Feasibility of a Novel 3D Ultrasound Imaging Technique for Intraoperative Margin Assessment during Tongue Cancer Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Ex Vivo 3D Ultrasound Imaging
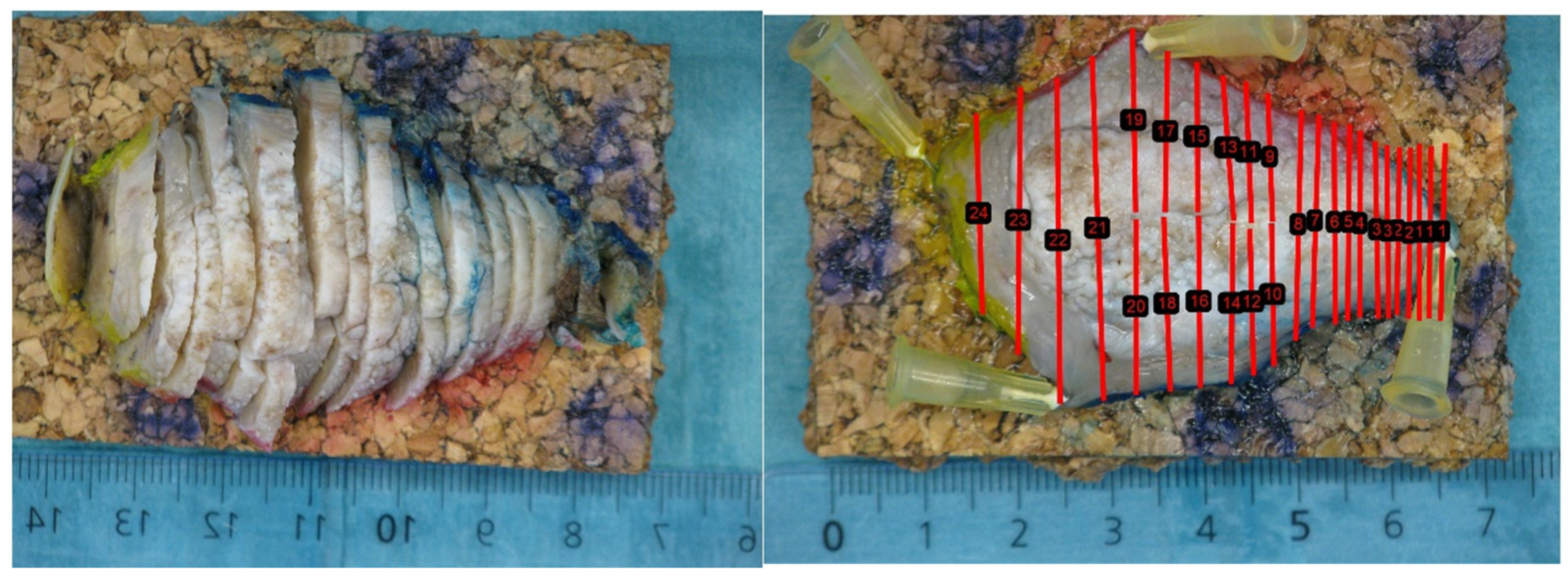
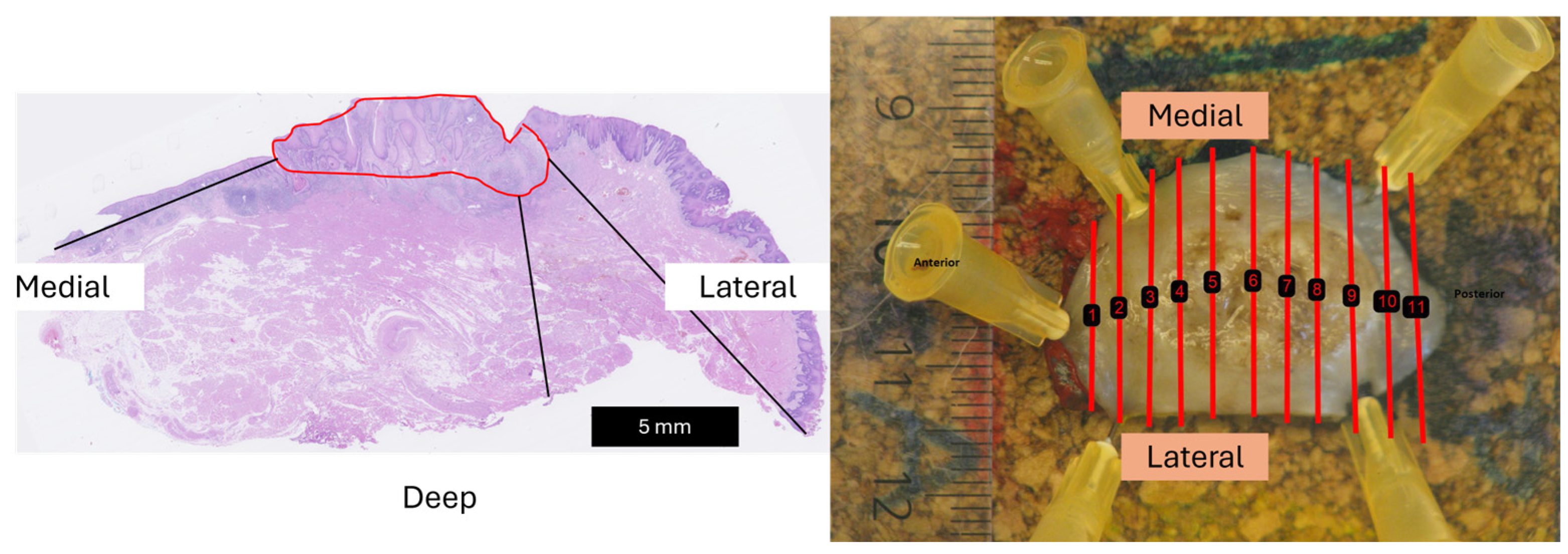
2.2. Three-Dimensional Ultrasound to Histopathology
2.3. Statistical Analysis
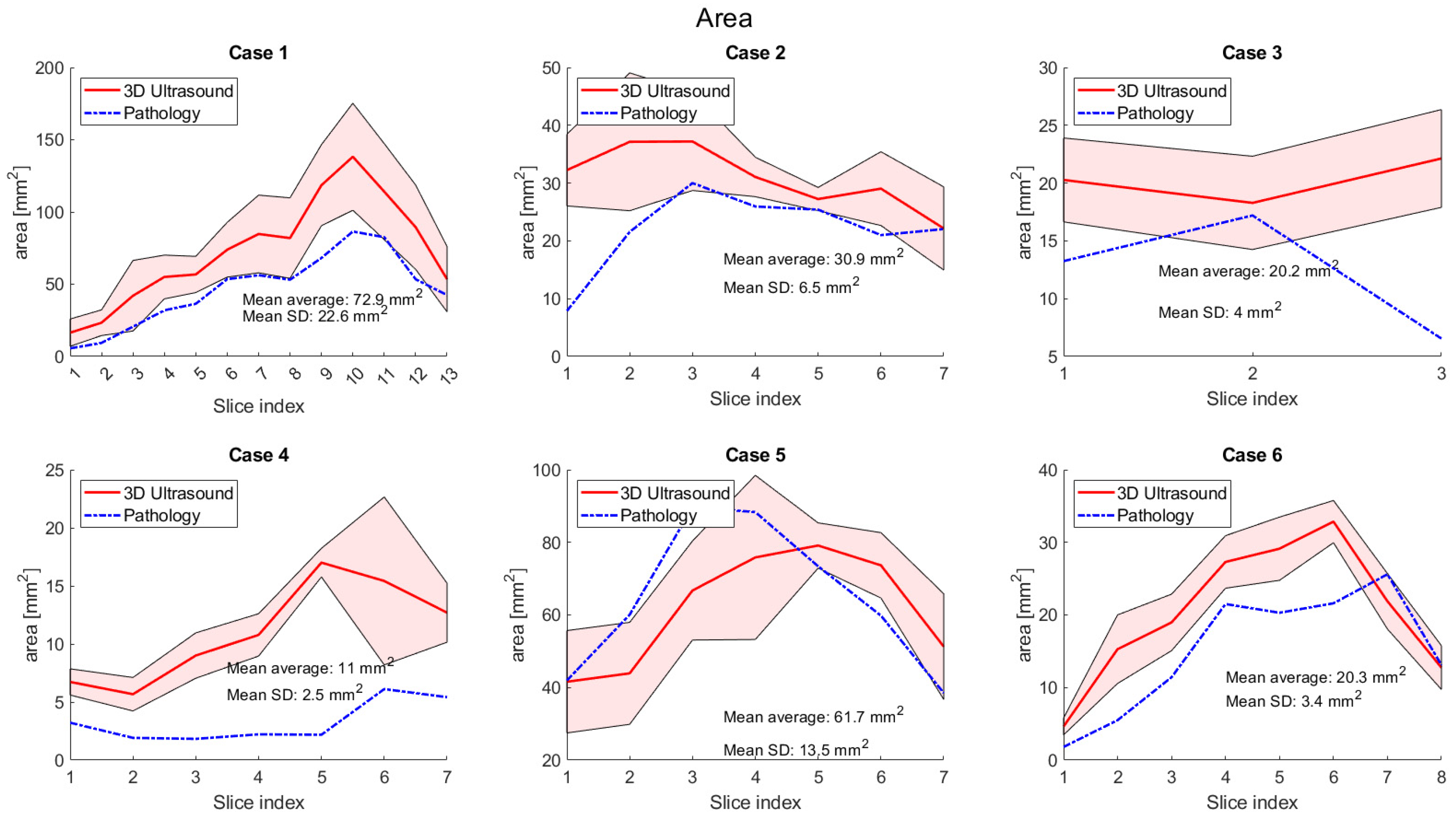
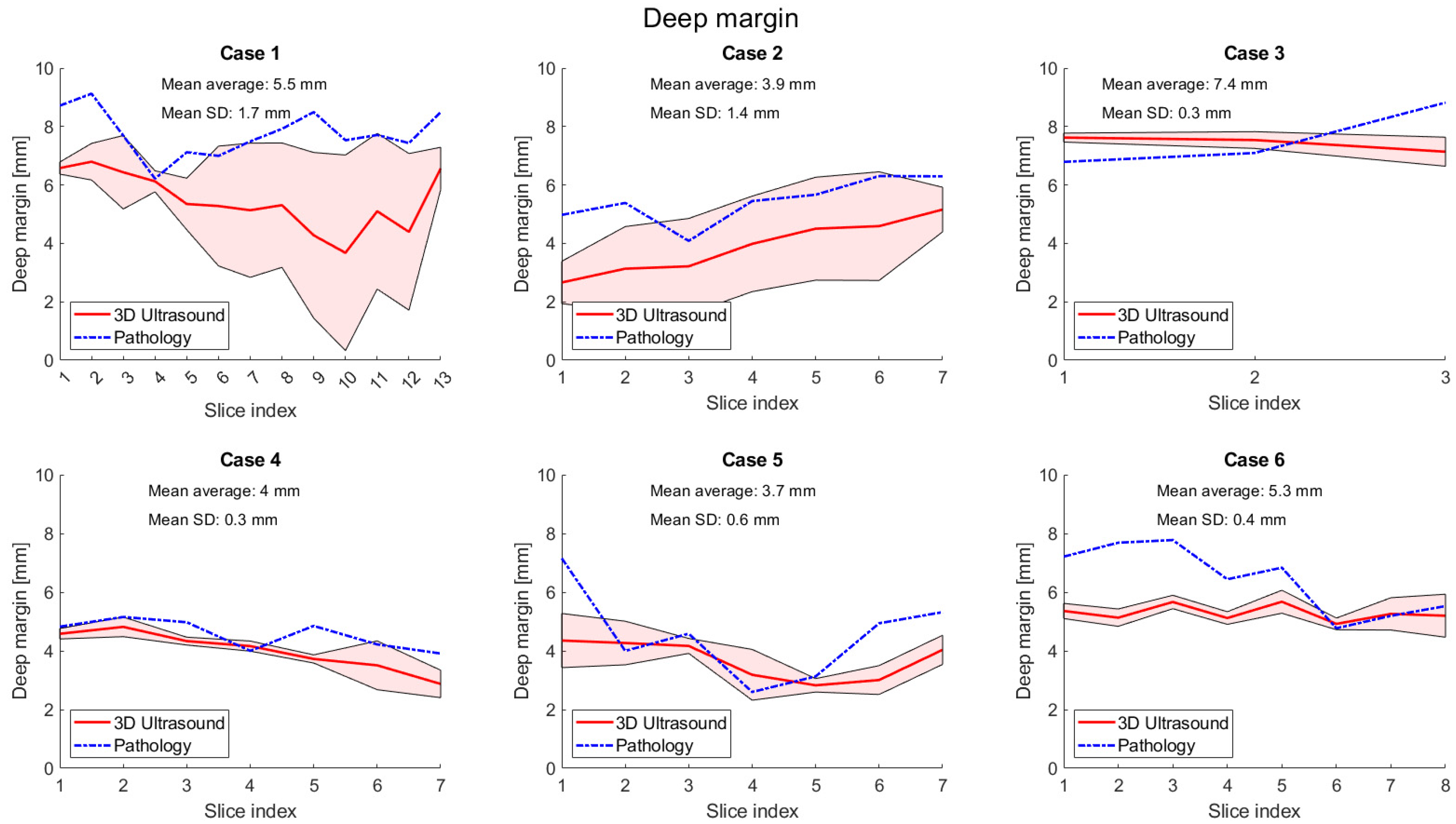
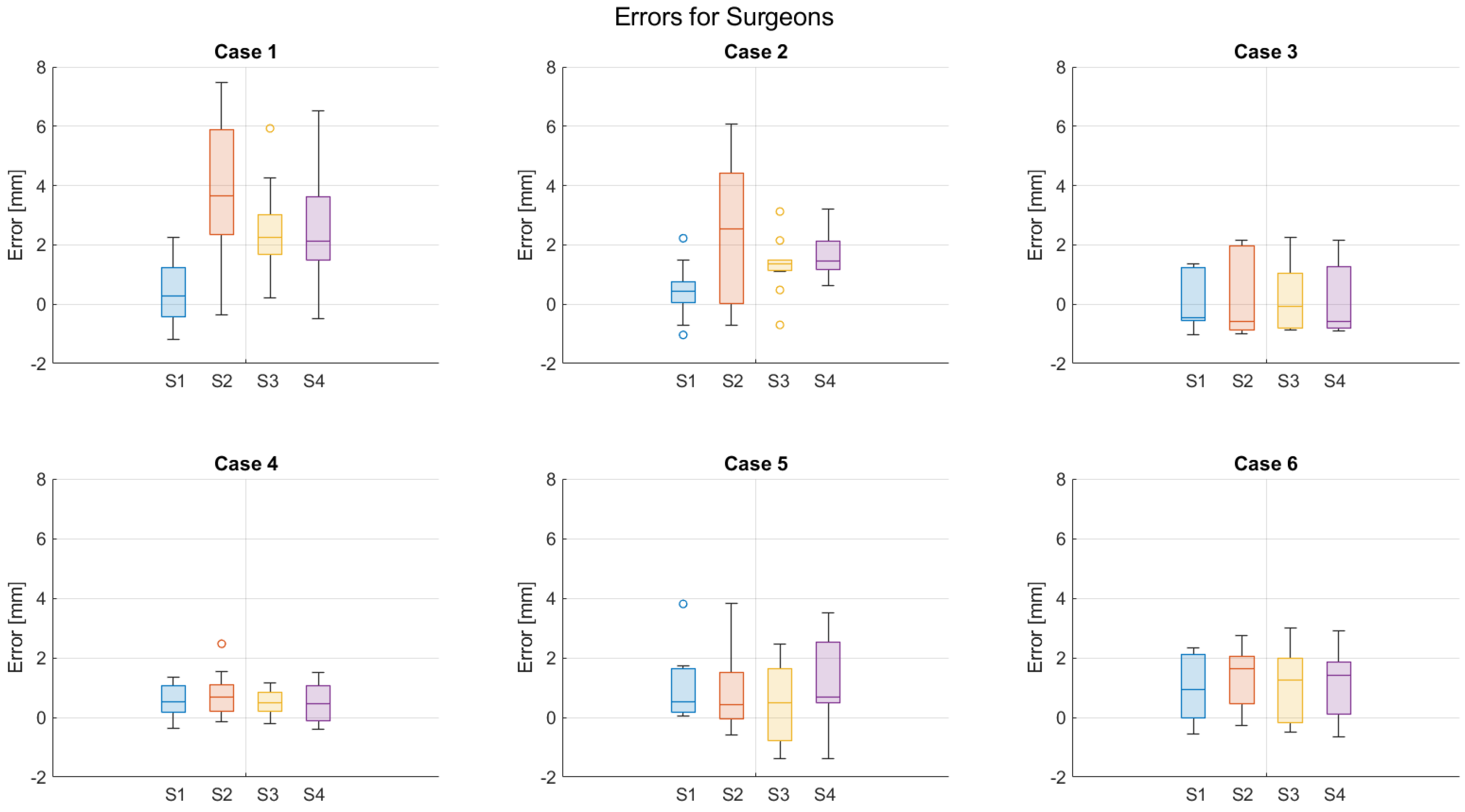
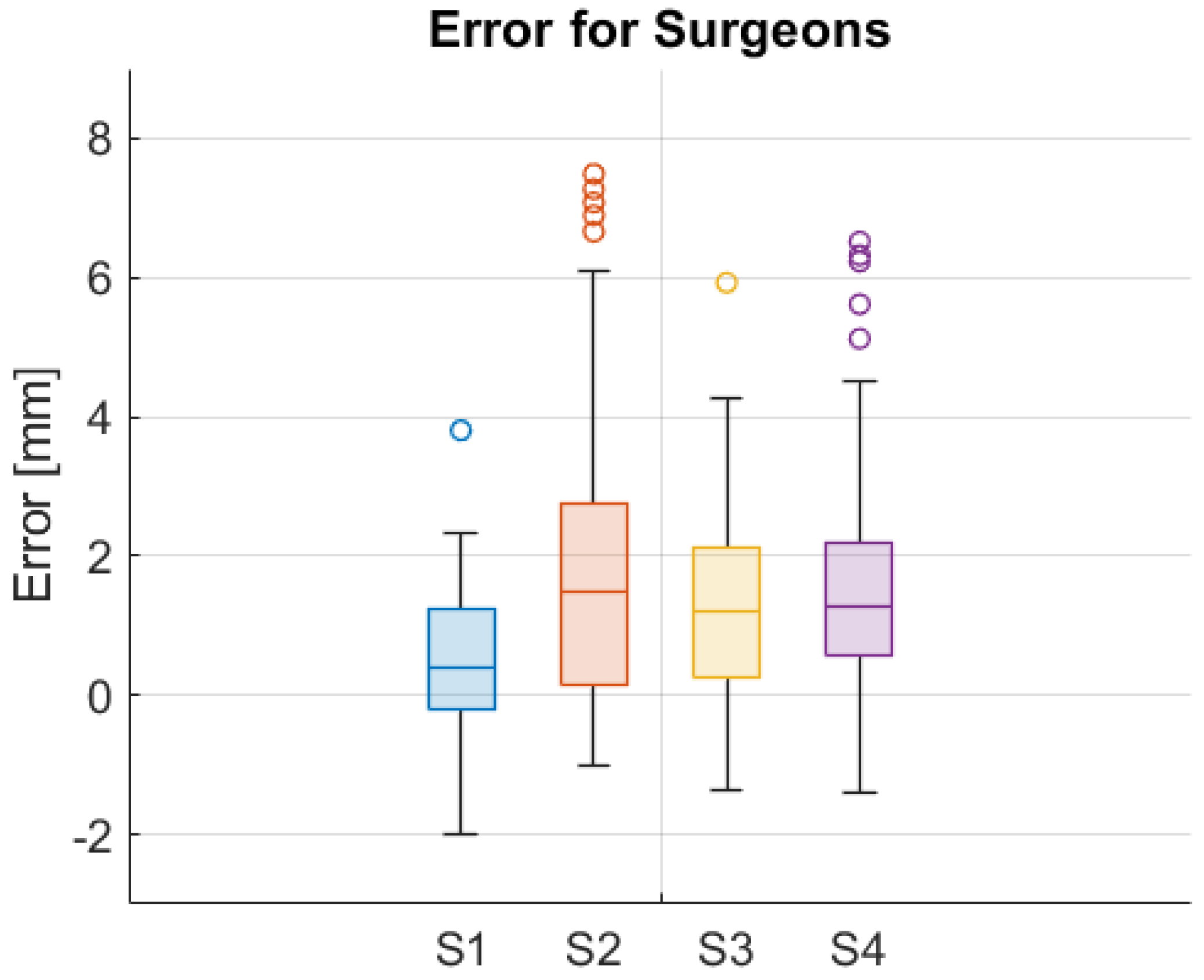
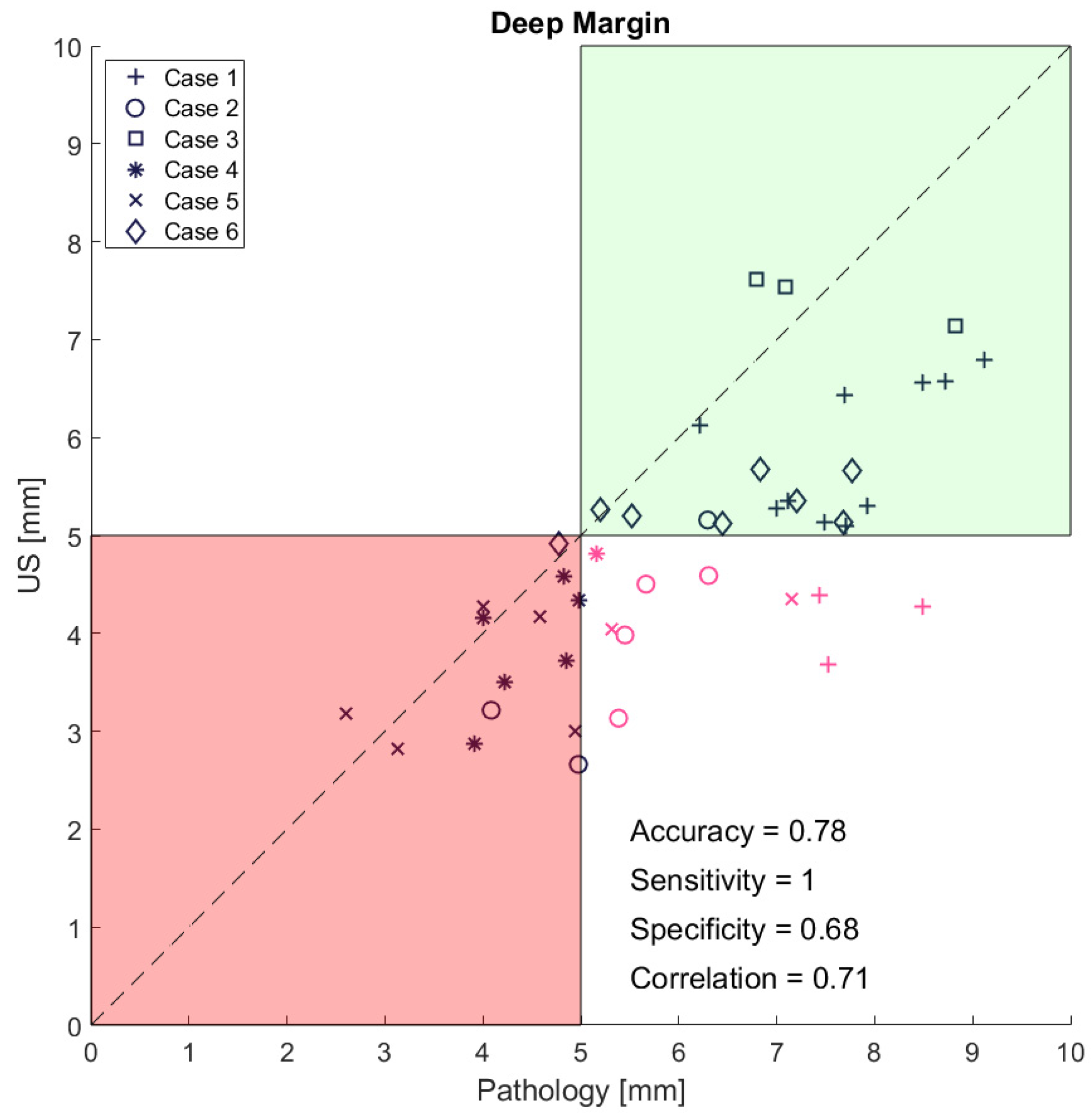
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
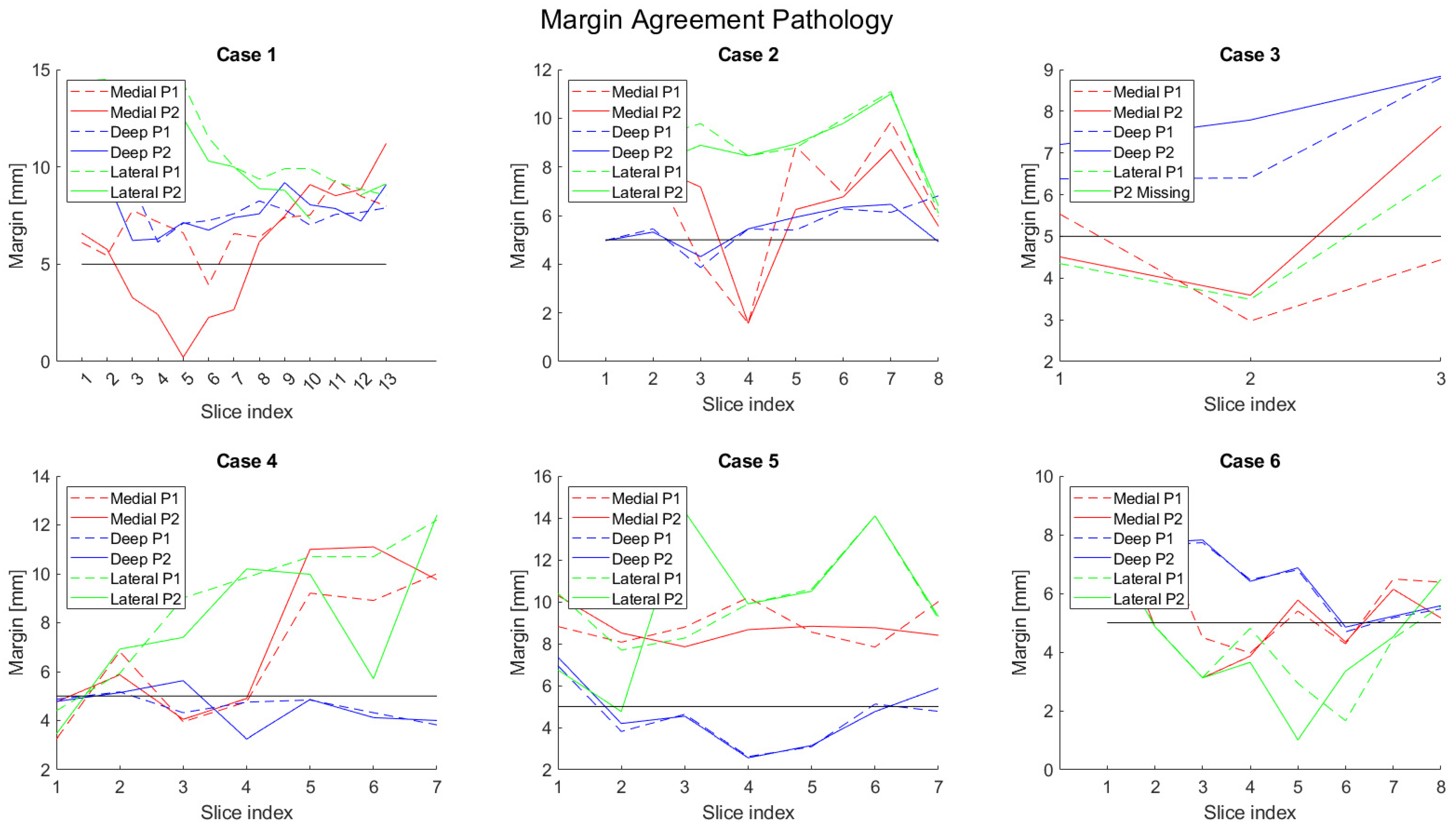
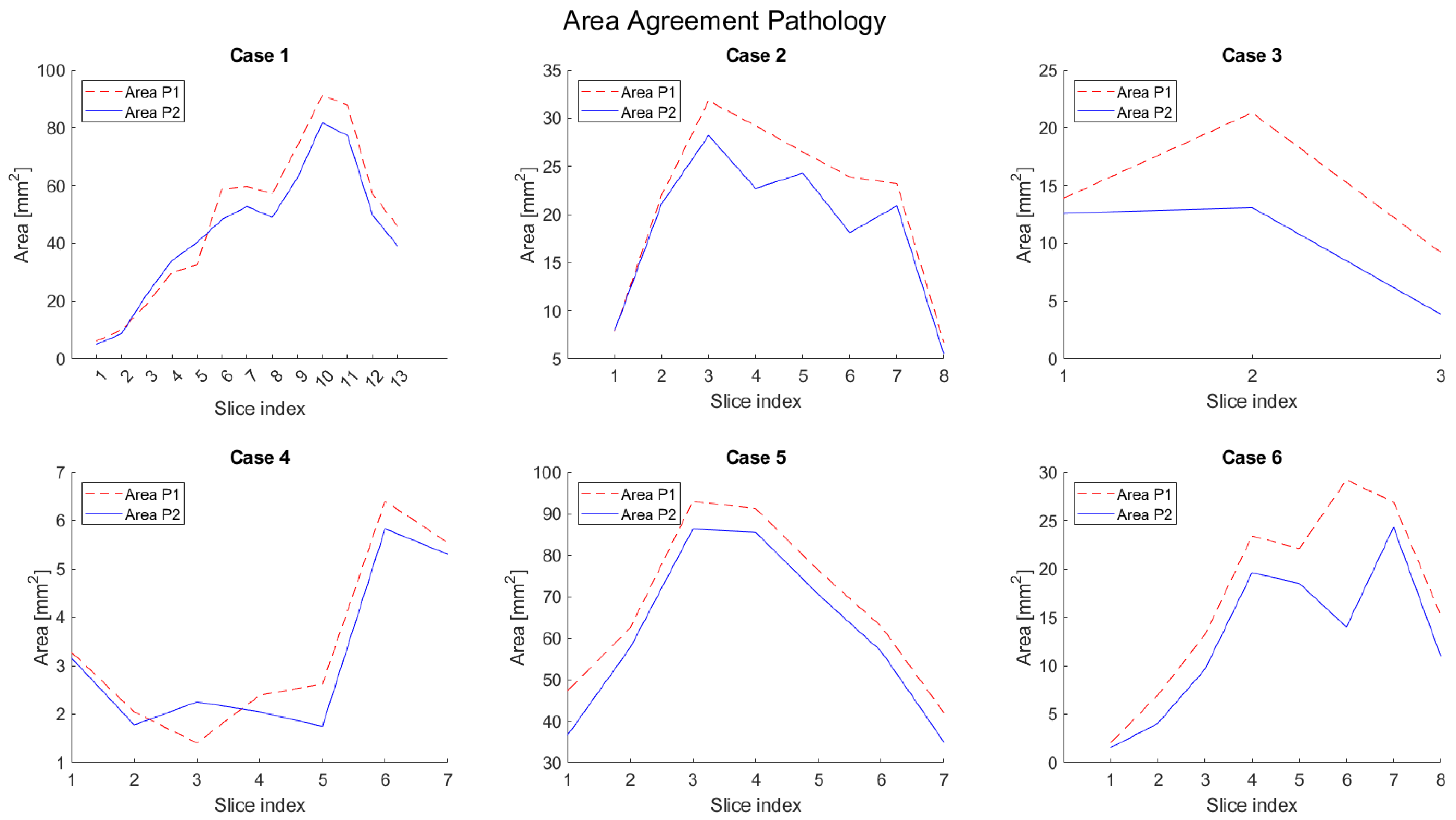
| Patient No. | Minimum Medial [mm] | Minimum Lateral [mm] | ||||||
|---|---|---|---|---|---|---|---|---|
| 3D US | HP | Error | Pearson (pval) | 3D US | HP | Error | Pearson (pval) | |
| 1 | 6.6 | 8.6 | 2.0 | −0.1 (<0.01) | 6.3 | 3.1 | −3.2 | 0.7 (<0.001) |
| 2 | 4.9 | 6.2 | 1.4 | 0.9 (<0.001) | 6.4 | 1.6 | −4.8 | 0.5 (<0.01) |
| 3 | 5.1 | 3.5 | −1.6 | 0.1 (ns) | 3.8 | 3.3 | −0.5 | −0.5 (ns) |
| 4 | 4.9 | 3.9 | −1.0 | 0.9 (ns) | 5.8 | 4.0 | −1.8 | 0.9 (ns) |
| 5 | 6.3 | 6.1 | −0.1 | −0.6 (<0.05) | 11.7 | 8.3 | −3.4 | 0.1 (<0.001) |
| 6 | 6.9 | 2.0 | −5.0 | 0.6 (<0.001) | 4.0 | 3.8 | −0.2 | 0.6 (ns) |
| Mean Absolute Difference [mm] | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Average over Cases |
|---|---|---|---|---|---|---|---|
| Medial | 2.2 | 1.0 | 1.6 | 1.0 | 1.0 | 0.9 | 1.3 |
| Deep | 0.7 | 0.4 | 0.7 | 0.5 | 0.4 | 0.1 | 0.5 |
| Lateral | 0.9 | 0.3 | 0.2 | 1.4 | 1.8 | 0.7 | 0.9 |
| Area | 6.8 | 2.8 | 5.0 | 0.5 | 6.7 | 4.5 | 4.4 |
| Case | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Deep | 0.3 | 0.3 | 0.1 | 0.1 | 0.2 | 0.2 |
| Medial | 0.3 | 0.4 | 0.2 | 0.2 | 0.4 | 0.2 |
| Lateral | 0.2 | 0.3 | 0.2 | 0.1 | 0.3 | 0.5 |
| Area | 0.3 | 0.4 | 0.6 | 1.3 | 0.2 | 0.3 |


References
- Ng, J.H.; Iyer, N.G.; Tan, M.-H.; Edgren, G. Changing epidemiology of oral squamous cell carcinoma of the tongue: A global study. Head Neck 2017, 39, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Chinn, S.B.; Myers, J.N. Oral Cavity Carcinoma: Current Management, Controversies, and Future Directions. J. Clin. Oncol. 2015, 33, 3269–3276. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, O.; Knutsson, J.; Landström, F.J.; Magnuson, A.; Von Beckerath, M. Ultrasound accurately assesses depth of invasion in T1-T2 oral tongue cancer. Laryngoscope Investig. Otolaryngol. 2022, 7, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.K. Postoperative pathologic assessment of surgical margins in oral cancer: A contemporary review. J. Oral Maxillofac. Pathol. 2018, 22, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Smits, R.W.H.; Koljenović, S.; Hardillo, J.A.; Ten Hove, I.; Meeuwis, C.A.; Sewnaik, A.; Dronkers, E.A.C.; Bakker Schut, T.C.; Langeveld, T.P.M.; Molenaar, J.; et al. Resection margins in oral cancer surgery: Room for improvement. Head Neck 2016, 38, E2197–E2203. [Google Scholar] [CrossRef]
- Bulbul, M.G.; Tarabichi, O.; Sethi, R.K.; Parikh, A.S.; Varvares, M.A. Does Clearance of Positive Margins Improve Local Control in Oral Cavity Cancer? A Meta-analysis. Otolaryngol.-Head Neck Surg. 2019, 161, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Dinardo, L.J.; Lin, J.; Karageorge, L.S.; Powers, C.N. Accuracy, Utility, and Cost of Frozen Section Margins in Head and Neck Cancer Surgery. Laryngoscope 2000, 110, 1773–1776. [Google Scholar] [CrossRef] [PubMed]
- De Koning, K.J.; Koppes, S.A.; De Bree, R.; Dankbaar, J.W.; Willems, S.M.; Van Es, R.J.J.; Noorlag, R. Feasibility study of ultrasound-guided resection of tongue cancer with immediate specimen examination to improve margin control—Comparison with conventional treatment. Oral Oncol. 2021, 116, 105249. [Google Scholar] [CrossRef] [PubMed]
- Heidkamp, J.; Scholte, M.; Rosman, C.; Manohar, S.; Fütterer, J.J.; Rovers, M.M. Novel imaging techniques for intraoperative margin assessment in surgical oncology: A systematic review. Int. J. Cancer 2021, 149, 635–645. [Google Scholar] [CrossRef] [PubMed]
- de Koning, K.J.; van Es, R.J.J.; Klijn, R.J.; Breimer, G.E.; Willem Dankbaar, J.; Braunius, W.W.; van Cann, E.M.; Dieleman, F.J.; Rijken, J.A.; Tijink, B.M.; et al. Application and accuracy of ultrasound-guided resections of tongue cancer. Oral Oncol. 2022, 133, 106023. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Krishnan, G.; Nishio, N.; Berg, N.S.; Lu, G.; Martin, B.A.; Keulen, S.; Colevas, A.D.; Kapoor, S.; Liu, J.T.C.; et al. Intraoperative Fluorescence-Guided Surgery in Head and Neck Squamous Cell Carcinoma. Laryngoscope 2021, 131, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Steinkamp, P.J.; Voskuil, F.J.; Van Der Vegt, B.; Doff, J.J.; Schepman, K.-P.; De Visscher, S.A.H.J.; Kelder, W.; Jayalakshmi, Y.; Gao, J.; Sumer, B.D.; et al. A Standardized Framework for Fluorescence-Guided Margin Assessment for Head and Neck Cancer Using a Tumor Acidosis Sensitive Optical Imaging Agent. Mol. Imaging Biol. 2021, 23, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Woolgar, J.A.; Triantafyllou, A. A histopathological appraisal of surgical margins in oral and oropharyngeal cancer resection specimens. Oral Oncol. 2005, 41, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Noorlag, R.; de Bree, R.; Witjes, M.J.H. Image-guided surgery in oral cancer: Toward improved margin control. Curr. Opin. Oncol. 2022, 34, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Heidkamp, J.; Weijs, W.L.J.; Engen-Van Grunsven, A.C.H.; Laak-De Vries, I.; Maas, M.C.; Rovers, M.M.; Fütterer, J.J.; Steens, S.C.A.; Takes, R.P. Assessment of surgical tumor-free resection margins in fresh squamous-cell carcinoma resection specimens of the tongue using a clinical MRI system. Head Neck 2020, 42, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Steens, S.C.; Bekers, E.M.; Weijs, W.L.; Litjens, G.J.; Veltien, A.; Maat, A.; van den Broek, G.B.; van der Laak, J.A.; Fütterer, J.J.; van der Kaa, C.A.H. Evaluation of tongue squamous cell carcinoma resection margins using ex-vivo MR. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Kahng, P.W.; Wu, X.; Ramesh, N.P.; Pastel, D.A.; Halter, R.J.; Paydarfar, J.A. Improving target localization during trans-oral surgery with use of intraoperative imaging. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Klein Nulent, T.J.W.; Noorlag, R.; Van Cann, E.M.; Pameijer, F.A.; Willems, S.M.; Yesuratnam, A.; Rosenberg, A.; de Bree, R.; van Es, R.J.J. Intraoral ultrasonography to measure tumor thickness of oral cancer: A systematic review and meta-analysis. Oral Oncol. 2018, 77, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kaltoft, M.; Hahn, C.H.; Wessman, M.; Hansen, M.L.; Agander, T.K.; Makouei, F.; Wessel, I.; Todsen, T. Intraoral Ultrasound versus MRI for Depth of Invasion Measurement in Oral Tongue Squamous Cell Carcinoma: A Prospective Diagnostic Accuracy Study. Cancers 2024, 16, 637. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Kim, J.H.; Choi, S.H.; Yun, T.J.; Wi, J.Y.; Kim, S.A.; Sun, H.Y.; Ryoo, I.; Park, S.W.; Sohn, C.H. Off-site evaluation of three-dimensional ultrasound for the diagnosis of thyroid nodules: Comparison with two-dimensional ultrasound. Eur. Radiol. 2016, 26, 3353–3360. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.; Carreira, J.; Thompson, R.; Morais, A.; Miller, C.; Wein, W.; Ghosh, J.; McCollum, C. An Ex Vivo Evaluation of Tomographic 3-D Ultrasound, B-Mode Ultrasound, CT And MR Imaging to Measure Artery Diameter, Length and Wall Volume. Ultrasound Med. Biol. 2019, 45, 2819–2829. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.; Bandarkar, A.; Rana, M.S.; Tabrizi, P.R.; Preciado, D.; Jago, J.; Linguraru, M.G.; Reilly, B.K. Pilot study of the potential of 3D ultrasound to measure tonsillar volume and hypertrophy. Int. J. Pediatr. Otorhinolaryngol. 2019, 126, 109612. [Google Scholar] [CrossRef] [PubMed]
- Makouei, F.; Ewertsen, C.; Agander, T.K.; Olesen, M.V.; Pakkenberg, B.; Todsen, T. 3D Ultrasound versus Computed Tomography for Tumor Volume Measurement Compared to Gross Pathology—A Pilot Study on an Animal Model. J. Imaging 2022, 8, 329. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Prior, S.J.; Kampmann, M.; Sorkin, J.D.; Caldwell, K.; Braganza, M.; McEvoy, S.; Lal, B.K. Measurement of thrombus resolution using three-dimensional ultrasound assessment of deep vein thrombosis volume. J. Vasc. Surg. Venous Lymphat. Disord. 2014, 2, 140–147. [Google Scholar] [CrossRef]
- Bekedam, N.; Smit, J.; de Koekkoek-Doll, P.; van Alphen, M.; van Veen, R.; Karssemakers, L.; Karakullukcu, M.; Smeele, L. Intra-operative resection margin model of tongue carcinoma using 3D reconstructed ultrasound. Adv. Oral Maxillofac. Surg. 2021, 4, 100154. [Google Scholar] [CrossRef]
- Ying, M.; Pang, S.F.; Sin, M.H. Reliability of 3-D ultrasound measurements of cervical lymph node volume. Ultrasound Med. Biol. 2006, 32, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Bekedam, N.M.; Idzerda, L.H.W.; van Alphen, M.J.A.; van Veen, R.L.P.; Karssemakers, L.H.E.; Karakullukcu, M.B.; Smeele, L.E. Implementing deep learning model for automatic tongue tumour segmentation in ex-vivo 3D ultrasound volumes. Br. J. Oral Maxillofac. Surg. 2024, 62, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Cenni, F.; Monari, D.; Desloovere, K.; Aertbeliën, E.; Schless, S.-H.; Bruyninckx, H. The reliability and validity of a clinical 3D freehand ultrasound system. Comput. Methods Programs Biomed. 2016, 136, 179–187. [Google Scholar] [CrossRef]
- Makouei, F.; Agander, T.K.; Ewertsen, C.; Søndergaard Svendsen, M.B.; Norling, R.; Kaltoft, M.; Hansen, A.E.; Rasmussen, J.H.; Wessel, I.; Todsen, T. 3D Ultrasound and MRI in Assessing Resection Margins during Tongue Cancer Surgery: A Research Protocol for a Clinical Diagnostic Accuracy Study. J. Imaging 2023, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Orchard, G.E.; Shams, M.; Nwokie, T.; Bulut, C.; D’Amico, C.; Gabriel, J.; Ramji, Z.; Georgaki, A.; Neichcial, A.; Shams, F.; et al. Development of new and accurate measurement devices (TruSlice and TruSlice Digital) for use in histological dissection: An attempt to improve specimen dissection precision. Br. J. Biomed Sci. 2015, 72, 140–145. [Google Scholar] [CrossRef] [PubMed]
- El-Fol, H.A.; Noman, S.A.; Beheiri, M.G.; Khalil, A.M.; Kamel, M.M. Significance of post-resection tissue shrinkage on surgical margins of oral squamous cell carcinoma. J. Craniomaxillofac. Surg. 2015, 43, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Limongelli, L.; Capodiferro, S.; Tempesta, A.; Sportelli, P.; Dell’Olio, F.; Angelelli, G.; Maiorano, E.; Favia, G. Early tongue carcinomas (clinical stage I and II): Echo-guided three-dimensional diode laser mini-invasive surgery with evaluation of histological prognostic parameters. A study of 85 cases with prolonged follow-up. Lasers Med. Sci. 2020, 35, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Adriaansens, C.M.E.M.; De Koning, K.J.; De Bree, R.; Dankbaar, J.W.; Breimer, G.E.; Van Es, R.J.J.; Noorlag, R. Ultrasound-guided resection for squamous cell carcinoma of the buccal mucosa: A feasibility study. Head Neck 2022, 45, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Tarabichi, O.; Kanumuri, V.; Juliano, A.F.; Faquin, W.C.; Cunnane, M.E.; Varvares, M.A. Intraoperative Ultrasound in Oral Tongue Cancer Resection: Feasibility Study and Early Outcomes. Otolaryngol. Head Neck Surg. 2018, 158, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, L.; Kujan, O.; Farah, C.S. Optical fluorescence imaging in oral cancer and potentially malignant disorders: A systematic review. Oral Dis. 2020, 26, 491–510. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.S.; McIntosh, L.; Georgiou, A.; McCullough, M.J. Efficacy of tissue autofluorescence imaging (velscope) in the visualization of oral mucosal lesions. Head Neck 2012, 34, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.S.; Dalley, A.J.; Nguyen, P.; Batstone, M.; Kordbacheh, F.; Perry-Keene, J.; Fielding, D. Improved surgical margin definition by narrow band imaging for resection of oral squamous cell carcinoma: A prospective gene expression profiling study. Head Neck 2016, 38, 832–839. [Google Scholar] [CrossRef] [PubMed]

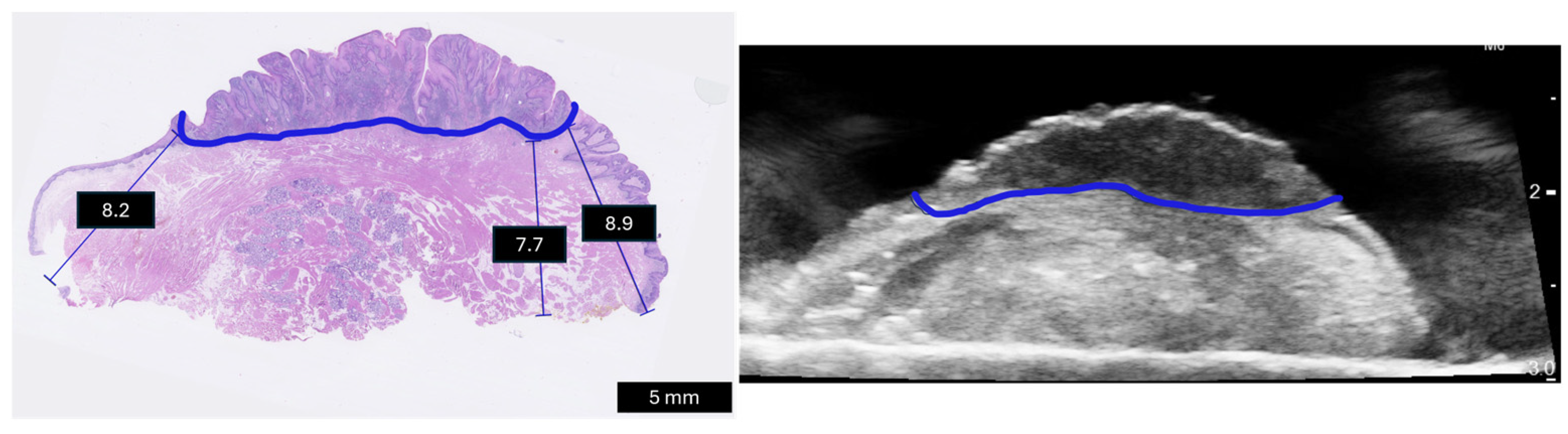
| Patient No. | Gender | Age | cTNM or pTNM | No. Pathology Slices | Invasion Pattern | Perineural Invasion | Pathology Margin Status | Ultrasound Machine |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 64 | T2N0M0 | 13 | Non-cohesive | Yes | Free | BK5000 (BK Ultrasound, Burlington, MA, USA) |
| 2 | Female | 73 | T2N0M0 | 7 | Non-cohesive | Yes | Close | Arietta 850 (Fujifilm, Ratingen, Germany) |
| 3 | Male | 81 | T1N0M0 | 3 | Non-cohesive | No | Free | Arietta 850 (Fujifilm, Ratingen, Germany) |
| 4 | Female | 68 | T1N0M0 | 7 | Cohesive | No | Close | Arietta 850 (Fujifilm, Ratingen, Germany) |
| 5 | Male | 50 | T2N1M0 | 7 | Non-cohesive | Yes | Close | Arietta 850 (Fujifilm, Ratingen, Germany) |
| 6 | Male | 56 | T1N0M0 | 8 | Non-cohesive | No | Close | Arietta 850 (Fujifilm, Ratingen, Germany) |
| Patient No. | Pathologist Minimum Deep [mm] | 3D Ultrasound Minimum Deep [mm] | Margin Status | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | Difference | Mean | A | B | C | D | Mean | Mean Abs Error | 3D US | HP | Diagnosis | |
| 1 | 6.1 | 6.2 | 0.1 | 0.7 | 5.9 | 0.4 | 3.8 | 1.8 | 3.0 | 2.3 | Close | Free | FP |
| 2 | 3.9 | 4.3 | 0.5 | 0.4 | 3.1 | 1.9 | 2.7 | 2.4 | 2.5 | 2.2 | Close | Close | TP |
| 3 | 6.4 | 7.2 | 0.8 | 0.8 | 7.5 | 6.7 | 7.2 | 7.1 | 7.1 | 2.4 | Free | Free | TN |
| 4 | 3.8 | 3.3 | 0.6 | 0.5 | 2.8 | 2.6 | 3.2 | 2.6 | 2.8 | 2.0 | Close | Close | TP |
| 5 | 2.6 | 2.6 | 0.1 | 0.4 | 2.7 | 2.8 | 3.1 | 2.3 | 2.6 | 2.1 | Close | Close | TP |
| 6 | 4.7 | 4.9 | 0.2 | 0.1 | 5.1 | 4.4 | 4.9 | 4.8 | 4.8 | 0.2 | Close | Close | TP |
| Overall | 4.6 | 4.8 | 0.4 | 0.5 | 4.5 | 3.1 | 4.2 | 3.5 | 3.8 | 1.9 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makouei, F.; Frehr, T.D.; Agander, T.K.; Lelkaitis, G.; Hyldig Dal, M.; Kaltoft, M.; Orloff, L.; Sebelik, M.; Søndergaard Svendsen, M.B.; Wessel, I.; et al. Feasibility of a Novel 3D Ultrasound Imaging Technique for Intraoperative Margin Assessment during Tongue Cancer Surgery. Curr. Oncol. 2024, 31, 4414-4431. https://doi.org/10.3390/curroncol31080330
Makouei F, Frehr TD, Agander TK, Lelkaitis G, Hyldig Dal M, Kaltoft M, Orloff L, Sebelik M, Søndergaard Svendsen MB, Wessel I, et al. Feasibility of a Novel 3D Ultrasound Imaging Technique for Intraoperative Margin Assessment during Tongue Cancer Surgery. Current Oncology. 2024; 31(8):4414-4431. https://doi.org/10.3390/curroncol31080330
Chicago/Turabian StyleMakouei, Fatemeh, Theresa Dahl Frehr, Tina Klitmøller Agander, Giedrius Lelkaitis, Mette Hyldig Dal, Mikkel Kaltoft, Lisa Orloff, Merry Sebelik, Morten Bo Søndergaard Svendsen, Irene Wessel, and et al. 2024. "Feasibility of a Novel 3D Ultrasound Imaging Technique for Intraoperative Margin Assessment during Tongue Cancer Surgery" Current Oncology 31, no. 8: 4414-4431. https://doi.org/10.3390/curroncol31080330
APA StyleMakouei, F., Frehr, T. D., Agander, T. K., Lelkaitis, G., Hyldig Dal, M., Kaltoft, M., Orloff, L., Sebelik, M., Søndergaard Svendsen, M. B., Wessel, I., & Todsen, T. (2024). Feasibility of a Novel 3D Ultrasound Imaging Technique for Intraoperative Margin Assessment during Tongue Cancer Surgery. Current Oncology, 31(8), 4414-4431. https://doi.org/10.3390/curroncol31080330








