.; Etzioni, R. Personalized risks of over diagnosis for screen detected prostate cancer incorporating patient comorbidities: Estimation and communication. J. Urol. 2019, 202, 936–943. [Google Scholar] [CrossRef]
- Loeb, S.; Bjurlin, M.A.; Nicholson, J.; Tammela, T.L.; Penson, D.F.; Carter, H.B.; Carroll, P.; Etzioni, R. Overdiagnosis and overtreatment of prostate cancer. Eur. Urol. 2014, 65, 1046–1055. [Google Scholar] [CrossRef]
- Krausewitz, P.; Fostitsch, D.; Weiten, R.; Kluemper, N.; Stein, J.; Luetkens, J.; Kristiansen, G.; Ellinger, J.; Ritter, M. Current role of systematic biopsy in diagnosis of clinically significant prostate cancer in primary combined MRI-targeted biopsy: A high-volume single-center study. World J. Urol. 2023, 41, 19–25. [Google Scholar] [CrossRef]
- Djavan, B.; Margreiter, M. Biopsy standards for detection of prostate cancer. World J. Urol. 2007, 25, 11–17. [Google Scholar] [CrossRef]
- Deng, Y.S.; He, Y.H.; Ying, W.W.; Liu, H.L.; Li, P.Z.; Ma, C.Y.; Ding, Z.S.; Chen, X.; Wang, J.F.; Zhou, X.F. Value of three biopsy methods in prostate cancer detection: A meta-analysis and systematic review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2221–2234. [Google Scholar] [PubMed]
- Eichler, K.; Hempel, S.; Wilby, J.; Myers, L.; Bachmann, L.M.; Kleijnen, J. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: A systematic review. J. Urol. 2006, 175, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; Margolis, D.; Schnall, M.D.; Shtern, F.; Tempany, C.M. PI-RADS prostate imaging–reporting and data system: 2015, version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar] [CrossRef]
- Elkhoury, F.F.; Felker, E.R.; Kwan, L.; Sisk, A.E.; Delfin, M.; Natarajan, S.; Marks, L.S. Comparison of targeted vs systematic prostate biopsy in men who are biopsy naive: The prospective assessment of image registration in the diagnosis of prostate cancer (PAIREDCAP) study. JAMA Surg. 2019, 154, 811–818. [Google Scholar] [CrossRef]
- Presti, J.C.; O’DOWD, G.J.; Miller, M.C.; Mattu, R.; Veltri, R.W. Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: Results of a community multi-practice study. J. Urol. 2003, 169, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Bosaily, A.E.-S.; Parker, C.; Brown, L.; Gabe, R.; Hindley, R.; Kaplan, R.; Emberton, M.; Ahmed, H.; Group, P. PROMIS—Prostate MR imaging study: A paired validating cohort study evaluating the role of multi-parametric MRI in men with clinical suspicion of prostate cancer. Contemp. Clin. Trials 2015, 42, 26–40. [Google Scholar] [CrossRef]
- Alqahtani, S.; Wei, C.; Zhang, Y.; Szewczyk-Bieda, M.; Wilson, J.; Huang, Z.; Nabi, G. Prediction of prostate cancer Gleason score upgrading from biopsy to radical prostatectomy using pre-biopsy multiparametric MRI PIRADS scoring system. Sci. Rep. 2020, 10, 7722. [Google Scholar] [CrossRef]
- Hu, Y.; Ahmed, H.U.; Carter, T.; Arumainayagam, N.; Lecornet, E.; Barzell, W.; Freeman, A.; Nevoux, P.; Hawkes, D.J.; Villers, A. A biopsy simulation study to assess the accuracy of several transrectal ultrasonography (TRUS)-biopsy strategies compared with template prostate mapping biopsies in patients who have undergone radical prostatectomy. BJU Int. 2012, 110, 812–820. [Google Scholar] [CrossRef]
- Taira, A.V.; Merrick, G.S.; Bennett, A.; Andreini, H.; Taubenslag, W.; Galbreath, R.W.; Butler, W.M.; Bittner, N.; Adamovich, E. Transperineal template-guided mapping biopsy as a staging procedure to select patients best suited for active surveillance. Am. J. Clin. Oncol. 2013, 36, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Barzell, W.E.; Melamed, M.R.; Cathcart, P.; Moore, C.M.; Ahmed, H.U.; Emberton, M. Identifying candidates for active surveillance: An evaluation of the repeat biopsy strategy for men with favorable risk prostate cancer. J. Urol. 2012, 188, 762–768. [Google Scholar] [CrossRef]
- Cheng, E.; Davuluri, M.; Lewicki, P.J.; Hu, J.C.; Basourakos, S.P. Developments in optimizing transperineal prostate biopsy. Curr. Opin. Urol. 2022, 32, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.F.; Luan, Y.; Lu, S.M.; Zhou, G.C.; Huang, T.B.; Zhu, L.Y.; Guo, C.H. Risk factors for infection complications after transrectal ultrasound-guided transperineal prostate biopsy. World J. Urol. 2021, 39, 2463–2467. [Google Scholar] [CrossRef]
- Scattoni, V.; Maccagnano, C.; Zanni, G.; Angiolilli, D.; Raber, M.; Rigatti, P.; Montorsi, F. Systematic extended and saturation prostate biopsy: When and how. Minerva Urol. Nefrol. 2010, 62, 179–192. [Google Scholar] [PubMed]
- Kuru, T.H.; Wadhwa, K.; Chang, R.T.M.; Echeverria, L.M.C.; Roethke, M.; Polson, A.; Rottenberg, G.; Koo, B.; Lawrence, E.M.; Seidenader, J. Definitions of terms, processes and a minimum dataset for transperineal prostate biopsies: A standardization approach of the Ginsburg Study Group for Enhanced Prostate Diagnostics. BJU Int. 2013, 112, 568. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhu, S.; Feng, G.; Zhang, Z.; Li, C.; Li, H.; Wang, C.; Xu, Y. Is an initial saturation prostate biopsy scheme better than an extended scheme for detection of prostate cancer? A systematic review and meta-analysis. Eur. Urol. 2013, 63, 1031–1039. [Google Scholar] [CrossRef]
- Taneja, S.S.; Bjurlin, M.A.; Carter, H.B.; Cookson, M.; Gomella, L.G.; Penson, D.F.; Schellhammer, P.; Schlossberg, S.M.; Troyer, D.A.; Wheeler, T.; et al. AUA/Optimal Techniques of Prostate Biopsy and Specimen Handling. 2013. Available online: https://www.auanet.org/Documents/education/clinical-guidance/Prostate-Biopsy-WhitePaper.pdf (accessed on 22 July 2024).
- Abdulmajed, M.I.; Hughes, D.; Shergill, I.S. The role of transperineal template biopsies of the prostate in the diagnosis of prostate cancer: A review. Expert Rev. Med. Devices 2015, 12, 175–182. [Google Scholar] [CrossRef]
- Martorana, E.; Pirola, G.M.; Aisa, M.C.; Scialpi, P.; Di Blasi, A.; Saredi, G.; D’Andrea, A.; Signore, S.; Grisanti, R.; Scialpi, M. Prostate MRI and transperineal TRUS/MRI fusion biopsy for prostate cancer detection: Clinical practice updates. Turk. J. Urol. 2019, 45, 237. [Google Scholar] [CrossRef]
- Meyer, A.R.; Mamawala, M.; Winoker, J.S.; Landis, P.; Epstein, J.I.; Macura, K.J.; Allaf, M.E.; Partin, A.W.; Pavlovich, C.P.; Gorin, M.A. Transperineal prostate biopsy improves the detection of clinically significant prostate cancer among men on active surveillance. J. Urol. 2021, 205, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Yan, H.; Li, J.; Wang, X.; Chen, H.; Zheng, X. Transperineal versus transrectal prostate biopsy in the diagnosis of prostate cancer: A systematic review and meta-analysis. World J. Surg. Oncol. 2019, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; van den Bergh, R.C.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.C.; Assel, M.; Allaf, M.E.; Ehdaie, B.; Vickers, A.J.; Cohen, A.J.; Ristau, B.T.; Green, D.A.; Han, M.; Rezaee, M.E.; et al. Transperineal Versus Transrectal Magnetic Resonance Imaging-targeted and Systematic Prostate Biopsy to Prevent Infectious Complications: The PREVENT Randomized Trial. Eur. Urol. 2024, 86, 61–68. [Google Scholar] [CrossRef]
- Wei, J.T.; Barocas, D.; Carlsson, S.; Coakley, F.; Eggener, S.; Etzioni, R.; Fine, S.W.; Han, M.; Kim, S.K.; Kirkby, E.; et al. Early detection of prostate cancer: AUA/SUO guideline part I: Prostate cancer screening. J. Urol. 2023, 210, 45–53. [Google Scholar] [CrossRef]
- Roberts, M.J.; Macdonald, A.; Ranasinghe, S.; Bennett, H.; Teloken, P.E.; Harris, P.; Paterson, D.; Coughlin, G.; Dunglison, N.; Esler, R. Transrectal versus transperineal prostate biopsy under intravenous anaesthesia: A clinical, microbiological and cost analysis of 2048 cases over 11 years at a tertiary institution. Prostate Cancer Prostatic Dis. 2021, 24, 169–176. [Google Scholar] [CrossRef]
- Urkmez, A.; Demirel, C.; Altok, M.; Bathala, T.K.; Shapiro, D.D.; Davis, J.W. Freehand versus Grid-Based Transperineal Prostate Biopsy: A Comparison of Anatomical Region Yield and Complications. J. Urol. 2021, 206, 894–902. [Google Scholar] [CrossRef]
- Basourakos, S.P.; Allaway, M.J.; Ross, A.E.; Schaeffer, E.M.; Hu, J.C.; Gorin, M.A. Local anaesthetic techniques for performing transperineal prostate biopsy. Nat. Rev. Urol. 2021, 18, 315–317. [Google Scholar] [CrossRef]
- Schaufler, C.; Daigle, R.; Singhaviranon, S.; Gjertson, C.K.; Albertsen, P.C.; Ristau, B.T. How many cores are enough? Optimizing the transperineal prostate biopsy template. Urol. Oncol. 2022, 40, 191.e1–191.e7. [Google Scholar] [CrossRef]
- Meng, M.V.; Elkin, E.P.; DuChane, J.; Carroll, P.R.; Investigators, C. Impact of increased number of biopsies on the nature of prostate cancer identified. J. Urol. 2006, 176, 63–69. [Google Scholar] [CrossRef]
- Kwon, H.J.; Rhew, S.A.; Yoon, C.E.; Shin, D.; Bang, S.; Park, Y.H.; Cho, H.J.; Ha, U.S.; Hong, S.-H.; Lee, J.Y.; et al. Comparing 12-core and 20-core biopsy for prostate cancer diagnosis with transperineal MR/US fusion biopsy: Assessing the effective number of systemic cores using propensity score matching. Int. Urol. Nephrol. 2023, 55, 2465–2471. [Google Scholar] [CrossRef]
- European Association of Urology. Prostate Cancer; European Association of Urology: Arnhem, The Netherlands, 2022. [Google Scholar]
- Ravery, V.; Goldblatt, L.; Royer, B.; Blanc, E.; Toublanc, M.; Boccon-Gibod, L. Extensive biopsy protocol improves the detection rate of prostate cancer. J. Urol. 2000, 164, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.M.; Chen, K.; Tan, Y.G.; Lee, H.J.; Shutchaidat, V.; Fook-Chong, S.; Cheng, C.W.S.; Ho, H.S.S.; Yuen, J.S.P.; Ngo, N.T.; et al. Reducing the number of systematic biopsy cores in the era of MRI targeted biopsy—Implications on clinically-significant prostate cancer detection and relevance to focal therapy planning. Prostate Cancer Prostatic Dis. 2022, 25, 720–726. [Google Scholar] [CrossRef]
- Gore, J.L.; Shariat, S.F.; Miles, B.J.; Kadmon, D.; Jiang, N.; Wheeler, T.M.; Slawin, K.M. Optimal combinations of systematic sextant and laterally directed biopsies for the detection of prostate cancer. J. Urol. 2001, 165, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Canto, E.I.; Shariat, S.F.; Kadmon, D.; Miles, B.J.; Wheeler, T.M.; Slawin, K.M. Six additional systematic lateral cores enhance sextant biopsy prediction of pathological features at radical prostatectomy. J. Urol. 2004, 171, 204–209. [Google Scholar] [CrossRef]
- Ahdoot, M.; Lebastchi, A.H.; Turkbey, B.; Wood, B.; Pinto, P.A. Contemporary treatments in prostate cancer focal therapy. Curr. Opin. Oncol. 2019, 31, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Tay, K.J.; Amin, M.B.; Ghai, S.; Jimenez, R.E.; Kench, J.G.; Klotz, L.; Montironi, R.; Muto, S.; Rastinehad, A.R.; Turkbey, B. Surveillance after prostate focal therapy. World J. Urol. 2019, 37, 397–407. [Google Scholar] [CrossRef]
- Aslim, E.J.; Law, Y.X.T.; Fook-Chong, S.M.C.; Ho, H.S.S.; Yuen, J.S.P.; Lau, W.K.O.; Lee, L.S.; Cheng, C.W.S.; Ngo, N.T.; Law, Y.M. Defining prostate cancer size and treatment margin for focal therapy: Does intralesional heterogeneity impact the performance of multiparametric MRI? BJU Int. 2021, 128, 178–186. [Google Scholar] [CrossRef]
- Wise, A.M.; Stamey, T.A.; McNeal, J.E.; Clayton, J.L. Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology 2002, 60, 264–269. [Google Scholar] [CrossRef]
- Bott, S.R.; Ahmed, H.U.; Hindley, R.G.; Abdul-Rahman, A.; Freeman, A.; Emberton, M. The index lesion and focal therapy: An analysis of the pathological characteristics of prostate cancer. BJU Int. 2010, 106, 1607–1611. [Google Scholar] [CrossRef]
- Yu, A.; Yamany, T.; Mojtahed, A.; Hanna, N.; Nicaise, E.; Harisinghani, M.; Wu, C.-L.; Dahl, D.M.; Wszolek, M.; Blute, M.L. Combination MRI-targeted and systematic prostate biopsy may overestimate gleason grade on final surgical pathology and impact risk stratification. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Mischinger, J.; Schöllnast, H.; Zurl, H.; Geyer, M.; Fischereder, K.; Adelsmayr, G.; Igrec, J.; Fritz, G.; Merdzo-Hörmann, M.; Elstner, J. Combining targeted and systematic prostate biopsy improves prostate cancer detection and correlation with the whole mount histopathology in biopsy naïve and previous negative biopsy patients. Front. Surg. 2022, 9, 1013389. [Google Scholar] [CrossRef]
- Ahdoot, M.; Wilbur, A.R.; Reese, S.E.; Lebastchi, A.H.; Mehralivand, S.; Gomella, P.T.; Bloom, J.; Gurram, S.; Siddiqui, M.; Pinsky, P. MRI-targeted, systematic, and combined biopsy for prostate cancer diagnosis. N. Engl. J. Med. 2020, 382, 917–928. [Google Scholar] [CrossRef]
- Brisbane, W.G.; Priester, A.M.; Ballon, J.; Kwan, L.; Delfin, M.K.; Felker, E.R.; Sisk, A.E.; Hu, J.C.; Marks, L.S. Targeted Prostate Biopsy: Umbra, Penumbra, and Value of Perilesional Sampling. Eur. Urol. 2022, 82, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Bryk, D.J.; Llukani, E.; Taneja, S.S.; Rosenkrantz, A.B.; Huang, W.C.; Lepor, H. The role of ipsilateral and contralateral transrectal ultrasound-guided systematic prostate biopsy in men with unilateral magnetic resonance imaging lesion undergoing magnetic resonance imaging-ultrasound fusion-targeted prostate biopsy. Urology 2017, 102, 178–182. [Google Scholar] [CrossRef]
- Feuer, Z.; Meng, X.; Rosenkrantz, A.B.; Kasivisvanathan, V.; Moore, C.M.; Huang, R.; Deng, F.-M.; Lepor, H.; Wysock, J.S.; Huang, W.C. Application of the PRECISION trial biopsy strategy to a contemporary magnetic resonance imaging-targeted biopsy cohort—How many clinically significant prostate cancers are missed? J. Urol. 2021, 205, 740–747. [Google Scholar] [CrossRef]
- Tay, K.; Scheltema, M.; Ahmed, H.; Barret, E.; Coleman, J.; Dominguez-Escrig, J.; Ghai, S.; Huang, J.; Jones, J.; Klotz, L. Patient selection for prostate focal therapy in the era of active surveillance: An International Delphi Consensus Project. Prostate Cancer Prostatic Dis. 2017, 20, 294–299. [Google Scholar] [CrossRef]
- Patel, N.; Cricco-Lizza, E.; Kasabwala, K.; Xu, C.; Robinson, B.D.; Khani, F.; Wang, Y.; Margolis, D.; Hu, J.C. The role of systematic and targeted biopsies in light of overlap on magnetic resonance imaging ultrasound fusion biopsy. Eur. Urol. Oncol. 2018, 1, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Yang, X.Y.; Lee, H.J.; Law, Y.M.; Huang, H.H.; Sim, A.S.; Lau, W.K.; Lee, L.S.; Cheng, C.W.; Ho, H.S. Limitations of overlapping cores in systematic and MRI-US fusion biopsy. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Chung, J.H.; Park, B.K.; Song, W.; Kang, M.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M. TRUS-Guided Target Biopsy for a PI-RADS 3–5 Index Lesion to Reduce Gleason Score Underestimation: A Propensity Score Matching Analysis. Front. Oncol. 2022, 11, 824204. [Google Scholar] [CrossRef]
- Cata, E.; Andras, I.; Ferro, M.; Kadula, P.; Leucuta, D.; Musi, G.; Matei, D.-V.; De Cobelli, O.; Tamas-Szora, A.; Caraiani, C. Systematic sampling during MRI-US fusion prostate biopsy can overcome errors of targeting—Prospective single center experience after 300 cases in first biopsy setting. Transl. Androl. Urol. 2020, 9, 2510. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.L.; Barrett, T.; Lloyd, T.; Warren, A.; Samel, C.; Bratt, O.; Kastner, C. Optimising the number of cores for magnetic resonance imaging-guided targeted and systematic transperineal prostate biopsy. BJU Int. 2020, 125, 260–269. [Google Scholar] [CrossRef]
- Briganti, A.; Blute, M.L.; Eastham, J.H.; Graefen, M.; Heidenreich, A.; Karnes, J.R.; Montorsi, F.; Studer, U.E. Pelvic lymph node dissection in prostate cancer. Eur. Urol. 2009, 55, 1251–1265. [Google Scholar] [CrossRef]
- Walz, J.; Epstein, J.I.; Ganzer, R.; Graefen, M.; Guazzoni, G.; Kaouk, J.; Menon, M.; Mottrie, A.; Myers, R.P.; Patel, V.; et al. A Critical Analysis of the Current Knowledge of Surgical Anatomy of the Prostate Related to Optimisation of Cancer Control and Preservation of Continence and Erection in Candidates for Radical Prostatectomy: An Update. Eur. Urol. 2016, 70, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Ploussard, G.; Valerio, M.; Marra, G.; Moschini, M.; Martini, A.; Roumiguié, M.; Fossati, N.; Stabile, A.; Beauval, J.-B. Prognostic implications of multiparametric magnetic resonance imaging and concomitant systematic biopsy in predicting biochemical recurrence after radical prostatectomy in prostate cancer patients diagnosed with magnetic resonance imaging–targeted biopsy. Eur. Urol. Oncol. 2020, 3, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Martini, A.; Ploussard, G.; Fossati, N.; Stabile, A.; De Visschere, P.; Borgmann, H.; Heidegger, I.; Steinkohl, F.; Kretschmer, A. External validation of the 2019 Briganti nomogram for the identification of prostate cancer patients who should be considered for an extended pelvic lymph node dissection. Eur. Urol. 2020, 78, 138–142. [Google Scholar] [CrossRef]
- Rouvière, O.; Puech, P.; Renard-Penna, R.; Claudon, M.; Roy, C.; Mège-Lechevallier, F.; Decaussin-Petrucci, M.; Dubreuil-Chambardel, M.; Magaud, L.; Remontet, L.; et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): A prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019, 20, 100–109. [Google Scholar] [CrossRef]
- Hanna, N.; Wszolek, M.F.; Mojtahed, A.; Nicaise, E.; Wu, B.; Gelpi-Hammerschmidt, F.J.; Salari, K.; Dahl, D.M.; Blute, M.L.; Harisinghani, M. Multiparametric magnetic resonance imaging-ultrasound fusion biopsy improves but does not replace standard template biopsy for the detection of prostate cancer. J. Urol. 2019, 202, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.M.; Rais-Bahrami, S.; Turkbey, B.; George, A.K.; Rothwax, J.; Shakir, N.; Okoro, C.; Raskolnikov, D.; Parnes, H.L.; Linehan, W.M. Comparison of MR/ultrasound fusion–guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015, 313, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Hugosson, J.; Månsson, M.; Wallström, J.; Axcrona, U.; Carlsson, S.V.; Egevad, L.; Geterud, K.; Khatami, A.; Kohestani, K.; Pihl, C.-G. Prostate cancer screening with PSA and MRI followed by targeted biopsy only. N. Engl. J. Med. 2022, 387, 2126–2137. [Google Scholar] [CrossRef]
- Johnson, D.C.; Raman, S.S.; Mirak, S.A.; Kwan, L.; Bajgiran, A.M.; Hsu, W.; Maehara, C.K.; Ahuja, P.; Faiena, I.; Pooli, A. Detection of individual prostate cancer foci via multiparametric magnetic resonance imaging. Eur. Urol. 2019, 75, 712–720. [Google Scholar] [CrossRef]
- Cash, H.; Maxeiner, A.; Stephan, C.; Fischer, T.; Durmus, T.; Holzmann, J.; Asbach, P.; Haas, M.; Hinz, S.; Neymeyer, J. The detection of significant prostate cancer is correlated with the Prostate Imaging Reporting and Data System (PI-RADS) in MRI/transrectal ultrasound fusion biopsy. World J. Urol. 2016, 34, 525–532. [Google Scholar] [CrossRef]
- Stabile, A.; Dell’Oglio, P.; De Cobelli, F.; Esposito, A.; Gandaglia, G.; Fossati, N.; Brembilla, G.; Cristel, G.; Cardone, G.; Losa, A. Association between Prostate Imaging Reporting and Data System (PI-RADS) score for the index lesion and multifocal, clinically significant prostate cancer. Eur. Urol. Oncol. 2018, 1, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Malewski, W.; Milecki, T.; Szempliński, S.; Tayara, O.; Kuncman, Ł.; Kryst, P.; Nyk, Ł. Prostate Biopsy in the Case of PIRADS5—Is Systematic Biopsy Mandatory? J. Clin. Med. 2023, 12, 5612. [Google Scholar] [CrossRef] [PubMed]
- van der Leest, M.; Cornel, E.; Israel, B.; Hendriks, R.; Padhani, A.R.; Hoogenboom, M.; Zamecnik, P.; Bakker, D.; Setiasti, A.Y.; Veltman, J. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naïve men with elevated prostate-specific antigen: A large prospective multicenter clinical study. Eur. Urol. 2019, 75, 570–578. [Google Scholar]
- Zhang, Y.; Zeng, N.; Zhang, F.; Huang, Y.; Tian, Y. How to make clinical decisions to avoid unnecessary prostate screening in biopsy-naïve men with PI-RADs v2 score ≤ 3? Int. J. Clin. Oncol. 2020, 25, 175–186. [Google Scholar] [CrossRef]
- Sfoungaristos, S.; Katafigiotis, I.; Perimenis, P. The role of PSA density to predict a pathological tumour upgrade between needle biopsy and radical prostatectomy for low risk clinical prostate cancer in the modified Gleason system era. Can. Urol. Assoc. J. 2013, 7, E722. [Google Scholar] [CrossRef] [PubMed]
- Magheli, A.; Hinz, S.; Hege, C.; Stephan, C.; Jung, K.; Miller, K.; Lein, M. Prostate specific antigen density to predict prostate cancer upgrading in a contemporary radical prostatectomy series: A single center experience. J. Urol. 2010, 183, 126–132. [Google Scholar] [CrossRef]
- Drost, F.-J.H.; Osses, D.; Nieboer, D.; Bangma, C.H.; Steyerberg, E.W.; Roobol, M.J.; Schoots, I.G. Prostate magnetic resonance imaging, with or without magnetic resonance imaging-targeted biopsy, and systematic biopsy for detecting prostate cancer: A Cochrane systematic review and meta-analysis. Eur. Urol. 2020, 77, 78–94. [Google Scholar] [CrossRef]
- Ljungberg, B.; Albiges, L.; Bensalah, K.; Bex, A.; Giles, R.; Hora, M.; Kuczyk, M.; Lam, T.; Marconi, L.; Canfield, S. EAU guidelines. Edn. presented at the EAU annual congress Amsterdam 2020. Eur. Urol. 2020, 67, 913–924. [Google Scholar] [CrossRef]
- Exterkate, L.; Wegelin, O.; Barentsz, J.O.; van der Leest, M.G.; Kummer, J.A.; Vreuls, W.; de Bruin, P.C.; Bosch, J.; van Melick, H.H.E.; Somford, D.M. Is There Still a Need for Repeated Systematic Biopsies in Patients with Previous Negative Biopsies in the Era of Magnetic Resonance Imaging-targeted Biopsies of the Prostate? Eur. Urol. Oncol. 2020, 3, 216–223. [Google Scholar] [CrossRef]
- Matulevičius, A.; Bakavičius, A.; Ulys, A.; Trakymas, M.; Ušinskienė, J.; Naruševičiūtė, I.; Sabaliauskaitė, R.; Žukauskaitė, K.; Jarmalaitė, S.; Jankevičius, F. Multiparametric MRI Fusion-Guided Prostate Biopsy for Detection of Clinically Significant Prostate Cancer Eliminates the Systemic Prostate Biopsy. Appl. Sci. 2022, 12, 10151. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Emberton, M.; Moore, C.M. There Is No Longer a Role for Systematic Biopsies in Prostate Cancer Diagnosis. Eur. Urol. Open Sci. 2022, 38, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, S.; Jäderling, F.; Wallerstedt, A.; Nyberg, T.; Stranne, J.; Thorsteinsdottir, T.; Carlsson, S.V.; Bjartell, A.; Hugosson, J.; Haglind, E. Oncological and functional outcomes 1 year after radical prostatectomy for very-low-risk prostate cancer: Results from the prospective LAPPRO trial. BJU Int. 2016, 118, 205–212. [Google Scholar] [CrossRef]
- Chang, S.D.; Ghai, S.; Kim, C.K.; Oto, A.; Giganti, F.; Moore, C.M. MRI Targeted Prostate Biopsy Techniques: AJR Expert Panel Narrative Review. AJR Am. J. Roentgenol. 2021, 217, 1263–1281. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Metcalfe, C.; Davis, M.; Turner, E.L.; Martin, R.M.; Young, G.J.; Walsh, E.I.; Bryant, R.J.; et al. Fifteen-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N. Engl. J. Med. 2023, 388, 1547–1558. [Google Scholar] [CrossRef]
- Sazuka, T.; Imamoto, T.; Namekawa, T.; Utsumi, T.; Yanagisawa, M.; Kawamura, K.; Kamiya, N.; Suzuki, H.; Ueda, T.; Ota, S. Analysis of preoperative detection for apex prostate cancer by transrectal biopsy. Prostate Cancer 2013, 2013, 705865. [Google Scholar] [CrossRef]
- Lane, B.R.; Zippe, C.D.; Abouassaly, R.; Schoenfield, L.; Magi-Galluzzi, C.; Jones, J.S. Saturation technique does not decrease cancer detection during followup after initial prostate biopsy. J. Urol. 2008, 179, 1746–1750, discussion 1750. [Google Scholar] [CrossRef] [PubMed]
- Roehl, K.A.; Antenor, J.A.; Catalona, W.J. Serial biopsy results in prostate cancer screening study. J. Urol. 2002, 167, 2435–2439. [Google Scholar] [CrossRef] [PubMed]
- Schoots, I.G.; Roobol, M.J.; Nieboer, D.; Bangma, C.H.; Steyerberg, E.W.; Hunink, M.G. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: A systematic review and meta-analysis. Eur. Urol. 2015, 68, 438–450. [Google Scholar] [CrossRef]
- Tu, X.; Liu, Z.; Zhang, C.; Chang, T.; Xu, H.; Bao, Y.; Li, J.; Jin, K.; Yuan, Q.; Qiu, S.; et al. Diagnostic Role of Magnetic Resonance Imaging-Targeted Biopsy for Prostate Cancer in Biopsy-Naïve Men: A Meta-Analysis. Urol. Int. 2020, 104, 187–198. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, C.; Lu, L.; Ruan, L.; Lan, J.; Feng, S.; Luo, J. Comparison of ultrasound-guided transrectal and transperineal prostate biopsies in clinical application. Zhonghua Nan Ke Xue = Natl. J. Androl. 2014, 20, 1004–1007. [Google Scholar]
- Grummet, J.P.; Weerakoon, M.; Huang, S.; Lawrentschuk, N.; Frydenberg, M.; Moon, D.A.; O’Reilly, M.; Murphy, D. Sepsis and ‘superbugs’: Should we favour the transperineal over the transrectal approach for prostate biopsy? BJU Int. 2014, 114, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Perumalla, C.; Heap, G. Complications of transrectal versus transperineal prostate biopsy. ANZ J. Surg. 2005, 75, 48–50. [Google Scholar] [CrossRef]
- Ghafoori, M.; Velayati, M.; Ghasabeh, M.A.; Shakiba, M.; Alavi, M. Prostate biopsy using transrectal ultrasonography; the optimal number of cores regarding cancer detection rate and complications. Iran. J. Radiol. 2015, 12, e13257. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, A.; Sivaraman, A.; Sánchez-Salas, R.; Nunes-Silva, I.; Baghdadi, M.; Srougi, V.; Di Trapani, E.; Pizzaro, F.U.; Doizi, S.; Barret, E. Un mayor número de cilindros de biopsia transrectal de próstata guiada por ultrasonido se asocia con una mayor pérdida de sangre y complicaciones perioperatorias en la prostatectomía radical asistida por robot. Actas Urológicas Españolas 2017, 41, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Szabo, R.J. “Free-Hand” transperineal prostate biopsy under local anesthesia: Review of the literature. J. Endourol. 2021, 35, 525–543. [Google Scholar] [CrossRef]
- Sathianathen, N.J.; Omer, A.; Harriss, E.; Davies, L.; Kasivisvanathan, V.; Punwani, S.; Moore, C.M.; Kastner, C.; Barrett, T.; Van Den Bergh, R.C. Negative predictive value of multiparametric magnetic resonance imaging in the detection of clinically significant prostate cancer in the prostate imaging reporting and data system era: A systematic review and meta-analysis. Eur. Urol. 2020, 78, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.U.; Bosaily, A.E.-S.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef]
- Lombardo, R.; Tema, G.; Nacchia, A.; Mancini, E.; Franco, S.; Zammitti, F.; Franco, A.; Cash, H.; Gravina, C.; Guidotti, A.; et al. Role of Perilesional Sampling of Patients Undergoing Fusion Prostate Biopsies. Life 2023, 13, 1719. [Google Scholar] [CrossRef]
- Priester, A.; Natarajan, S.; Khoshnoodi, P.; Margolis, D.J.; Raman, S.S.; Reiter, R.E.; Huang, J.; Grundfest, W.; Marks, L.S. Magnetic resonance imaging underestimation of prostate cancer geometry: Use of patient specific molds to correlate images with whole mount pathology. J. Urol. 2017, 197, 320–326. [Google Scholar] [CrossRef]
- Noujeim, J.-P.; Belahsen, Y.; Lefebvre, Y.; Lemort, M.; Deforche, M.; Sirtaine, N.; Martin, R.; Roumeguere, T.; Peltier, A.; Diamand, R. Optimizing multiparametric magnetic resonance imaging-targeted biopsy and detection of clinically significant prostate cancer: The role of perilesional sampling. Prostate Cancer Prostatic Dis. 2023, 26, 575–580. [Google Scholar] [CrossRef]
- Tschirdewahn, S.; Wiesenfarth, M.; Bonekamp, D.; Püllen, L.; Reis, H.; Panic, A.; Kesch, C.; Darr, C.; Heß, J.; Giganti, F. Detection of significant prostate cancer using target saturation in transperineal magnetic resonance imaging/transrectal ultrasonography–fusion biopsy. Eur. Urol. Focus 2021, 7, 1300–1307. [Google Scholar] [CrossRef]
- Diamand, R.; Hollans, M.; Lefebvre, Y.; Sirtaine, N.; Limani, K.; Hawaux, E.; Abou Zahr, R.; Mattlet, A.; Albisinni, S.; Roumeguère, T. The role of perilesional and multiparametric resonance imaging-targeted biopsies to reduce the risk of upgrading at radical prostatectomy pathology: A retrospective monocentric study. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Padhani, A.R.; Weinreb, J.; Rosenkrantz, A.B.; Villeirs, G.; Turkbey, B.; Barentsz, J. Prostate imaging-reporting and data system steering committee: PI-RADS v2 status update and future directions. Eur. Urol. 2019, 75, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Cool, D.W.; Romagnoli, C.; Izawa, J.I.; Chin, J.; Gardi, L.; Tessier, D.; Mercado, A.; Mandel, J.; Ward, A.D.; Fenster, A. Comparison of prostate MRI-3D transrectal ultrasound fusion biopsy for first-time and repeat biopsy patients with previous atypical small acinar proliferation. Can. Urol. Assoc. J. 2016, 10, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Marks, L.S.; Margolis, D.J.; Huang, J.; Macairan, M.L.; Lieu, P.; Fenster, A. Clinical application of a 3D ultrasound-guided prostate biopsy system. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Ukimura, O.; Desai, M.M.; Palmer, S.; Valencerina, S.; Gross, M.; Abreu, A.L.; Aron, M.; Gill, I.S. 3-Dimensional elastic registration system of prostate biopsy location by real-time 3-dimensional transrectal ultrasound guidance with magnetic resonance/transrectal ultrasound image fusion. J. Urol. 2012, 187, 1080–1086. [Google Scholar] [CrossRef]
- Mendhiratta, N.; Rosenkrantz, A.B.; Meng, X.; Wysock, J.S.; Fenstermaker, M.; Huang, R.; Deng, F.M.; Melamed, J.; Zhou, M.; Huang, W.C.; et al. Magnetic Resonance Imaging-Ultrasound Fusion Targeted Prostate Biopsy in a Consecutive Cohort of Men with No Previous Biopsy: Reduction of Over Detection through Improved Risk Stratification. J. Urol. 2015, 194, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef]
- Mozer, P.; Rouprêt, M.; Le Cossec, C.; Granger, B.; Comperat, E.; de Gorski, A.; Cussenot, O.; Renard-Penna, R. First round of targeted biopsies using magnetic resonance imaging/ultrasonography fusion compared with conventional transrectal ultrasonography-guided biopsies for the diagnosis of localised prostate cancer. BJU Int. 2015, 115, 50–57. [Google Scholar] [CrossRef]
- Ukimura, O.; Marien, A.; Palmer, S.; Villers, A.; Aron, M.; de Castro Abreu, A.L.; Leslie, S.; Shoji, S.; Matsugasumi, T.; Gross, M.; et al. Trans-rectal ultrasound visibility of prostate lesions identified by magnetic resonance imaging increases accuracy of image-fusion targeted biopsies. World J. Urol. 2015, 33, 1669–1676. [Google Scholar] [CrossRef]
- Baco, E.; Rud, E.; Eri, L.M.; Moen, G.; Vlatkovic, L.; Svindland, A.; Eggesbø, H.B.; Ukimura, O. A Randomized Controlled Trial To Assess and Compare the Outcomes of Two-core Prostate Biopsy Guided by Fused Magnetic Resonance and Transrectal Ultrasound Images and Traditional 12-core Systematic Biopsy. Eur. Urol. 2016, 69, 149–156. [Google Scholar] [CrossRef]
- Verma, S.; Choyke, P.L.; Eberhardt, S.C.; Oto, A.; Tempany, C.M.; Turkbey, B.; Rosenkrantz, A.B. The current state of MR imaging–targeted biopsy techniques for detection of prostate cancer. Radiology 2017, 285, 343–356. [Google Scholar] [CrossRef]
- Tilak, G.; Tuncali, K.; Song, S.E.; Tokuda, J.; Olubiyi, O.; Fennessy, F.; Fedorov, A.; Penzkofer, T.; Tempany, C.; Hata, N. 3T MR-guided in-bore transperineal prostate biopsy: A comparison of robotic and manual needle-guidance templates. J. Magn. Reson. Imaging 2015, 42, 63–71. [Google Scholar] [CrossRef]
- Pokorny, M.; Kua, B.; Esler, R.; Yaxley, J.; Samaratunga, H.; Dunglison, N.; Gianduzzo, T.; Coughlin, G.; Holt, R.; Laing, B. MRI-guided in-bore biopsy for prostate cancer: What does the evidence say? A case series of 554 patients and a review of the current literature. World J. Urol. 2019, 37, 1263–1279. [Google Scholar] [CrossRef]
- Grummet, J.; Pepdjonovic, L.; Huang, S.; Anderson, E.; Hadaschik, B. Transperineal vs. transrectal biopsy in MRI targeting. Transl. Androl. Urol. 2017, 6, 368. [Google Scholar] [CrossRef] [PubMed]
- Cicione, A.; De Nunzio, C.; Manno, S.; Damiano, R.; Posti, A.; Lima, E.; Tubaro, A.; Balloni, F. An update on prostate biopsy in the era of magnetic resonance imaging. Minerva Urol. Nefrol. = Ital. J. Urol. Nephrol. 2018, 70, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perdomo, H.A.; Mejia, N.G.; Fernandez, L.; Carbonell, J. Effectiveness of periprostatic block to prevent pain in transrectal prostate biopsy: A systematic review and a network meta-analysis. Cent. Eur. J. Urol. 2019, 72, 121. [Google Scholar]
- Yang, Y.; Liu, Z.; Wei, Q.; Cao, D.; Yang, L.; Zhu, Y.; Wei, X.; Tang, Z.; Liu, L.; Han, P. The efficiency and safety of intrarectal topical anesthesia for transrectal ultrasound-guided prostate biopsy: A systematic review and meta-analysis. Urol. Int. 2017, 99, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Pradere, B.; Veeratterapillay, R.; Dimitropoulos, K.; Yuan, Y.; Omar, M.I.; MacLennan, S.; Cai, T.; Bruyère, F.; Bartoletti, R.; Köves, B.; et al. Nonantibiotic Strategies for the Prevention of Infectious Complications following Prostate Biopsy: A Systematic Review and Meta-Analysis. J. Urol. 2021, 205, 653–663. [Google Scholar] [CrossRef]
- Liss, M.A.; Ehdaie, B.; Loeb, S.; Meng, M.V.; Raman, J.D.; Spears, V.; Stroup, S.P. An update of the American Urological Association white paper on the prevention and treatment of the more common complications related to prostate biopsy. J. Urol. 2017, 198, 329–334. [Google Scholar] [CrossRef]
- Borghesi, M.; Ahmed, H.; Nam, R.; Schaeffer, E.; Schiavina, R.; Taneja, S.; Weidner, W.; Loeb, S. Complications After Systematic, Random, and Image-guided Prostate Biopsy. Eur. Urol. 2017, 71, 353–365. [Google Scholar] [CrossRef]
- Anastasiadis, E.; van der Meulen, J.; Emberton, M. Hospital admissions after transrectal ultrasound-guided biopsy of the prostate in men diagnosed with prostate cancer: A database analysis in E ngland. Int. J. Urol. 2015, 22, 181–186. [Google Scholar] [CrossRef]
- Solomon, S.L.; Oliver, K.B. Antibiotic resistance threats in the United States: Stepping back from the brink. Am. Fam. Physician 2014, 89, 938–941. [Google Scholar]
- The Food and Drug Administration (FDA). FDA Drug Safety Communication: FDA Updates Warnings for Oral and Injectable Fluoroquinolone Antibiotics Due to Disabling Side Effects. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-updates-warnings-oral-and-injectable-fluoroquinolone-antibiotics (accessed on 2 June 2019).
- The Food and Drug Administration (FDA). Drug Safety Communication: FDA Warns about Increased Risk of Ruptures or Tears in the Aorta Blood Vessel with Fluoroquinolone Antibiotics in Certain Patients. 2018. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-increased-risk-ruptures-or-tears-aorta-blood-vessel-fluoroquinolone-antibiotics (accessed on 3 June 2024).
- Disabling and Potentially Permanent Side Effects Lead to Suspension or Restrictions of Quinolone and Fluoroquinolone Antibiotics. EMA/175398/2019. 2019. Available online: https://www.ema.europa.eu/en/news/disabling-potentially-permanent-side-effects-lead-suspension-or-restrictions-quinolone-fluoroquinolone-antibiotics (accessed on 22 July 2024).
- Pilatz, A.; Dimitropoulos, K.; Veeratterapillay, R.; Yuan, Y.; Omar, M.I.; MacLennan, S.; Cai, T.; Bruyère, F.; Bartoletti, R.; Köves, B.; et al. Antibiotic Prophylaxis for the Prevention of Infectious Complications following Prostate Biopsy: A Systematic Review and Meta-Analysis. J. Urol. 2020, 204, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ma, L.; Yu, H. Impact of insertion timing of iodophor cotton ball on the control of infection complications after transrectal ultrasound guided prostate biopsy. Zhonghua Yi Xue Za Zhi 2014, 94, 609–611. [Google Scholar] [PubMed]
- Davis, P.; Paul, E.; Grummet, J. Current practice of prostate biopsy in Australia and New Zealand: A survey. Urol. Ann. 2015, 7, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Günzel, K.; Magheli, A.; Baco, E.; Cash, H.; Heinrich, S.; Neubert, H.; Schlegel, J.; Schostak, M.; Henkel, T.; Asbach, P. Infection rate and complications after 621 transperineal MRI-TRUS fusion biopsies in local anesthesia without standard antibiotic prophylaxis. World J. Urol. 2021, 39, 3861–3866. [Google Scholar] [CrossRef] [PubMed]
- Jahnen, M.; Amiel, T.; Kirchoff, F.; Büchler, J.W.; Herkommer, K.; Rothe, K.; Meissner, V.H.; Gschwend, J.E.; Lunger, L. Cotrimoxazole and targeted antibiotic prophylaxis for transrectal prostate biopsy: A single-center study. World J. Urol. 2024, 42, 260. [Google Scholar] [CrossRef] [PubMed]
- Elshal, A.M.; Atwa, A.M.; El-Nahas, A.R.; El-Ghar, M.A.; Gaber, A.; Elsawy, E.; Hashem, A.; Farag, Y.; Farg, H.; Elsorougy, A. Chemoprophylaxis during transrectal prostate needle biopsy: Critical analysis through randomized clinical trial. World J. Urol. 2018, 36, 1845–1852. [Google Scholar] [CrossRef]
- Bhanji, Y.; Allaway, M.J.; Gorin, M.A. Recent advances and current role of transperineal prostate biopsy. Urol. Clin. N. Am. 2021, 48, 25–33. [Google Scholar] [CrossRef]
- Thomson, A.; Li, M.; Grummet, J.; Sengupta, S. Transperineal prostate biopsy: A review of technique. Transl. Androl. Urol. 2020, 9, 3009. [Google Scholar] [CrossRef]
- Pooli, A.; Johnson, D.C.; Shirk, J.; Markovic, D.; Sadun, T.Y.; Sisk Jr, A.E.; Mohammadian Bajgiran, A.; Afshari Mirak, S.; Felker, E.R.; Hughes, A.K. Predicting pathological tumor size in prostate cancer based on multiparametric prostate magnetic resonance imaging and preoperative findings. J. Urol. 2021, 205, 444–451. [Google Scholar] [CrossRef]
- Triquell, M.; Regis, L.; Winkler, M.; Valdés, N.; Cuadras, M.; Celma, A.; Planas, J.; Morote, J.; Trilla, E. Multiparametric MRI for staging of prostate cancer: A multicentric analysis of predictive factors to improve identification of extracapsular extension before radical prostatectomy. Cancers 2022, 14, 3966. [Google Scholar] [CrossRef] [PubMed]
- Kuru, T.H.; Fütterer, J.J.; Schiffmann, J.; Porres, D.; Salomon, G.; Rastinehad, A.R. Transrectal ultrasound (US), contrast-enhanced US, real-time elastography, HistoScanning, magnetic resonance imaging (MRI), and MRI-US fusion biopsy in the diagnosis of prostate cancer. Eur. Urol. Focus 2015, 1, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk-Bieda, M.; Wei, C.; Coll, K.; Gandy, S.; Donnan, P.; Ragupathy, S.K.A.; Singh, P.; Wilson, J.; Nabi, G. A multicentre parallel-group randomised trial assessing multiparametric MRI characterisation and image-guided biopsy of prostate in men suspected of having prostate cancer: MULTIPROS study protocol. Trials 2019, 20, 638. [Google Scholar] [CrossRef] [PubMed]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Chin, J.; Black, P.C.; Finelli, A.; Anidjar, M.; Bladou, F.; Mercado, A.; Levental, M.; Ghai, S.; Chang, S.D. Comparison of multiparametric magnetic resonance imaging–targeted biopsy with systematic transrectal ultrasonography biopsy for biopsy-naive men at risk for prostate cancer: A phase 3 randomized clinical trial. JAMA Oncol. 2021, 7, 534–542. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Stabile, A.; Neves, J.B.; Giganti, F.; Valerio, M.; Shanmugabavan, Y.; Clement, K.D.; Sarkar, D.; Philippou, Y.; Thurtle, D. Magnetic resonance imaging-targeted biopsy versus systematic biopsy in the detection of prostate cancer: A systematic review and meta-analysis. Eur. Urol. 2019, 76, 284–303. [Google Scholar] [CrossRef]
- Porpiglia, F.; Manfredi, M.; Mele, F.; Cossu, M.; Bollito, E.; Veltri, A.; Cirillo, S.; Regge, D.; Faletti, R.; Passera, R. Diagnostic pathway with multiparametric magnetic resonance imaging versus standard pathway: Results from a randomized prospective study in biopsy-naïve patients with suspected prostate cancer. Eur. Urol. 2017, 72, 282–288. [Google Scholar] [CrossRef]
- Tonttila, P.P.; Lantto, J.; Pääkkö, E.; Piippo, U.; Kauppila, S.; Lammentausta, E.; Ohtonen, P.; Vaarala, M.H. Prebiopsy Multiparametric Magnetic Resonance Imaging for Prostate Cancer Diagnosis in Biopsy-naive Men with Suspected Prostate Cancer Based on Elevated Prostate-specific Antigen Values: Results from a Randomized Prospective Blinded Controlled Trial. Eur. Urol. 2016, 69, 419–425. [Google Scholar] [CrossRef]
- Preisser, F.; Cooperberg, M.R.; Crook, J.; Feng, F.; Graefen, M.; Karakiewicz, P.I.; Klotz, L.; Montironi, R.; Nguyen, P.L.; D’Amico, A.V. Intermediate-risk prostate cancer: Stratification and management. Eur. Urol. Oncol. 2020, 3, 270–280. [Google Scholar] [CrossRef]
- Valerio, M.; Donaldson, I.; Emberton, M.; Ehdaie, B.; Hadaschik, B.A.; Marks, L.S.; Mozer, P.; Rastinehad, A.R.; Ahmed, H.U. Detection of clinically significant prostate cancer using magnetic resonance imaging–ultrasound fusion targeted biopsy: A systematic review. Eur. Urol. 2015, 68, 8–19. [Google Scholar] [CrossRef]
- Pezelj, I.; Pirša, M.; Svaguša, I.; Nikles, S.; Tomić, M.; Knežević, M.; Tomašković, I.; Krušlin, B. Comparison of Grading Accuracy of Prostate Cancer in Samples Acquired by a Targeted and Systemic Prostate Biopsy. Acta Clin. Croat. 2022, 61 (Suppl. S3), 28–31. [Google Scholar] [CrossRef] [PubMed]
- Overland, M.R.; Washington III, S.L.; Carroll, P.R.; Cooperberg, M.R.; Herlemann, A. Active surveillance for intermediate-risk prostate cancer: Yes, but for whom? Curr. Opin. Urol. 2019, 29, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; You, S.; Nguyen, C.; Wang, Y.; Kim, J.; Sirohi, D.; Ziembiec, A.; Luthringer, D.; Lin, S.-C.; Daskivich, T. Genes involved in prostate cancer progression determine MRI visibility. Theranostics 2018, 8, 1752. [Google Scholar] [CrossRef] [PubMed]
- Stavrinides, V.; Giganti, F.; Trock, B.; Punwani, S.; Allen, C.; Kirkham, A.; Freeman, A.; Haider, A.; Ball, R.; McCartan, N. Five-year outcomes of magnetic resonance imaging–based active surveillance for prostate cancer: A large cohort study. Eur. Urol. 2020, 78, 443–451. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Ito, M.; Fukushima, H.; Yokoyama, M.; Kataoka, M.; Ikuta, S.; Sakamoto, K.; Takemura, K.; Suzuki, H.; Tobisu, K.-i. Who can avoid systematic biopsy without missing clinically significant prostate cancer in men who undergo magnetic resonance imaging-targeted biopsy? Clin. Genitourin. Cancer 2019, 17, e664–e671. [Google Scholar] [CrossRef]
- Derigs, F.; Kowalewski, K.F.; Hartung, F.O.; Waldbillig, F.; Neuberger, M.; von Hardenberg, J.; Westhoff, N. A Matched-pair Analysis Comparing Systematic Prostate Biopsy by Conventional Transrectal Ultrasound-guidance Versus Software-based Predefined 3D-Guidance. Urology 2023, 177, 128–133. [Google Scholar] [CrossRef]
- Ristau, B.T.; Chen, D.Y.T.; Ellis, J.; Malhotra, A.; DeMora, L.; Parsons, R.B.; Milestone, B.; Brody, M.; Viterbo, R.; Greenberg, R.; et al. Defining Novel and Practical Metrics to Assess the Deliverables of Multiparametric Magnetic Resonance Imaging/Ultrasound Fusion Prostate Biopsy. J. Urol. 2018, 199, 969–975. [Google Scholar] [CrossRef]
- Shin, T.; Smyth, T.B.; Ukimura, O.; Ahmadi, N.; de Castro Abreu, A.L.; Oishi, M.; Mimata, H.; Gill, I.S. Detection of prostate cancer using magnetic resonance imaging/ultrasonography image-fusion targeted biopsy in African-American men. BJU Int. 2017, 120, 233–238. [Google Scholar] [CrossRef]
- Vourganti, S.; Rastinehad, A.; Yerram, N.; Nix, J.; Volkin, D.; Hoang, A.; Turkbey, B.; Gupta, G.N.; Kruecker, J.; Linehan, W.M.; et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J. Urol. 2012, 188, 2152–2157. [Google Scholar] [CrossRef]
- Ber, Y.; Segal, N.; Tamir, S.; Benjaminov, O.; Yakimov, M.; Sela, S.; Halstauch, D.; Baniel, J.; Kedar, D.; Margel, D. A noninferiority within-person study comparing the accuracy of transperineal to transrectal MRI-US fusion biopsy for prostate-cancer detection. Prostate Cancer Prostatic Dis. 2020, 23, 449–456. [Google Scholar] [CrossRef]
- Rai, B.P.; Mayerhofer, C.; Somani, B.K.; Kallidonis, P.; Nagele, U.; Tokas, T. Magnetic resonance imaging/ultrasound fusion-guided transperineal versus magnetic resonance imaging/ultrasound fusion-guided transrectal prostate biopsy—A systematic review. Eur. Urol. Oncol. 2021, 4, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Rastinehad, A.R.; Turkbey, B.; Salami, S.S.; Yaskiv, O.; George, A.K.; Fakhoury, M.; Beecher, K.; Vira, M.A.; Kavoussi, L.R.; Siegel, D.N.; et al. Improving detection of clinically significant prostate cancer: Magnetic resonance imaging/transrectal ultrasound fusion guided prostate biopsy. J. Urol. 2014, 191, 1749–1754. [Google Scholar] [CrossRef]
- Borkowetz, A.; Platzek, I.; Toma, M.; Laniado, M.; Baretton, G.; Froehner, M.; Koch, R.; Wirth, M.; Zastrow, S. Comparison of systematic transrectal biopsy to transperineal magnetic resonance imaging/ultrasound-fusion biopsy for the diagnosis of prostate cancer. BJU Int. 2015, 116, 873–879. [Google Scholar] [CrossRef]
- Eklund, M.; Jäderling, F.; Discacciati, A.; Bergman, M.; Annerstedt, M.; Aly, M.; Glaessgen, A.; Carlsson, S.; Grönberg, H.; Nordström, T. MRI-Targeted or Standard Biopsy in Prostate Cancer Screening. N. Engl. J. Med. 2021, 385, 908–920. [Google Scholar] [CrossRef] [PubMed]
- Deniffel, D.; Perlis, N.; Ghai, S.; Girgis, S.; Healy, G.M.; Fleshner, N.; Hamilton, R.; Kulkarni, G.; Toi, A.; van der Kwast, T.; et al. Prostate biopsy in the era of MRI-targeting: Towards a judicious use of additional systematic biopsy. Eur. Radiol. 2022, 32, 7544–7554. [Google Scholar] [CrossRef]
- Barrett, T.; de Rooij, M.; Giganti, F.; Allen, C.; Barentsz, J.O.; Padhani, A.R. Quality checkpoints in the MRI-directed prostate cancer diagnostic pathway. Nat. Rev. Urol. 2023, 20, 9–22. [Google Scholar] [CrossRef]
- Borkowetz, A.; Hadaschik, B.; Platzek, I.; Toma, M.; Torsev, G.; Renner, T.; Herout, R.; Baunacke, M.; Laniado, M.; Baretton, G.; et al. Prospective comparison of transperineal magnetic resonance imaging/ultrasonography fusion biopsy and transrectal systematic biopsy in biopsy-naïve patients. BJU Int. 2018, 121, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Hakozaki, Y.; Matsushima, H.; Kumagai, J.; Murata, T.; Masuda, T.; Hirai, Y.; Oda, M.; Kawauchi, N.; Yokoyama, M.; Homma, Y. A prospective study of magnetic resonance imaging and ultrasonography (MRI/US)-fusion targeted biopsy and concurrent systematic transperineal biopsy with the average of 18-cores to detect clinically significant prostate cancer. BMC Urol. 2017, 17, 117. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Z.; Tang, L.; Zhang, R.; Lu, Y.; Liang, J.; Zou, Z.; Zhou, C.; Wang, Y. Significance of MRI/Transrectal Ultrasound Fusion Three-Dimensional Model-Guided, Targeted Biopsy Based on Transrectal Ultrasound-Guided Systematic Biopsy in Prostate Cancer Detection: A Systematic Review and Meta-Analysis. Urol. Int. 2018, 100, 57–65. [Google Scholar] [CrossRef]
- Krausewitz, P.; Schmeller, H.; Luetkens, J.; Dabir, D.; Ellinger, J.; Ritter, M.; Conrad, R. Prospective analysis of pain expectancy and experience during MR-fusion prostate biopsy: Does reality match patients’ expectancy? World J. Urol. 2022, 40, 2239–2244. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer—2020 update. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Williams, C.; Ahdoot, M.; Daneshvar, M.A.; Hague, C.; Wilbur, A.R.; Gomella, P.T.; Shih, J.; Khondakar, N.; Yerram, N.; Mehralivand, S.; et al. Why does magnetic resonance imaging-targeted biopsy miss clinically significant cancer? J. Urol. 2022, 207, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, N.; Baeßler, B.; von Hardenberg, J.; Hetjens, S.; Porubsky, S.; Siegel, F.; Martini, T.; Michel, M.S.; Attenberger, U.; Ritter, M. Systematic prostate biopsy still matters: A comprehensive analysis of MRI/TRUS-fusion targeted prostate biopsies across different indications. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Westhoff, N.; Haumann, H.; Kriegmair, M.C.; Von Hardenberg, J.; Budjan, J.; Porubsky, S.; Michel, M.S.; Honeck, P.; Ritter, M. Association of training level and outcome of software-based image fusion-guided targeted prostate biopsies. World J. Urol. 2019, 37, 2119–2127. [Google Scholar] [CrossRef]
- Tomašković, I.; Pezelj, I.; Bolanča Čulo, K.; Novosel, L.; Nikles, S.; Tomić, M.; Reljić, A.; Katušić, J.; Knežević, M.; Pirša, M. Diagnostic value of cognitive-registration multiparametric magnetic resonance guided biopsy for the detection of prostate cancer after initial negative biopsy. Acta Clin. Croat. 2018, 57 (Suppl. 1), 40–45. [Google Scholar] [PubMed]
- Moore, C.M.; Kasivisvanathan, V.; Eggener, S.; Emberton, M.; Fütterer, J.J.; Gill, I.S.; Grubb Iii, R.L.; Hadaschik, B.; Klotz, L.; Margolis, D.J. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: Recommendations from an International Working Group. Eur. Urol. 2013, 64, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Oerther, B.; Engel, H.; Nedelcu, A.; Schlett, C.L.; Grimm, R.; von Busch, H.; Sigle, A.; Gratzke, C.; Bamberg, F.; Benndorf, M. Prediction of upgrade to clinically significant prostate cancer in patients under active surveillance: Performance of a fully automated AI-algorithm for lesion detection and classification. Prostate 2023, 83, 871–878. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malewski, W.; Milecki, T.; Tayara, O.; Poletajew, S.; Kryst, P.; Tokarczyk, A.; Nyk, Ł. Role of Systematic Biopsy in the Era of Targeted Biopsy: A Review. Curr. Oncol. 2024, 31, 5171-5194. https://doi.org/10.3390/curroncol31090383
Malewski W, Milecki T, Tayara O, Poletajew S, Kryst P, Tokarczyk A, Nyk Ł. Role of Systematic Biopsy in the Era of Targeted Biopsy: A Review. Current Oncology. 2024; 31(9):5171-5194. https://doi.org/10.3390/curroncol31090383
Chicago/Turabian StyleMalewski, Wojciech, Tomasz Milecki, Omar Tayara, Sławomir Poletajew, Piotr Kryst, Andrzej Tokarczyk, and Łukasz Nyk. 2024. "Role of Systematic Biopsy in the Era of Targeted Biopsy: A Review" Current Oncology 31, no. 9: 5171-5194. https://doi.org/10.3390/curroncol31090383
APA StyleMalewski, W., Milecki, T., Tayara, O., Poletajew, S., Kryst, P., Tokarczyk, A., & Nyk, Ł. (2024). Role of Systematic Biopsy in the Era of Targeted Biopsy: A Review. Current Oncology, 31(9), 5171-5194. https://doi.org/10.3390/curroncol31090383





