Ovarian Mesonephric-like Adenocarcinoma: Its Prevalence in a Japanese High-Volume Cancer Center and a Literature Review on Therapeutic Targets
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Tissue Section Preparation
2.2. Pathological Diagnosis
2.3. Literature Review
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
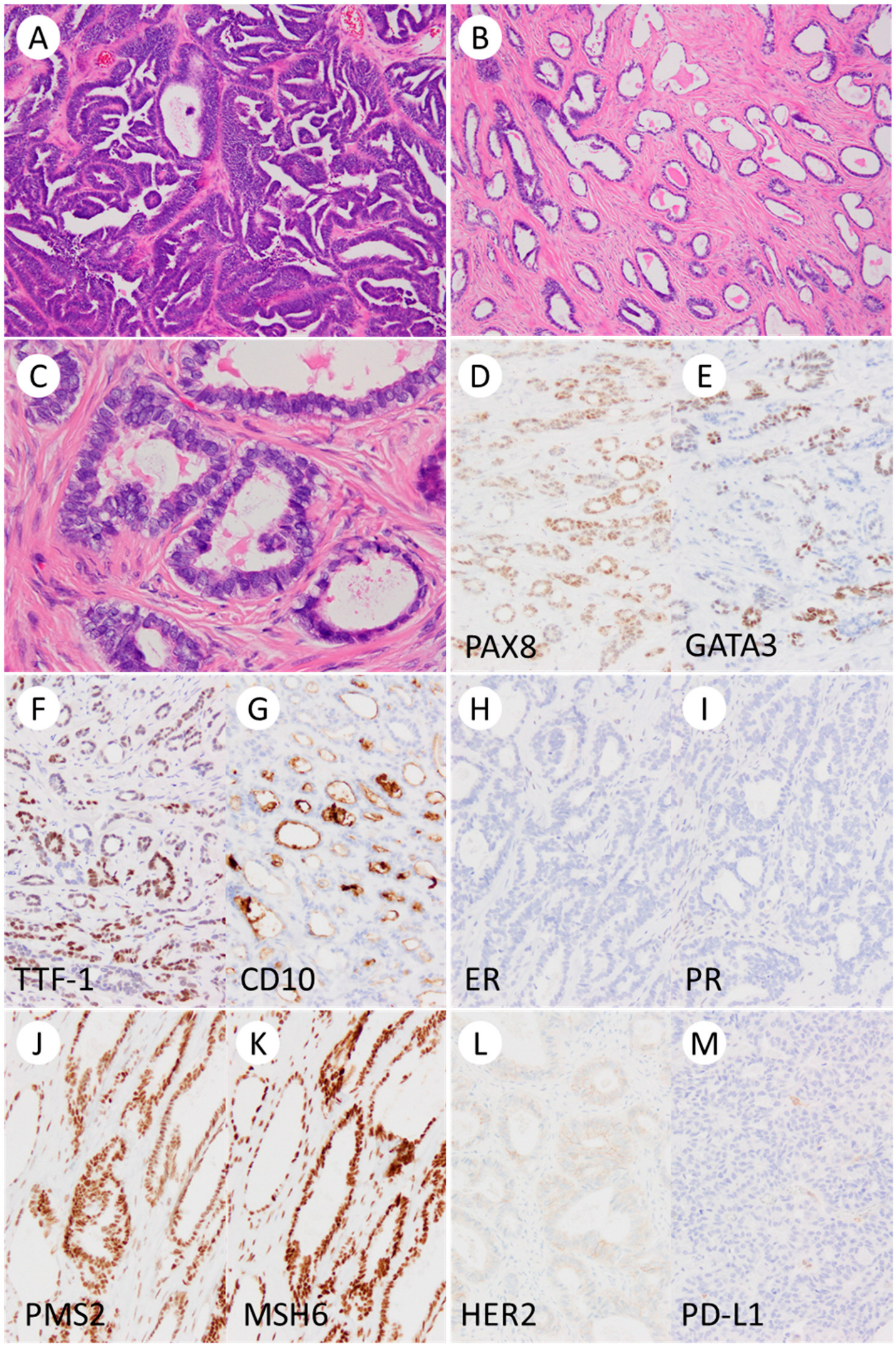
3.2. Pathological Findings
3.3. Literature Review and Summary of Previously Reported Cases
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McFarland, M.; Quick, C.M.; McCluggage, W.G. Hormone receptor-negative, thyroid transcription factor 1-positive uterine and ovarian adenocarcinomas: Report of a series of mesonephric-like adenocarcinomas. Histopathology 2016, 68, 1013–1020. [Google Scholar] [CrossRef]
- McCluggage, W.G. Endometriosis-related pathology: A discussion of selected uncommon benign, premalignant and malignant lesions. Histopathology 2020, 76, 76–92. [Google Scholar] [CrossRef]
- Yano, M.; Shintani, D.; Katoh, T.; Hamada, M.; Ito, K.; Kozawa, E.; Hasegawa, K.; Yasuda, M. Coexistence of endometrial mesonephric-like adenocarcinoma and endometrioid carcinoma suggests a Müllerian duct lineage: A case report. Diagn. Pathol. 2019, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.G. Mesonephric-like Adenocarcinoma of the Female Genital Tract: From Morphologic Observations to a Well-characterized Carcinoma with Aggressive Clinical Behavior. Adv. Anat. Pathol. 2022, 29, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Pors, J.; Segura, S.; Chiu, D.S.; Almadani, N.; Ren, H.; Fix, D.J.; Howitt, B.E.; Kolin, D.; McCluggage, W.G.; Mirkovic, J.; et al. Clinicopathologic Characteristics of Mesonephric Adenocarcinomas and Mesonephric-like Adenocarcinomas in the Gynecologic Tract: A Multi-institutional Study. Am. J. Surg. Pathol. 2021, 45, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Sugitani, A.; Ogawa, A.; Yoshida, H.; Kobayashi-Kato, M.; Kikkawa, N.; Tanase, Y.; Uno, M.; Ishikawa, M.; Kato, T. Ovarian Mesonephric-Like Adenocarcinoma with Recurrent Liver Metastases: A Case Report with Analysis of Therapeutic Molecular Targets. Int. J. Surg. Pathol. 2024, 32, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Mirkovic, J.; McFarland, M.; Garcia, E.; Sholl, L.M.; Lindeman, N.; MacConaill, L.; Dong, F.; Hirsch, M.; Nucci, M.R.; Quick, C.M.; et al. Targeted Genomic Profiling Reveals Recurrent KRAS Mutations in Mesonephric-like Adenocarcinomas of the Female Genital Tract. Am. J. Surg. Pathol. 2018, 42, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Pors, J.; Cheng, A.; Leo, J.M.; Kinloch, M.A.; Gilks, B.; Hoang, L. A Comparison of GATA3, TTF1, CD10, and Calretinin in Identifying Mesonephric and Mesonephric-like Carcinomas of the Gynecologic Tract. Am. J. Surg. Pathol. 2018, 42, 1596–1606. [Google Scholar] [CrossRef]
- Chapel, D.B.; Joseph, N.M.; Krausz, T.; Lastra, R.R. An Ovarian Adenocarcinoma with Combined Low-grade Serous and Mesonephric Morphologies Suggests a Müllerian Origin for Some Mesonephric Carcinomas. Int. J. Gynecol. Pathol. 2018, 37, 448–459. [Google Scholar] [CrossRef]
- McCluggage, W.G.; Vosmikova, H.; Laco, J. Ovarian Combined Low-grade Serous and Mesonephric-like Adenocarcinoma: Further Evidence for A Mullerian Origin of Mesonephric-like Adenocarcinoma. Int. J. Gynecol. Pathol. 2020, 39, 84–92. [Google Scholar] [CrossRef]
- Dundr, P.; Gregová, M.; Němejcová, K.; Bártů, M.; Hájková, N.; Hojný, J.; Stružinská, I.; Fischerová, D. Ovarian mesonephric-like adenocarcinoma arising in serous borderline tumor: A case report with complex morphological and molecular analysis. Diagn. Pathol. 2020, 15, 91. [Google Scholar] [CrossRef]
- Seay, K.; Akanbi, T.; Bustamante, B.; Chaudhary, S.; Goldberg, G.L. Mesonephric-like adenocarcinoma of the ovary with co-existent endometriosis: A case report and review of the literature. Gynecol. Oncol. Rep. 2020, 34, 100657. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shen, Y.; Xie, C. Mesonephric-like adenocarcinoma of the ovary: A case report and a review of the literature. Medicine 2020, 99, e23450. [Google Scholar] [CrossRef] [PubMed]
- Qazi, M.; Movahedi-Lankarani, S.; Wang, B.G. Cytohistopathologic correlation of ovarian mesonephric-like carcinoma and female adnexal tumor of probable Wolffian origin. Diagn. Cytopathol. 2021, 49, E207–E213. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.M.; Fix, D.J.; Sebastiao, A.P.M.; Selenica, P.; Ferrando, L.; Kim, S.H.; Stylianou, A.; Da Cruz Paula, A.; Pareja, F.; Smith, E.S.; et al. Mesonephric and mesonephric-like carcinomas of the female genital tract: Molecular characterization including cases with mixed histology and matched metastases. Mod. Pathol. 2021, 34, 1570–1587. [Google Scholar] [CrossRef]
- Kim, H.; Bae, G.E.; Jung, Y.Y.; Kim, H.S. Ovarian Mesonephric-like Adenocarcinoma with Multifocal Microscopic Involvement of the Fimbrial Surface: Potential for Misdiagnosis of Tubal Intraepithelial Metastasis as Serous Tubal Intraepithelial Carcinoma Associated with Ovarian High-grade Serous Carcinoma. In Vivo 2021, 35, 3613–3622. [Google Scholar] [CrossRef]
- Karpathiou, G.; Chauleur, C.; Picot, T.; Achour, S.; Corsini, T.; Devouassoux-Shisheboran, M.; Peoc’h, M. Ovarian mesonephric-like adenocarcinoma: Morphological diversity and histogenetic considerations of an unusual tumour. Pathology 2022, 54, 647–650. [Google Scholar] [CrossRef]
- Ujita, M.; Abiko, K.; Kuwahara, R.; Emoto, I.; Amano, Y.; Konishi, I. Mesonephric-like adenocarcinoma of the ovary in an elderly woman: A case report and a review of the literature. J. Obstet. Gynaecol. Res. 2021, 47, 4490–4495. [Google Scholar] [CrossRef]
- Deolet, E.; Arora, I.; Van Dorpe, J.; Van der Meulen, J.; Desai, S.; Van Roy, N.; Kaur, B.; Van de Vijver, K.; McCluggage, W.G. Extrauterine Mesonephric-like Neoplasms: Expanding the Morphologic Spectrum. Am. J. Surg. Pathol. 2022, 46, 124–133. [Google Scholar] [CrossRef]
- Koh, H.H.; Park, E.; Kim, H.S. Mesonephric-like Adenocarcinoma of the Ovary: Clinicopathological and Molecular Characteristics. Diagnostics 2022, 12, 326. [Google Scholar] [CrossRef]
- Ishida, K.; Ashihara, T.; So, M.; Minamiguchi, S.; Matsumura, N.; Nonogaki, T. Synchronous ovarian and uterine mesonephric-like carcinoma that potentially arose from endometrioid adenofibroma: A case report. J. Obstet. Gynaecol. Res. 2023, 49, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Arslanian, E.; Singh, K.; James Sung, C.; Quddus, M.R. Somatic mutation analysis of Mesonephric-Like adenocarcinoma and associated putative precursor Lesions: Insight into pathogenesis and potential molecular treatment targets. Gynecol. Oncol. Rep. 2022, 42, 101049. [Google Scholar] [CrossRef] [PubMed]
- Nilforoushan, N.; Liu, L.; Cheang, G.; Sui, A.C.; Andersen, J.; Finkelman, B.S.; Liu, Y.; Nasseri-Nik, N.; Vang, R.; Ronnett, B.M.; et al. Mucinous Tumor Coexisting with Mesonephric-like Proliferation/Tumor in the Ovary: A Novel Association. Am. J. Surg. Pathol. 2022, 46, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Mirkovic, J.; Olkhov-Mitsel, E.; Amemiya, Y.; Al-Hussaini, M.; Nofech-Mozes, S.; Djordjevic, B.; Kupets, R.; Seth, A.; McCluggage, W.G. Mesonephric-like adenocarcinoma of the female genital tract: Novel observations and detailed molecular characterisation of mixed tumours and mesonephric-like carcinosarcomas. Histopathology 2023, 82, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Park, K.J.; Rehrauer, W.M.; Weisman, P.S. Mesonephric-like adenocarcinoma of the ovary with squamoid morular metaplasia, aberrant β-catenin expression, and concurrent FGFR2 and CTNNB1 mutations: A case report. Virchows Arch. 2024, 484, 147–150. [Google Scholar] [CrossRef]
- Nilforoushan, N.; Liu, L.; Finkelman, B.S.; Andersen, J.; Liu, Y.; James, J.; Hung, C.F.; Wu, T.C.; Vang, R.; Xing, D. Ovarian Combined Serous Borderline Tumor/Low-grade Serous Carcinoma and Mesonephric-like Lesion: Report of 2 Cases with New Observations. Int. J. Gynecol. Pathol. 2023, 42, 182–191. [Google Scholar] [CrossRef]
- Stolnicu, S.; Bartalis, R.J.; Ye, Q.; Da Cruz Paula, A.; Weigelt, B.; Soslow, R.A. Ovarian RASoma With Mesonephric-like Adenocarcinoma and Mixed Mullerian Components: A Case Report with Molecular Analysis Demonstrating Multidirectional Mullerian Differentiation. Int. J. Gynecol. Pathol. 2023, 42, 620–626. [Google Scholar] [CrossRef]
- Kommoss, F.K.; Lee, C.H.; Tessier-Cloutier, B.; Gilks, C.B.; Stewart, C.J.; von Deimling, A.; Köbel, M. Mesonephric-like adenocarcinoma harbours characteristic copy number variations and a distinct DNA methylation signature closely related to mesonephric adenocarcinoma of the cervix. J. Pathol. 2024, 262, 4–9. [Google Scholar] [CrossRef]
- Zhao, Z.; Nadarajah, R.; Busmanis, I. Synchronous Bilateral Ovarian Mesonephric-like Adenocarcinomas with Separate Origins from High-Grade Mullerian Adenosarcoma and Endometriosis: Report of a Rare Case. Int. J. Gynecol. Pathol. 2024, 43, 382–388. [Google Scholar] [CrossRef]
- Chang, C.S.; Carney, M.E.; Killeen, J.L. Two Cases of Mesonephric-like Carcinoma Arising from Endometriosis: Case Report and Review of the Literature. Int. J. Gynecol. Pathol. 2023, 42, 101–107. [Google Scholar] [CrossRef]
- Linck, J.; Torres, W. Mesonephric-like adenocarcinoma arising from endometrioid adenofibroma in a patient with in-utero exposure to diethylstilbestrol: A case report. Case Rep. Women’s Health 2023, 39, e00531. [Google Scholar] [CrossRef] [PubMed]
- Nagase, S.; Saeki, H.; Ura, A.; Terao, Y.; Matsumoto, T.; Yao, T. Mixed Mesonephric-like Adenocarcinoma, Clear Cell Carcinoma, and Endometrioid Carcinoma Arising from an Endometriotic Cyst. Int. J. Surg. Pathol. 2023, 32, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Kolin, D.L.; Costigan, D.C.; Dong, F.; Nucci, M.R.; Howitt, B.E. A Combined Morphologic and Molecular Approach to Retrospectively Identify KRAS-Mutated Mesonephric-like Adenocarcinomas of the Endometrium. Am. J. Surg. Pathol. 2019, 43, 389–398. [Google Scholar] [CrossRef]
- Peres, L.C.; Cushing-Haugen, K.L.; Köbel, M.; Harris, H.R.; Berchuck, A.; Rossing, M.A.; Schildkraut, J.M.; Doherty, J.A. Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage. J. Natl. Cancer Inst. 2019, 111, 60–68. [Google Scholar] [CrossRef]
- Swift, B.E.; Covens, A.; Mintsopoulos, V.; Parra-Herran, C.; Bernardini, M.Q.; Nofech-Mozes, S.; Hogen, L. The effect of complete surgical staging and adjuvant chemotherapy on survival in stage I, grade 1 and 2 endometrioid ovarian carcinoma. Int. J. Gynecol. Cancer 2022, 32, 525–531. [Google Scholar] [CrossRef]
- Paver, E.C.; Cooper, W.A.; Colebatch, A.J.; Ferguson, P.M.; Hill, S.K.; Lum, T.; Shin, J.S.; O’Toole, S.; Anderson, L.; Scolyer, R.A.; et al. Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: A guide to immunohistochemistry implementation and interpretation. Pathology 2021, 53, 141–156. [Google Scholar] [CrossRef]
- Soovares, P.; Pasanen, A.; Similä-Maarala, J.; Bützow, R.; Lassus, H. Clinical factors and biomarker profiles associated with patient outcome in endometrioid ovarian carcinoma—Emphasis on tumor grade. Gynecol. Oncol. 2022, 164, 187–194. [Google Scholar] [CrossRef]
- da Cunha Colombo Bonadio, R.R.; Fogace, R.N.; Miranda, V.C.; Diz, M.D.P.E. Homologous recombination deficiency in ovarian cancer: A review of its epidemiology and management. Clinics 2018, 73, e450s. [Google Scholar] [CrossRef] [PubMed]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
| Histological Type | n (%) |
|---|---|
| Total n = 501 | |
| High-grade serous carcinoma | 239 (46.3) |
| Clear cell carcinoma | 116 (22.5) |
| Endometrioid carcinoma | 57 (11.0) |
| Mucinous carcinoma | 30 (5.8) |
| Adenocarcinoma, unclassifiable * | 17 (3.3) |
| Low-grade serous carcinoma | 16 (3.1) |
| Mixed cell carcinoma | 11 (2.1) |
| Carcinosarcoma | 8 (1.6) |
| Mesonephric-like adenocarcinoma | 3 (0.6) |
| Undifferentiated carcinoma | 2 (0.4) |
| Malignant Brenner tumor | 1 (0.2) |
| Squamous cell carcinoma | 1 (0.2) |
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age [year] | 76 | 52 | 71 |
| Obstetric history | G3P3 | G0P0 | G0P0 |
| Menopause [year] | 58 | 50 | 51 |
| Previous medical history | rheumatoid arthritis | leiomyoma, lt shoulder fracture | breast cancer, dyslipidemia |
| Body mass index [kg/m2] | 18 | 22.9 | 22.5 |
| Symptom | pelvic pain, abdominal distension | adnexal mass | pelvic pain, pelvic mass |
| Tumor marker | CA19-9: 290 U/mL, CA125: 434 U/mL | CA19-9: 135 U/mL, CA125: 64 U/mL | CA125: 199 U/mL |
| Radiological diagnosis | rt ovarian cancer | lt ovarian cancer (s/o EM, CCC) | ovarian cancer |
| Clinical stage (FIGO 2008) | cT1N0M0 | cT1N0M0 | cT3bN0M0 |
| Surgical procedure | PDS, TAH+BSO+OMT+PLNB | PDS, TAH+BSO+OMT+PLNB | IDS, TAH+BSO+OMT+PLNB+LAR |
| Pathological stage | pT1c1N0M0 | pT1c1N0M0 | ypT2N0M0 |
| Tumor size [cm] | 15.5 | 13 | 16.5 |
| Recurrence [month] | Yes, 16 month | Yes, 1 month | No |
| Metastatic site | liver, lung | liver | - |
| Follow-up time [month] | 42 | 9 | 8 |
| Prognosis | DOD | AWD | NED |
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Laterality | bilateral | left | bilateral |
| Tumor size [cm] | rt. 15 × 11.5 × 9; lt. 4 × 3 × 1.5 | 16 × 10 × 4 | rt. 3.5 × 2.5 × 1; lt. 11.5 × 7 × 2 |
| Macroscopic type | solid and cystic | solid and cystic | solid and cystic |
| Glandular and papillary pattern | + | + | + |
| Intraluminal eosinophilic secretion | + | + | + |
| Spindled tumor cells | + | + | + |
| Sex cord-like pattern | + | - | + |
| Hyalinized/Fibrous stroma | + | + | + |
| Tumor infiltrating lymphocytes | a few | a few | a few |
| Glassy nucleus | + | + | + |
| Mitotic counts | up to 14/10HPFs | up to 10/10HPFs | up to 11/10HPFs |
| Metaplasia | no | no | no |
| Endometriosis | + | + | + |
| Endometrium | atrophic | atrophic | atrophic |
| Adenomyosis/Leiomyoma | −/+ | +/+ | +/+ |
| Immunohistochemistry | |||
| ER/PR/WT-1 | −/−/− | −/−/− | −/−/− |
| GATA3/TTF-1 positivity | focal/focal | focal/focal | diffuse/focal |
| CD10/Calretinin positivity | focal/focal | focal/focal | focal/rare |
| p53 | wild-type pattern | wild-type pattern | wild-type pattern |
| MMR | pMMR | pMMR | pMMR |
| PD-L1 (22C3) | CPS < 1 | CPS < 1 | CPS < 1 |
| HER2 | score 1+ | score 1+ | score 1+ |
| HR (myChoice®) | HRP (GIS = 4) | HRP (GIS = 1) | HRP (GIS = 2) |
| Author/Year | n | Age | Laterality | Size (cm) | Surgical Treatment | FIGO Stage | Recurrence | Survival | Follow-Up Time (Month) |
|---|---|---|---|---|---|---|---|---|---|
| McFarland/2016 [1]; Mirkovic/2018 [7] | 5 | 42–62 (4); N/A (1) | B (2); L (2); N/A (1) | 4–32 | TH + BSO (2); BSO (1); N/A (2) | IA (1); IC (1); IIB (1); IIIC (1); N/A (1) | Yes (1); No (4) | Alive (5) | 7–37 (4); N/A (1) |
| Pors/2018 [8] | 1 | 67 | N/A | N/A | N/A | IC | N/A | N/A | N/A |
| Chapel/2018 [9] | 1 | 80 | R | 10.6 | TH+BSO+OMT+P | IIIC | No | Alive | 3 |
| McCluggage/2020 [10] | 5 | 50–77 | R (1); L (3); N/A (1) | 6 (1); N/A (4) | TH+BSO+PLND+OMT+P (1); N/A (4) | IIIA (1); NA (4) | N/A | N/A | N/A |
| Dundr/2020 [11] | 1 | 61 | L | 3.5 | TH+BSO+OMT+P+A | IVB | No | Alive | N/A |
| Seay/2020 [12] | 1 | 67 | R | 11 | TH+RSO+PLND+OMT | IA | Yes, abdominopelvic | Alive | 18 |
| Chen/2020 [13] | 1 | 29 | R | 10 | TH+BSO+PLND+PALND+OMT | IC2 | No | Alive | 13 |
| Qazi/2020 [14] | 1 | 51 | N/A | 18 | N/A | N/A | N/A | N/A | N/A |
| Pors/2021 [5] | 25 | 36–81 | N/A | N/A | N/A | I (11); II–IV (7); N/A (7) | Yes (10); No (14); N/A (1) | 5-yr OS 71% (23) | 101 (mean) |
| da Silva/2021 [15] | 15 | 36–76 | B (1); R (2); N/A (12) | 3.5–18.5 (12); N/A (3) | N/A | IA (2); IC (3); IIB (2); IIIA (1); IIIC (2); IV (3); NA (2) | Yes (10: abdominopelvic, 6;distant metastasis, 4) | N/A | N/A |
| Kim/2021 [16] | 1 | 47 | L | 4.4 | PLND+PALND+OMT+P | IIIC | No | Alive | 11 |
| Karpathiou/2021 [17] | 1 | 74 | L | 19 | TH+OMT+P+LND | IIIB | No | Alive | 6 |
| Ujita/2021 [18] | 1 | 84 | L | 7 | TH+BSO+pOMT | IC3 | No | Alive | 4 |
| Deolet/2022 [19] | 4 | 33–75 | R (1); L (2); N/A (1) | 7–15 (3); N/A (1) | LSO (1); TH+BSO+OMT (1); BSO (1); cyctectomy (1) | IA (1); IC (1); IIIC (1); IVB (1) | Yes, abdominopelvic (1); No (3) | Alive (4) | 8–46 |
| Koh/2022 [20] | 5 | 42–61 | R (2); L (3) | 4.7–11.0 | TH+BSO+PLND+PALND+P+OMT (1); TH+BSO+PLND+P+OMT (1); BSO+PLNb+P+OMT (1); TH+BSO+PLND+PALND+Pb+OMT (1); TH+BSO+P (1) | IA (1); IC (3); IIB (1) | Yes, distant metastasis (1); No (3); N/A (1) | Dead (1); Alive (3); N/A (1) | 11–53 (4); N/A (1) |
| Ishida/2022 [21] | 1 | 69 | B | 3.2, 2.0 | TH+BSO | IIB | Yes, lung | N/A | N/A |
| Arslanian/2022 [22] | 2 | 66–67 | R (1); L (1) | 8, 18 | TH+BSO+infracolic omentectomy+rightPALND(1); TH+BSO+PLND+OMT (1) | IC (1); IIIA1 (1) | - | Dead (1); Alive (1) | 15–32 |
| Nilforoushan/2022 [23] | 2 | 55–58 | L (2) | 12, 13 | TH+BSO+OMT (1); TH+LSO+OMT+LND+pelvic staging biopsy (1) | N/A | N/A | N/A | N/A |
| Mirkovic/2023 [24] | 2 | 61–62 | R (2) | 9, 27 | TH+BSO+OMT+LAR (1); BSO+OMT+rectosigmoid and posterior vaginal ressection (1) | IIB (2) | N/A | Alive (2) | 12, 6 |
| Xu/2023 [25] | 1 | 78 | R | 4.3 | TH+BSO+OMT+PLND | IC2 | Yes, pelvic | N/A | 60 |
| Nilforoushan/2023 [26] | 1 | 70 | B | 6.2, 2.9 | TH+BSO+OMT | IVB | N/A | N/A | N/A |
| Stolnicu/2023 [27] | 1 | 63 | L | 12 | TH+BSO | IC | N/A | N/A | N/A |
| Kommoss/2023 [28] | 14 | 50–83 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Zhao/2023 [29] | 1 | 58 | B | 10, 24 | TH+BSO | IC, IIA | N/A | N/A | N/A |
| Chang/2023 [30] | 2 | 51–57 | R (2) | 9.6, 5.7 | TH+BSO+OMT+rt PLND (1); RATH+BSO+PLND (1) | IIIA1, IA1 | N/A | N/A | N/A |
| Linck/2023 [31] | 1 | 65 | L | 3.2 | RATH+BSO+OMT+P | IIB | - | Alive | N/A |
| Nagase/2023 [32] | 1 | 48 | R | 20 | RSO+OMT+P+colostomy (post TH+LSO) | IVB | - | Dead | 15 |
| The present study | 3 | 52–76 | L (1); R (1); B (1) | 13, 15.5, 16.5 | TH+BSO+OMT+PLNb (2); TH+BSO+OMT+PLNb+LAR (1) | IC1 (2); ypIIB (1) | Yes (2: liver, 2; lung, 1), No (1) | Alive (2); Dead (1) | 7–44 |
| Author/Year | n | HRD | MMR | PD-L1 | HER2 | ER | PR | Genetic Analysis | KRAS Mutation | Other Gene Alterations |
|---|---|---|---|---|---|---|---|---|---|---|
| McFarland/2016 [1]; Mirkovic/2018 [7] | 5 | N/A | N/A | N/A | N/A | Neg (4/4) | Neg (4/4) | TS (4); N/A (1) | 4/4 | PIK3CA |
| Pors/2018 [8] | 1 | N/A | N/A | N/A | N/A | Neg | N/A | N/A | N/A | N/A |
| Chapel/2018 [9] | 1 | N/A | N/A | N/A | N/A | Neg | Neg | TS | - | NRAS, BCOR |
| McCluggage/2020 [10] | 5 | N/A | N/A | N/A | N/A | Neg | Neg | TS (1); N/A (4) | 1/1 | - |
| Dundr/2020 [11] | 1 | N/A | N/A | N/A | N/A | Neg | Neg | TS | 1/1 | PIK3CA, CHEK2 |
| Seay/2020 [12] | 1 | N/A | N/A | score 0 | N/A | Neg | Neg | TS | - | - |
| Chen/2020 [13] | 1 | N/A | N/A | N/A | N/A | Neg | Neg | N/A | N/A | N/A |
| Qazi/2020 [14] | 1 | N/A | N/A | N/A | N/A | Neg (1/2); N/A (1/2) | Neg (1/2); N/A (1/2) | N/A | N/A | N/A |
| Pors/2021 [5] | 25 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| da Silva/2021 [15] | 15 | N/A | Intact (8); N/A (7) | N/A | N/A | Focal (2/15); Neg (13/15) | Neg (12/15); N/A (3/15) | TS | 13/15 | PIK3CA, SPOP, NRAS, SETD8, CTNNB1, CREBBP, NOTCH3, ARID1A, FBXW7, FANCA, AKT1, ASXL1, RAD54L |
| Kim/2021 [16] | 1 | N/A | Intact | N/A | N/A | Neg | Neg | TS | 1/1 | - |
| Karpathiou/2021 [17] | 1 | HRP | Intact | N/A | N/A | Neg | Neg | TS | 1/1 | CTNNB1 |
| Ujita/2021 [18] | 1 | N/A | N/A | N/A | N/A | Neg | Neg | N/A | N/A | N/A |
| Deolet/2022 [19] | 4 | N/A | N/A | N/A | N/A | Neg | Neg (3/4) N/A (1/4) | TS | 3/4 | PIK3CA, PTEN amplification, 12p isochromosome |
| Koh/2022 [20] | 5 | N/A | Intact (4); N/A (1) | N/A | N/A | Focal (3/5); Neg (2/5) | Focal (1/5); Neg (4/5) | TS (4); N/A (1) | 4/4 | - |
| Ishida/2022 [21] | 1 | N/A | N/A | N/A | N/A | Neg | Neg | TS | 1/1 | SPOP, FANCA |
| Arslanian/2022 [22] | 2 | N/A | Intact (2) | N/A | N/A | Focal (1/2); Neg (1/2) | Neg (1/2); N/A (1/2) | TS | 2/2 | PIK3CA |
| Nilforoushan/2022 [23] | 2 | N/A | N/A | N/A | N/A | Neg (2/2) | Neg (2/2) | TS (1); N/A (1) | 1/2; N/A (1) | CTNNB1, FGFR2 amplification, CDKN2A/ p16 deletion |
| Mirkovic/2023 [24] | 2 | N/A | Intact | N/A | N/A | Neg (2/2) | Neg (1/2); N/A (1/2) | TS | 2/2 | FANCA(1/2), CREBBP(2/2), POLE(1/2), PTEN(1/2) |
| Xu/2023 [25] | 1 | N/A | Intact | N/A | N/A | Neg | N/A | TS | - | FGFR2, CTNNB1 |
| Nilforoushan/2023 [26] | 1 | N/A | Intact | Neg | N/A | Neg | Neg | TS | 1/1 | NOTCH1 |
| Stolnicu/2023 [27] | 1 | N/A | N/A | N/A | N/A | Neg | Neg | TS | 1/1 | RRR2R1A, ARHGAP35, IRS1 |
| Kommoss/2023 [28] | 14 | N/A | Intact (14) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Zhao/2023 [29] | 1 | N/A | Intact | N/A | N/A | Neg | Neg | N/A | N/A | N/A |
| Chang/2023 [30] | 2 | N/A | N/A | N/A | N/A | Neg | Neg (1/2); N/A (1/2) | TS (1); N/A (1) | 1/1 | TP53, PPP2R1A, SPEN |
| Linck/2023 [31] | 1 | N/A | N/A | N/A | N/A | Neg | Neg | N/A | N/A | N/A |
| Nagase/2023 [32] | 1 | N/A | N/A | N/A | N/A | Neg | Neg | TS | 1/1 | PIK3CA, FBXW7, RAD21 |
| The present case | 3 | HRP (3) | Intact (3) | CPS < 1 (3) | Score 1+ (3) | Rare (1/3); Neg (2/3) | Neg (3/3) | N/A | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, A.; Yoshida, H.; Kawano, S.; Kikkawa, N.; Kobayashi-Kato, M.; Tanase, Y.; Uno, M.; Ishikawa, M. Ovarian Mesonephric-like Adenocarcinoma: Its Prevalence in a Japanese High-Volume Cancer Center and a Literature Review on Therapeutic Targets. Curr. Oncol. 2024, 31, 5107-5120. https://doi.org/10.3390/curroncol31090378
Ogawa A, Yoshida H, Kawano S, Kikkawa N, Kobayashi-Kato M, Tanase Y, Uno M, Ishikawa M. Ovarian Mesonephric-like Adenocarcinoma: Its Prevalence in a Japanese High-Volume Cancer Center and a Literature Review on Therapeutic Targets. Current Oncology. 2024; 31(9):5107-5120. https://doi.org/10.3390/curroncol31090378
Chicago/Turabian StyleOgawa, Ayako, Hiroshi Yoshida, Saria Kawano, Nao Kikkawa, Mayumi Kobayashi-Kato, Yasuhito Tanase, Masaya Uno, and Mitsuya Ishikawa. 2024. "Ovarian Mesonephric-like Adenocarcinoma: Its Prevalence in a Japanese High-Volume Cancer Center and a Literature Review on Therapeutic Targets" Current Oncology 31, no. 9: 5107-5120. https://doi.org/10.3390/curroncol31090378
APA StyleOgawa, A., Yoshida, H., Kawano, S., Kikkawa, N., Kobayashi-Kato, M., Tanase, Y., Uno, M., & Ishikawa, M. (2024). Ovarian Mesonephric-like Adenocarcinoma: Its Prevalence in a Japanese High-Volume Cancer Center and a Literature Review on Therapeutic Targets. Current Oncology, 31(9), 5107-5120. https://doi.org/10.3390/curroncol31090378





