Prostate-Specific Membrane Antigen Expression in Patients with Primary Prostate Cancer: Diagnostic and Prognostic Value in Positron Emission Tomography-Prostate-Specific Membrane Antigen
Abstract
1. Introduction
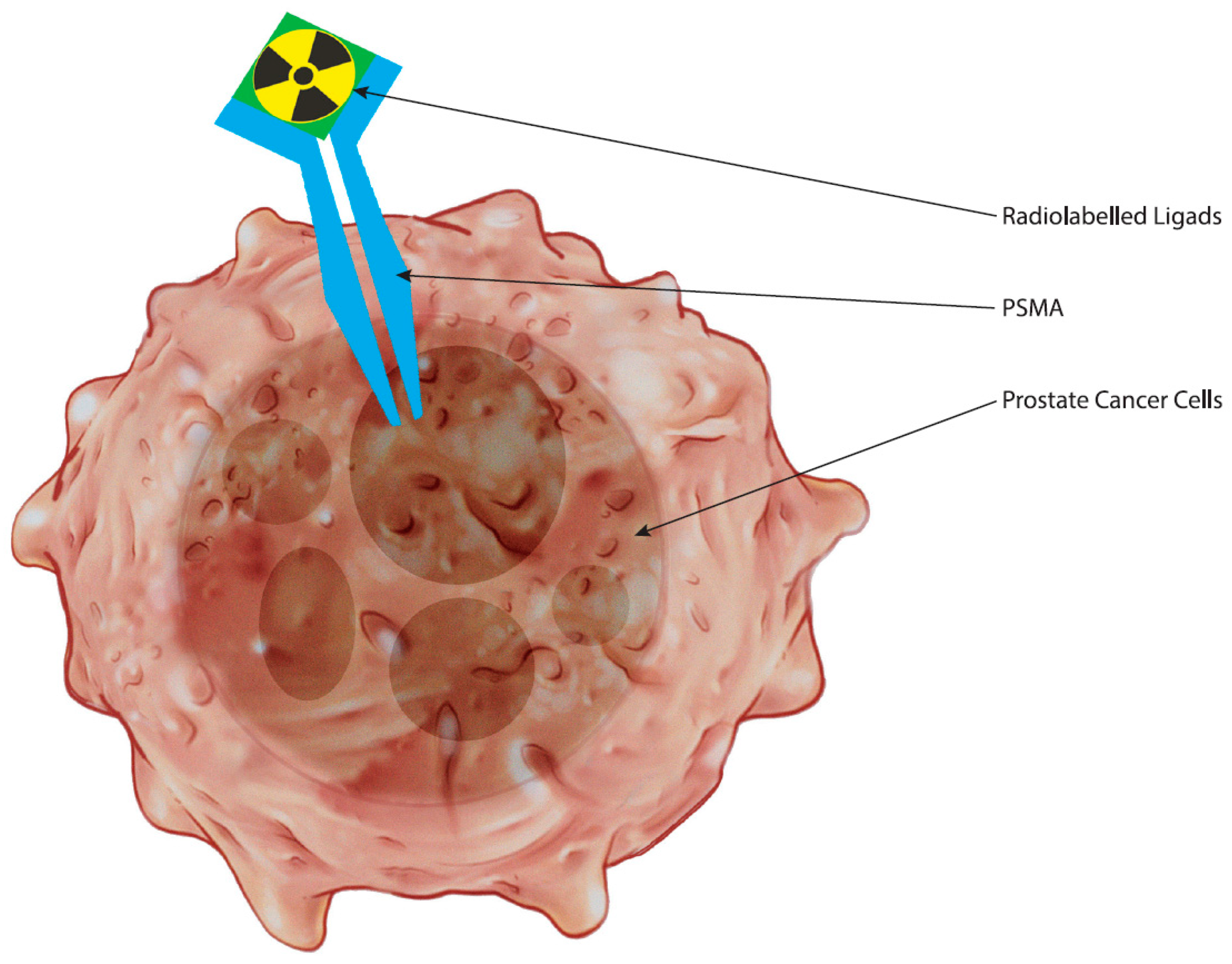
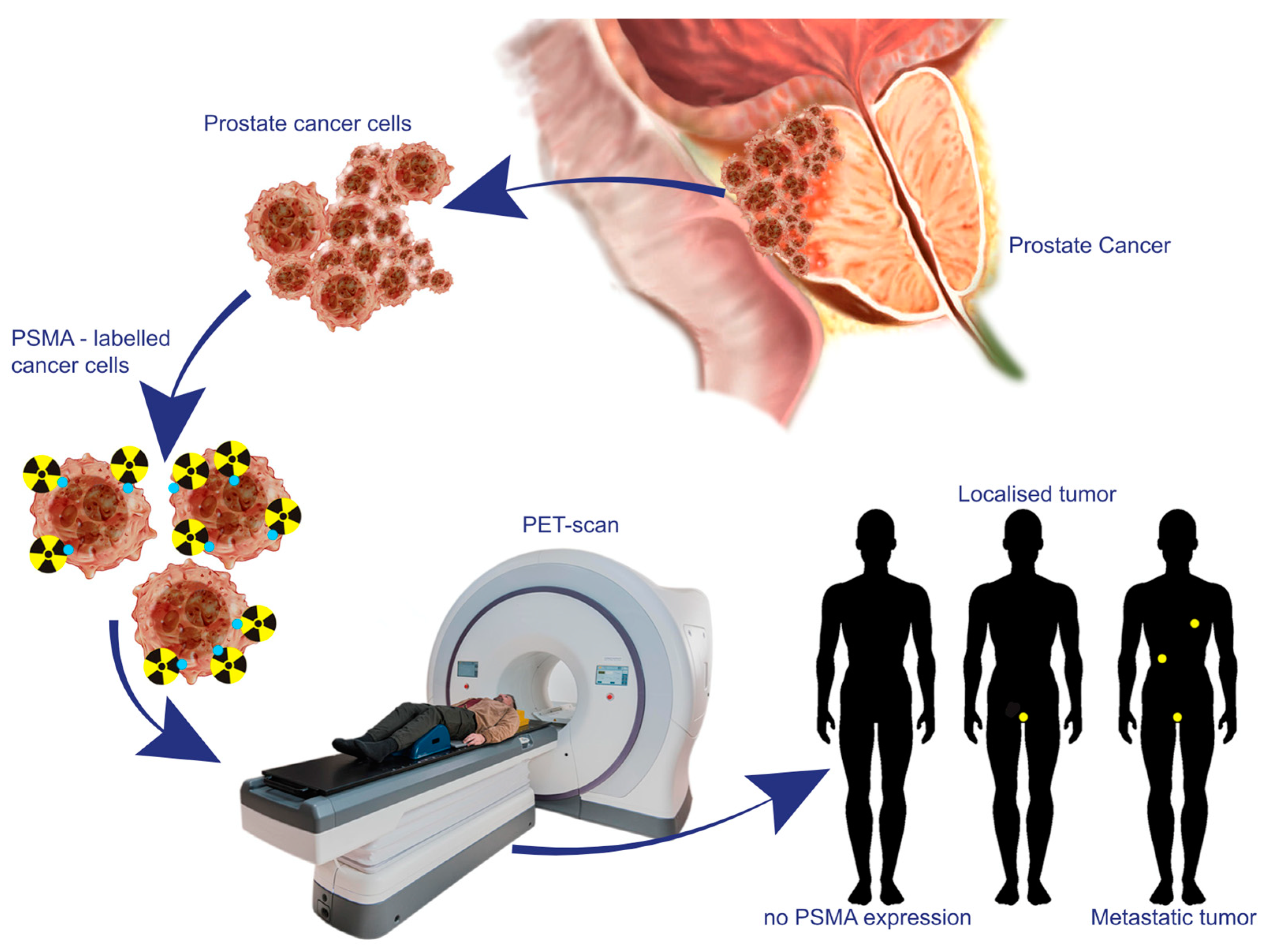
2. The Biological Underpinnings of PSMA as a Target in Prostate Cancer
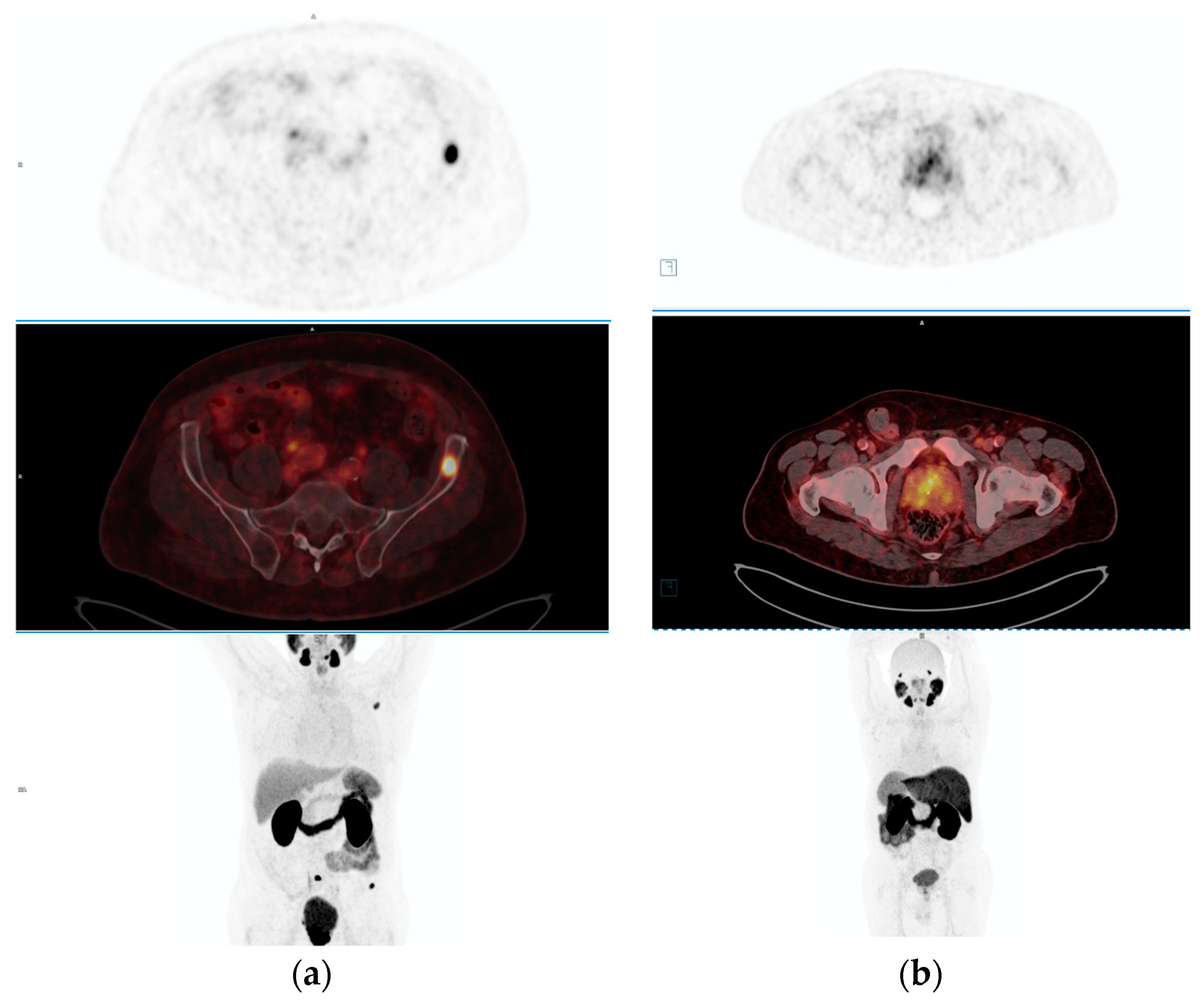
3. Advances in PET-PSMA Imaging Technologies and Methodologies
4. Diagnostic Accuracy and Clinical Utility of PET-PSMA across Different Stages of Prostate Cancer
5. The Predictive Value of PSMA Expression in Response to Therapy as Determined by PET-PSMA Imaging
6. Limitations and Future Directions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Unterrainer, L.M.; Calais, J.; Bander, N.H. Prostate-Specific Membrane Antigen: Gateway to Management of Advanced Prostate Cancer. Annu. Rev. Med. 2024, 75, 49–66. [Google Scholar] [CrossRef]
- Jochumsen, M.R.; Bouchelouche, K. PSMA PET/CT for Primary Staging of Prostate Cancer—An Updated Overview. Semin. Nucl. Med. 2024, 54, 39–45. [Google Scholar] [CrossRef]
- Tangel, M.R.; Rastinehad, A.R. Advances in prostate cancer imaging. F1000Research 2018, 7, 1337. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer—Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 77, 403–417. [Google Scholar] [CrossRef]
- The FDA Approves PSMA-targeted Drug for PET Imaging in Men with Prostate Cancer. BJU Int. 2021, 127, 267–268. [CrossRef]
- Schaeffer, E.M. NCCN Guidelines Version 3.2024 Prostate Cancer. 2024. Available online: https://www.nccn.org/home/ (accessed on 25 March 2024).
- CFoss, A.; Mease, R.C.; Cho, S.Y.; Kim, H.J.; Pomper, M.G. GCPII Imaging and Cancer. Curr. Med. Chem. 2012, 19, 1346–1359. [Google Scholar] [CrossRef]
- Caromile, L.A.; Shapiro, L.H. PSMA redirects MAPK to PI3K-AKT signaling to promote prostate cancer progression. Mol. Cell. Oncol. 2017, 4, e1321168. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Kuratsukuri, K.; Landas, S.; Imaida, K.; Rovito, P.M.; Wang, C.Y.; Haas, G.P. Expression of prostate-specific membrane antigen in normal and malignant human tissues. World J. Surg. 2006, 30, 628–636. [Google Scholar] [CrossRef]
- De Galiza Barbosa, F.; Queiroz, M.A.; Nunes, R.F.; Costa, L.B.; Zaniboni, E.C.; Marin, J.F.G.; Cerri, G.G.; Buchpiguel, C.A. Nonprostatic diseases on PSMA PET imaging: A spectrum of benign and malignant findings. Cancer Imaging 2020, 20, 23. [Google Scholar] [CrossRef]
- Kaittanis, C.; Andreou, C.; Hieronymus, H.; Mao, N.; Foss, C.A.; Eiber, M.; Weirich, G.; Panchal, P.; Gopalan, A.; Zurita, J.; et al. Prostate-specific membrane antigen cleavage of vitamin B9 stimulates oncogenic signaling through metabotropic glutamate receptors. J. Exp. Med. 2018, 215, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Conway, R.E.; Petrovic, N.; Li, Z.; Heston, W.; Wu, D.; Shapiro, L.H. Prostate-Specific Membrane Antigen Regulates Angiogenesis by Modulating Integrin Signal Transduction. Mol. Cell. Biol. 2006, 26, 5310–5324. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, A.; Sheehan, B.; Riisnaes, R.; Rodrigues, D.N.; Gurel, B.; Bertan, C.; Ferreira, A.; Lambros, M.B.; Seed, G.; Yuan, W.; et al. Prostate-specific Membrane Antigen Heterogeneity and DNA Repair Defects in Prostate Cancer. Eur. Urol. 2019, 76, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Bakht, M.K.; Derecichei, I.; Li, Y.; Ferraiuolo, R.-M.; Dunning, M.J.; Oh, S.W.; Hussein, A.; Youn, H.; Stringer, K.F.; Jeong, C.W.; et al. Neuroendocrine differentiation of prostate cancer leads to PSMA suppression. Endocr. Relat. Cancer 2019, 26, 131–146. [Google Scholar] [CrossRef]
- Hövels, A.M.; Heesakkers, R.; Adang, E.; Jager, G.; Strum, S.; Hoogeveen, Y.; Severens, J.; Barentsz, J. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin. Radiol. 2008, 63, 387–395. [Google Scholar] [CrossRef]
- Jetty, S.; Loftus, J.R.; Patel, A.; Gupta, A.; Puri, S.; Dogra, V. Prostate Cancer—PET Imaging Update. Cancers 2023, 15, 796. [Google Scholar] [CrossRef] [PubMed]
- Calais, J.; Fendler, W.P.; Herrmann, K.; Eiber, M.; Ceci, F. Comparison of 68Ga-PSMA-11 and 18F-Fluciclovine PET/CT in a Case Series of 10 Patients with Prostate Cancer Recurrence. J. Nucl. Med. 2018, 59, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Kesch, C.; Kratochwil, C.; Mier, W.; Kopka, K.; Giesel, F.L. 68Ga or 18F for Prostate Cancer Imaging? J. Nucl. Med. 2017, 58, 687–688. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, P.J.; Emmett, L.; Ho, B.; Delprado, W.; Ting, F.; Nguyen, Q.; Stricker, P.D. Prospective evaluation of 68Gallium-prostate-specific membrane antigen positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer. BJU Int. 2017, 119, 209–215. [Google Scholar] [CrossRef]
- Pienta, K.J.; Gorin, M.A.; Rowe, S.P.; Carroll, P.R.; Pouliot, F.; Probst, S.; Saperstein, L.; Preston, M.A.; Alva, A.S.; Patnaik, A.; et al. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY). J. Urol. 2021, 206, 52–61. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Calais, J.; Zhu, S.; Hirmas, N.; Eiber, M.; Hadaschik, B.; Stuschke, M.; Herrmann, K.; Czernin, J.; Kishan, A.U.; Nickols, N.G.; et al. Phase 3 multicenter randomized trial of PSMA PET/CT prior to definitive radiation therapy for unfavorable intermediate-risk or high-risk prostate cancer [PSMA dRT]: Study protocol. BMC Cancer 2021, 21, 512. [Google Scholar] [CrossRef]
- Matushita, C.S.; da Silva, A.M.M.; Schuck, P.N.; Bardisserotto, M.; Piant, D.B.; Pereira, J.L.; Cerci, J.J.; Filho, G.B.C.; Esteves, F.P.; Amorim, B.J.; et al. 68Ga-Prostate-specific membrane antigen (PSMA) positron emission tomography (pet) in prostate cancer: A systematic review and meta-analysis. Int. Braz. J. Urol. 2021, 47, 705–729. [Google Scholar] [CrossRef]
- Pattison, D.A.; Debowski, M.; Gulhane, B.; Arnfield, E.G.; Pelecanos, A.M.; Garcia, P.L.; Latter, M.J.; Lin, C.Y.; Roberts, M.J.; Ramsay, S.C.; et al. Prospective intra-individual blinded comparison of [18F]PSMA-1007 and [68 Ga]Ga-PSMA-11 PET/CT imaging in patients with confirmed prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 763–776. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Hofman, M.S.; Emmett, L.; Calais, J.; Osborne, J.R.; Iravani, A.; Koo, P.; Lindenberg, L.; et al. Joint EANM/SNMMI procedure guideline for the use of 177Lu-labeled PSMA-targeted radioligand-therapy (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2830–2845. [Google Scholar] [CrossRef]
- Ceci, F.; Oprea-Lager, D.E.; Emmett, L.; Adam, J.A.; Bomanji, J.; Czernin, J.; Eiber, M.; Haberkorn, U.; Hofman, M.S.; Hope, T.A.; et al. E-PSMA: The EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1626–1638. [Google Scholar] [CrossRef]
- Eiber, M.; Herrmann, K.; Calais, J.; Hadaschik, B.; Giesel, F.L.; Hartenbach, M.; Hope, T.A.; Reiter, R.; Maurer, T.; Weber, W.A.; et al. Prostate cancer molecular imaging standardized evaluation (PROMISE): Proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J. Nucl. Med. 2018, 59, 469–478. [Google Scholar] [CrossRef]
- Rowe, S.P.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A. Proposal for a structured reporting system for prostate-specific membrane antigen-targeted PET imaging: PSMA-RADS version 1.0. J. Nucl. Med. 2018, 59, 479–485. [Google Scholar] [CrossRef]
- Seifert, R.; Emmett, L.; Rowe, S.P.; Herrmann, K.; Hadaschik, B.; Calais, J.; Giesel, F.L.; Reiter, R.; Maurer, T.; Heck, M.; et al. Second Version of the Prostate Cancer Molecular Imaging Standardized Evaluation Framework Including Response Evaluation for Clinical Trials (PROMISE V2). Eur. Urol. 2023, 83, 405–412. [Google Scholar] [CrossRef]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Calais, J.; Ceci, F.; Cho, S.Y.; Fanti, S.; Giesel, F.L.; Goffin, K.; et al. PSMA PET/CT: Joint EANM procedure guideline/SNMMI procedure standard for prostate cancer imaging 2.0. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1466–1486. [Google Scholar] [CrossRef]
- Cornford, P.; Bergh, R.C.v.D.; Briers, E.; Broeck, T.V.D.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer—2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Kalapara, A.A.; Nzenza, T.; Pan, H.Y.; Ballok, Z.; Ramdave, S.; O’Sullivan, R.; Ryan, A.; Cherk, M.; Hofman, M.S.; Konety, B.R.; et al. Detection and localisation of primary prostate cancer using 68gallium prostate-specific membrane antigen positron emission tomography/computed tomography compared with multiparametric magnetic resonance imaging and radical prostatectomy specimen pathology. BJU Int. 2020, 126, 83–90. [Google Scholar] [CrossRef]
- Li, Y.; Han, D.; Wu, P.; Ren, J.; Ma, S.; Zhang, J.; Song, W.; Lin, X.; Jiao, D.; Shi, S.; et al. Comparison of 68Ga-PSMA-617 PET/CT with mpMRI for the detection of PCa in patients with a PSA level of 4–20 ng/ml before the initial biopsy. Sci. Rep. 2020, 10, 10963. [Google Scholar] [CrossRef]
- Sharma, A.P.; Kumar, R.; Chauhan, R.; Ziauddin, S.A.; Singh, S.; Singh, H.; Devana, S.K.; Gorsi, U.; Bora, G.S.; Mavuduru, R.S.; et al. Accuracy of combined multi-parametric MRI and PSMA PET-CT in diagnosing localized prostate cancer: Newer horizons for a biopsy-free pathway. Eur. J. Hybrid Imaging 2023, 7, 24. [Google Scholar] [CrossRef]
- Jeet, V.; Parkinson, B.; Song, R.; Sharma, R.; Hoyle, M. Histopathologically Validated Diagnostic Accuracy of PSMA-PET/CT in the Primary and Secondary Staging of Prostate Cancer and the Impact of PSMA-PET/CT on Clinical Management: A Systematic Review and Meta-analysis. Semin. Nucl. Med. 2023, 53, 706–718. [Google Scholar] [CrossRef]
- Klingenberg, S.; Fredsøe, J.; Sørensen, K.D.; Ulhøi, B.P.; Borre, M.; Jochumsen, M.R.; Bouchelouche, K. Recurrence rate after radical prostatectomy following primary staging of high-risk prostate cancer with 68Ga-PSMA PET/CT. Acta Oncol. 2022, 61, 1289–1294. [Google Scholar] [CrossRef]
- Karagiannis, V.; Wichmann, V.; Saarinen, J.; Eigeliene, N.; Andersen, H.; Jekunen, A. Radiotherapy treatment modification for prostate cancer patients based on PSMA-PET/CT. Radiat. Oncol. 2022, 17, 19. [Google Scholar] [CrossRef]
- Jilg, C.A.; Drendel, V.; Rischke, H.C.; Beck, T.I.R.; Reichel, K.; Krönig, M.; Wetterauer, U.; Schultze-Seemann, W.; Meyer, P.T.; Vach, W. Detection rate of 18F-choline PET/CT and 68Ga-PSMA-HBED-CC PET/CT for prostate cancer lymph node metastases with direct link from PET to histopathology: Dependence on the size of tumor deposits in lymph nodes. J. Nucl. Med. 2019, 60, 971–977. [Google Scholar] [CrossRef]
- Koseoglu, E.; Kordan, Y.; Kilic, M.; Sal, O.; Seymen, H.; Kiremit, M.C.; Armutlu, A.; Baydar, D.E.; Altinmakas, E.; Vural, M.; et al. Diagnostic ability of Ga-68 PSMA PET to detect dominant and non-dominant tumors, upgrading and adverse pathology in patients with PIRADS 4–5 index lesions undergoing radical prostatectomy. Prostate Cancer Prostatic Dis. 2021, 24, 202–209. [Google Scholar] [CrossRef]
- Tayara, O.M.; Pełka, K.; Kunikowska, J.; Malewski, W.; Sklinda, K.; Kamecki, H.; Poletajew, S.; Kryst, P.; Nyk, Ł. Comparison of Multiparametric MRI, [68Ga]Ga-PSMA-11 PET-CT, and Clinical Nomograms for Primary T and N Staging of Intermediate-to-High-Risk Prostate Cancer. Cancers 2023, 15, 5838. [Google Scholar] [CrossRef]
- Grubmüller, B.; Baltzer, P.; Hartenbach, S.; D’andrea, D.; Helbich, T.H.; Haug, A.R.; Goldner, G.M.; Wadsak, W.; Pfaff, S.; Mitterhauser, M.; et al. PSMA ligand PET/MRI for primary prostate cancer: Staging performance and clinical impact. Clin. Cancer Res. 2018, 24, 6300–6307. [Google Scholar] [CrossRef]
- Chow, K.M.; So, W.Z.; Lee, H.J.; Lee, A.; Yap, D.W.T.; Takwoingi, Y.; Tay, K.J.; Tuan, J.; Thang, S.P.; Lam, W.; et al. Head-to-head Comparison of the Diagnostic Accuracy of Prostate-specific Membrane Antigen Positron Emission Tomography and Conventional Imaging Modalities for Initial Staging of Intermediate- to High-risk Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2023, 84, 36–48. [Google Scholar] [CrossRef]
- Klingenberg, S.; Jochumsen, M.R.; Ulhøi, B.P.; Fredsøe, J.; Sørensen, K.D.; Borre, M.; Bouchelouche, K. 68Ga-PSMA PET/CT for primary lymph node and distant metastasis NM staging of high-risk prostate cancer. J. Nucl. Med. 2021, 62, 214–220. [Google Scholar] [CrossRef]
- Freitag, M.T.; Kesch, C.; Cardinale, J.; Flechsig, P.; Floca, R.; Eiber, M.; Bonekamp, D.; Radtke, J.P.; Kratochwil, C.; Kopka, K.; et al. Simultaneous whole-body 18F–PSMA-1007-PET/MRI with integrated high-resolution multiparametric imaging of the prostatic fossa for comprehensive oncological staging of patients with prostate cancer: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 340–347. [Google Scholar] [CrossRef]
- Solari, E.L.; Gafita, A.; Schachoff, S.; Bogdanović, B.; Asiares, A.V.; Amiel, T.; Hui, W.; Rauscher, I.; Visvikis, D.; Maurer, T.; et al. The added value of PSMA PET/MR radiomics for prostate cancer staging. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 527–538. [Google Scholar] [CrossRef]
- Eiber, M.; Weirich, G.; Holzapfel, K.; Souvatzoglou, M.; Haller, B.; Rauscher, I.; Beer, A.J.; Wester, H.-J.; Gschwend, J.; Schwaiger, M.; et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI Improves the Localization of Primary Prostate Cancer. Eur. Urol. 2016, 70, 829–836. [Google Scholar] [CrossRef]
- Roach, P.J.; Francis, R.; Emmett, L.; Hsiao, E.; Kneebone, A.; Hruby, G.; Eade, T.; Nguyen, Q.A.; Thompson, B.D.; Cusick, T.; et al. The impact of 68Ga-PSMA PET/CT on management intent in prostate cancer: Results of an australian prospective multicenter study. J. Nucl. Med. 2018, 59, 82–88. [Google Scholar] [CrossRef]
- Zamboglou, C.; Carles, M.; Fechter, T.; Kiefer, S.; Reichel, K.; Fassbender, T.F.; Bronsert, P.; Koeber, G.; Schilling, O.; Ruf, J.; et al. Radiomic features from PSMA PET for non-invasive intraprostatic tumor discrimination and characterization in patients with intermediate- and high-risk prostate cancer—A comparison study with histology reference. Theranostics 2019, 9, 2595–2605. [Google Scholar] [CrossRef]
- Lawal, I.O.; Ndlovu, H.; Kgatle, M.; Mokoala, K.M.G.; Sathekge, M.M. Prognostic Value of PSMA PET/CT in Prostate Cancer. Semin. Nucl. Med. 2024, 54, 46–59. [Google Scholar] [CrossRef]
- Hupe, M.C.; Philippi, C.; Roth, D.; Kümpers, C.; Ribbat-Idel, J.; Becker, F.; Joerg, V.; Duensing, S.; Lubczyk, V.H.; Kirfel, J.; et al. Expression of prostate-specific membrane antigen (PSMA) on biopsies is an independent risk stratifier of prostate cancer patients at time of initial diagnosis. Front. Oncol. 2018, 8, 623. [Google Scholar] [CrossRef]
- Mannweiler, S.; Amersdorfer, P.; Trajanoski, S.; Terrett, J.A.; King, D.; Mehes, G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol. Oncol. Res. 2009, 15, 167–172. [Google Scholar] [CrossRef]
- Seifert, R.; Seitzer, K.; Herrmann, K.; Kessel, K.; Schäfers, M.; Kleesiek, J.; Weckesser, M.; Boegemann, M.; Rahbar, K. Analysis of PSMA expression and outcome in patients with advanced Prostate Cancer receiving 177Lu-PSMA-617 Radioligand Therapy. Theranostics 2020, 10, 7812–7820. [Google Scholar] [CrossRef] [PubMed]
- Seifert, R.; Kessel, K.; Schlack, K.; Weber, M.; Herrmann, K.; Spanke, M.; Fendler, W.P.; Hadaschik, B.; Kleesiek, J.; Schäfers, M.; et al. PSMA PET total tumor volume predicts outcome of patients with advanced prostate cancer receiving [177Lu]Lu-PSMA-617 radioligand therapy in a bicentric analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Tosoian, J.J.; Gorin, M.A.; Ross, A.E.; Pienta, K.J.; Tran, P.T.; Schaeffer, E.M. Oligometastatic prostate cancer: Definitions, clinical outcomes, and treatment considerations. Nat. Rev. Urol. 2017, 14, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Smith-Jones, P.M.; Wongvipat, J.; Navarro, V.; Kim, S.; Bander, N.H.; Larson, S.M.; Sawyers, C.L. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc. Natl. Acad. Sci. USA 2011, 108, 9578–9582. [Google Scholar] [CrossRef] [PubMed]
- Vargas, H.A.; Wassberg, C.; Akin, O.; Hricak, H. MR imaging of treated prostate cancer. Radiology 2012, 262, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, P.; Ghezzo, S.; Spataro, A.; Bezzi, C.; Gajate, A.M.S.; Chiti, A.; Picchio, M. Systematic Review and Metanalysis on the Role of Prostate-Specific Membrane Antigen Positron Emission Tomography/Magnetic Resonance Imaging for Intraprostatic Tumour Assessment. Magn. Reson. Imaging Clin. N. Am. 2023, 31, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Chavoshi, M.; Mirshahvalad, S.A.; Metser, U.; Veit-Haibach, P. 68Ga-PSMA PET in prostate cancer: A systematic review and meta-analysis of the observer agreement. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1021–1029. [Google Scholar] [CrossRef]
- Gossili, F.; Mogensen, A.W.; Konnerup, T.C.; Bouchelouche, K.; Alberts, I.; Afshar-Oromieh, A.; Zacho, H.D. The diagnostic accuracy of radiolabeled PSMA-ligand PET for tumour staging in newly diagnosed prostate cancer patients compared to histopathology: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2023, 51, 281–294. [Google Scholar] [CrossRef]
- Anttinen, M.; Ettala, O.; Malaspina, S.; Jambor, I.; Sandell, M.; Kajander, S.; Rinta-Kiikka, I.; Schildt, J.; Saukko, E.; Rautio, P.; et al. A Prospective Comparison of 18F-prostate-specific Membrane Antigen-1007 Positron Emission Tomography Computed Tomography, Whole-body 1.5 T Magnetic Resonance Imaging with Diffusion-weighted Imaging, and Single-photon Emission Computed Tomography/Computed Tomography with Traditional Imaging in Primary Distant Metastasis Staging of Prostate Cancer (PROSTAGE). Eur. Urol. Oncol. 2021, 4, 635–644. [Google Scholar] [CrossRef]
- Yakar, D.; Noordzij, W.; Kwee, T.C. Potential Causes of False-Negative Interpretations in 68Ga-PSMA PET/CT for the Detection of Local and Recurrent Prostate Cancer: An Underexposed Issue. Clin. Nucl. Med. 2020, 45, e32–e35. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tayara, O.; Poletajew, S.; Malewski, W.; Kunikowska, J.; Pełka, K.; Kryst, P.; Nyk, Ł. Prostate-Specific Membrane Antigen Expression in Patients with Primary Prostate Cancer: Diagnostic and Prognostic Value in Positron Emission Tomography-Prostate-Specific Membrane Antigen. Curr. Oncol. 2024, 31, 4165-4177. https://doi.org/10.3390/curroncol31080311
Tayara O, Poletajew S, Malewski W, Kunikowska J, Pełka K, Kryst P, Nyk Ł. Prostate-Specific Membrane Antigen Expression in Patients with Primary Prostate Cancer: Diagnostic and Prognostic Value in Positron Emission Tomography-Prostate-Specific Membrane Antigen. Current Oncology. 2024; 31(8):4165-4177. https://doi.org/10.3390/curroncol31080311
Chicago/Turabian StyleTayara, Omar, Sławomir Poletajew, Wojciech Malewski, Jolanta Kunikowska, Kacper Pełka, Piotr Kryst, and Łukasz Nyk. 2024. "Prostate-Specific Membrane Antigen Expression in Patients with Primary Prostate Cancer: Diagnostic and Prognostic Value in Positron Emission Tomography-Prostate-Specific Membrane Antigen" Current Oncology 31, no. 8: 4165-4177. https://doi.org/10.3390/curroncol31080311
APA StyleTayara, O., Poletajew, S., Malewski, W., Kunikowska, J., Pełka, K., Kryst, P., & Nyk, Ł. (2024). Prostate-Specific Membrane Antigen Expression in Patients with Primary Prostate Cancer: Diagnostic and Prognostic Value in Positron Emission Tomography-Prostate-Specific Membrane Antigen. Current Oncology, 31(8), 4165-4177. https://doi.org/10.3390/curroncol31080311




