Phase-Based and Lifetime Health System Costs of Care for Patients Diagnosed with Leukemia and Lymphoma: A Population-Based Descriptive Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Data Sources
- Inpatient costs: Includes hospitalization-related costs.
- Outpatient costs: Includes general outpatient clinic costs.
- Physician services cost: Includes physician fees (including outpatient visits), shadow billings, and capitation payments.
- Prescription drug costs: Includes the costs of drugs covered under the ODB programme. ODB covers prescription drugs for patients meeting certain criteria including, age (65+ years), income, and residency status.
- Specialized cancer drug costs: Includes the costs of drugs that are covered under NDFP and includes intravenous systemic chemotherapy drugs.
- Emergency department, same-day surgery, and ambulatory care costs: refer to the costs incurred from same-day surgery visits, emergency department visits, and high-cost ambulatory care visits to the dialysis clinics and cancer clinics. This does not include physician billings incurred during any of the abovementioned visits.
- Home and community care costs: Includes the costs incurred from home care services, rehabilitation, complex and continuing care, and long-term care costs.
- Other costs: Includes laboratory billings, non-physician billings, and assisted device costs.
2.3. Cost Estimation
2.4. Statistical Analysis
- The initial phase: the first 9 months immediately after diagnosis;
- The terminal phase: 3 months before death;
- The continuing phase: time between the initial and terminal phase.
3. Results
3.1. Patient Characteristics
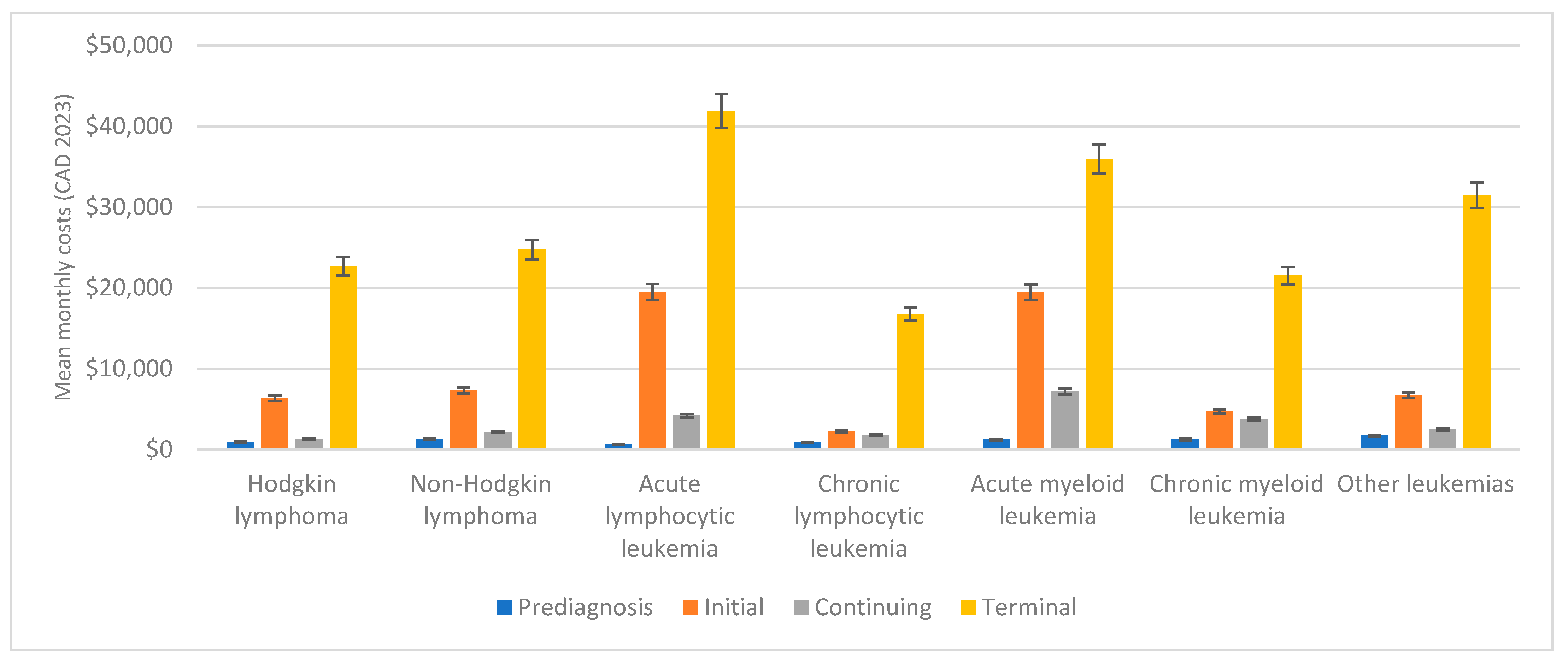
3.2. Phase-Specific Mean Monthly Costs
3.2.1. Prediagnosis Phase
3.2.2. Initial Phase
3.2.3. Continuing Phase
3.2.4. Terminal Phase
3.2.5. Trends in Phase-Specific Mean Monthly Costs
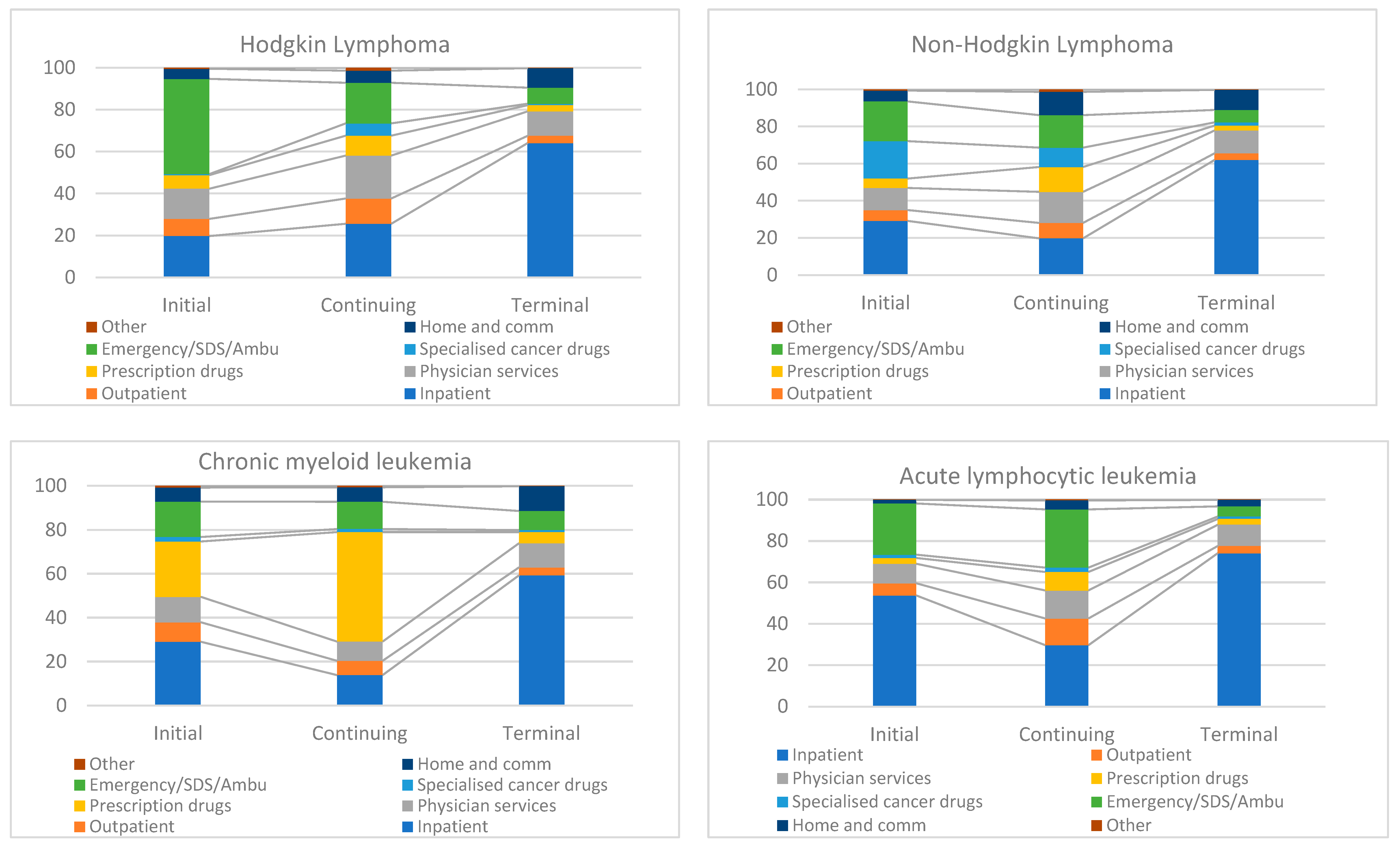
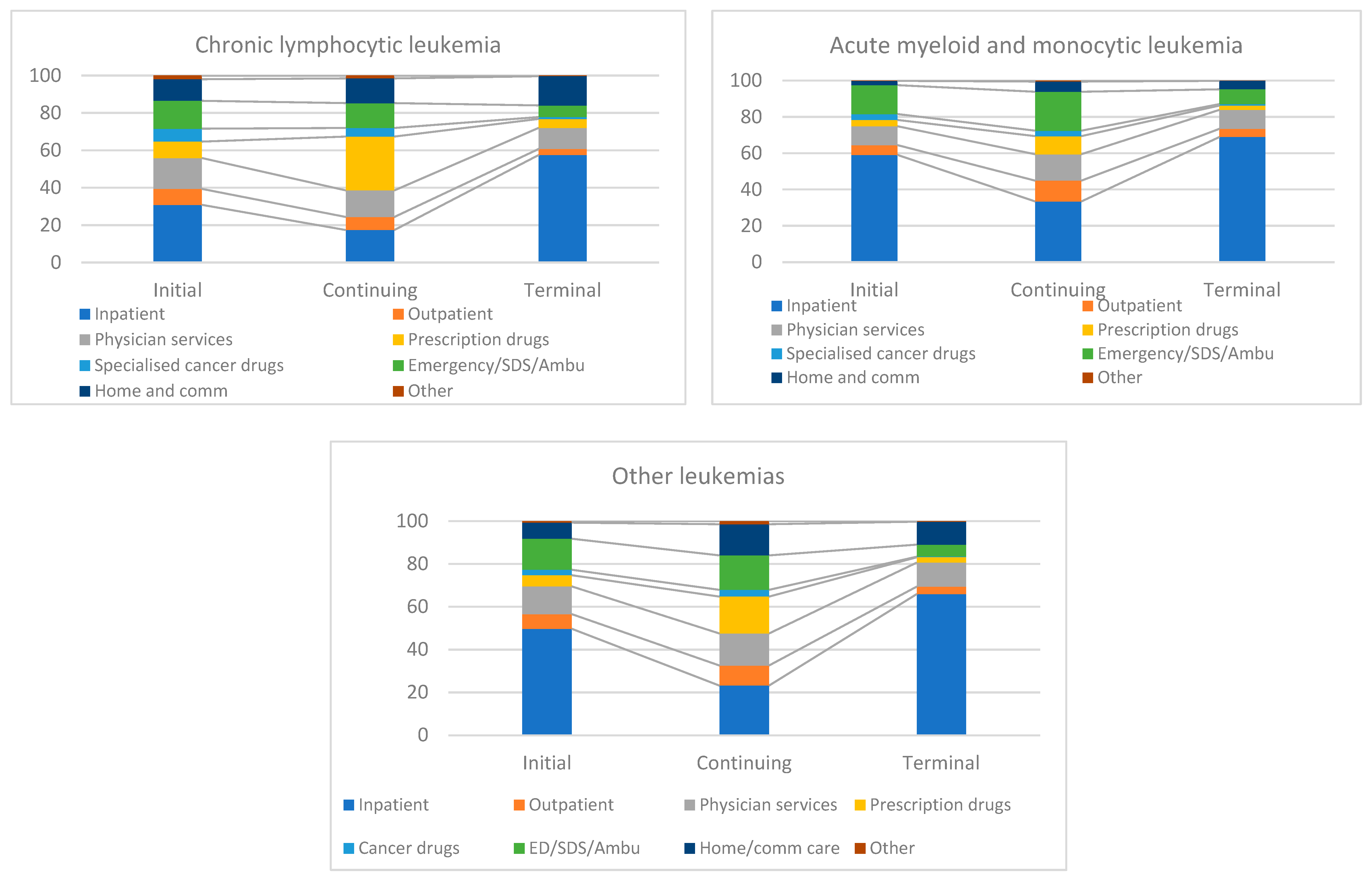
3.3. Component Contribution in Phase-Specific Costs
3.4. Lifetime Costs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canadian Cancer Statistics 2023; Canadian Cancer Statistics Advisory Committee: Toronto, ON. 2023. Available online: http://cancer.ca/Canadian-Cancer-Statistics-2023-EN (accessed on 17 April 2024).
- Canadian Cancer Society Statistics Canada. Canadian Cancer Statistics: A 2022 Special Report on Cancer Prevalence. 2022. Available online: http://cancer.ca/Canadian-Cancer-Statistics-2022-EN (accessed on 9 May 2023).
- Garaszczuk, R.; Yong, J.H.E.; Sun, Z.; de Oliveira, C. The Economic Burden of Cancer in Canada from a Societal Perspective. Curr. Oncol. 2022, 29, 2735–2748. [Google Scholar] [CrossRef] [PubMed]
- Dieguez, G.; Ferro, C.; Rotter, D. The Cost Burden of Blood Cancer Care: A Longitudinal Analysis of Commercially Insured Patients Diagnosed with Blood Cancer. 2018. Available online: https://us.milliman.com/en/insight/the-cost-burden-of-blood-cancer-care (accessed on 14 July 2024).
- de Oliveira, C.; Pataky, R.; Bremner, K.E.; Rangrej, J.; Chan, K.K.W.; Cheung, W.Y.; Hoch, J.S.; Peacock, S.; Krahn, M.D. Phase-specific and lifetime costs of cancer care in Ontario, Canada. BMC Cancer 2016, 16, 809. [Google Scholar] [CrossRef]
- Site Recode B ICD-O-3/WHO 2008 Definition—SEER Data Reporting Tools. Available online: https://seer.cancer.gov/siterecode_b/icdo3_who2008/ (accessed on 11 May 2023).
- Wodchis, W.P.; Bushmeneva, K.; Nikitovic, M.; Mckillop, I. Guidelines on Person-Level Costing Using Administrative Databases in Ontario. 2013. Available online: https://tspace.library.utoronto.ca/handle/1807/87373 (accessed on 11 May 2023).
- Seung, S.J.; Hurry, M.; Hassan, S.; Walton, R.N.; Evans, W.K. Cost-of-illness study for non-small-cell lung cancer using real-world data. Curr. Oncol. 2019, 26, 102. [Google Scholar] [CrossRef]
- Statistics Canada. Table 18-10-0005-01: Consumer Price Index, Annual Average, Not Seasonally Adjusted. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000501 (accessed on 15 July 2024).
- Brown, M.L.; Riley, G.F.; Schussler, N.; Etzioni, R. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med. Care 2002, 40, IV-104-17. [Google Scholar] [CrossRef]
- Blakely, T.; Atkinson, J.; Kvizhinadze, G.; Wilson, N.; Davies, A.; Clarke, P. Patterns of Cancer Care Costs in a Country with Detailed Individual Data. Med. Care 2015, 53, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Hornbrook, M.C. Definition and measurement of episodes of care in clinical and economic studies. In Cost Analysis Methodology for Clinical Practice Guidelines; Grady, M., Weis, K., Eds.; DHHS Pub No. (PHS) 95-001; Agency for Health Care Policy and Research: Rockville, MD, USA, 1995; pp. 15–40. [Google Scholar]
- Kim, H.; Fay, M.; Feuer, E.; Midthune, D. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Statistical Methodology and Applications Branch. Joinpoint Regression Program. Available online: https://surveillance.cancer.gov/joinpoint (accessed on 17 April 2024).
- Hornbrook, M.; Fishman, P.; Ritzwoller, D.; Elston-Lafata, J.; O’Keeffe-Rosetti, M.; Salloum, R. When does an episode of care for cancer begin? Med. Care 2013, 51, 324–329. [Google Scholar] [CrossRef]
- Wijeysundera, H.C.; Wang, X.; Tomlinson, G.; Ko, D.T.; Krahn, M.D. Techniques for estimating health care costs with censored data: An overview for the health services researcher. Clinicoecon. Outcomes Res. 2012, 4, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Yabroff, K.R.; Warren, J.L.; Schrag, D.; Mariotto, A.; Meekins, A.; Topor, M.; Brown, M.L. Comparison of approaches for estimating incidence costs of care for colorectal cancer patients. Med. Care 2009, 47 (Suppl. S1), S56–S63. [Google Scholar] [CrossRef]
- Brown, M.L.; Riley, G.F.; Potosky, A.L.; Etzioni, R.D. Obtaining long-term disease specific costs of care: Application to Medicare enrollees diagnosed with colorectal cancer. Med. Care 1999, 37, 1249–1259. [Google Scholar] [CrossRef]
- SAS Institute Inc. 2011. SAS® Enterprise Miner 7.1® Reference Help, Second Edition. 2011. Available online: https://support.sas.com/documentation/onlinedoc/miner/em71/emref.pdf (accessed on 14 July 2024).
- Lang, K.; Lines, L.M.; Lee, D.W.; Korn, J.R.; Earle, C.C.; Menzin, J. Lifetime and treatment-phase costs associated with colorectal cancer: Evidence from SEER-Medicare data. Clin. Gastroenterol. Hepatol. 2009, 7, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Yabroff, K.R.; Lamont, E.B.; Mariotto, A.; Warren, J.L.; Topor, M.; Meekins, A.; Brown, M.L. Cost of care for elderly cancer patients in the United States. J. Natl. Cancer Inst. 2008, 100, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.S.; Kessler, L.G.; Urban, N.; Smucker, R.C. Estimating the treatment costs of breast and lung cancer. Med. Care 1991, 29, 40–49. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.; Phd, M.A.; Weir, S.; Dphil, M.A.; Rangrej, J.; Mmath, M.; Krahn, M.D.; Mittmann, N.; Hoch, J.S.; Chan, K.K.W.; et al. The economic burden of cancer care in Canada: A population-based cost study. CMAJ Open 2018, 6, E1–E10. [Google Scholar] [CrossRef] [PubMed]
- Gotfrit, J.; Dempster, W.; Chambers, J.; Wheatley-Price, P. The Pathway for New Cancer Drug Access in Canada. Curr. Oncol. 2022, 29, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Al-Ali, B.; Madersbacher, S.; Zielonke, N.; Schauer, I.; Waldhoer, T.; Haidinger, G. Impact of gender on tumor stage and survival of upper urinary tract urothelial cancer: A population-based study. Wien. Klin. Wochenschr. 2017, 129, 385–390. [Google Scholar] [CrossRef]
- Micheli, A.; Ciampichini, R.; Oberaigner, W.; Ciccolallo, L.; de Vries, E.; Izarzugaza, I.; Zambon, P.; Gatta, G.; De Angelis, R.; Oberaigner, W.; et al. The advantage of women in cancer survival: An analysis of EUROCARE-4 data. Eur. J. Cancer 2009, 45, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Bugge, C.; Brustugun, O.T.; Sæther, E.M.; Kristiansen, I.S. Phase- and gender-specific, lifetime, and future costs of cancer: A retrospective population-based registry study. Medicine 2021, 100, E26523. [Google Scholar] [CrossRef] [PubMed]
- Kaye, D.R.; Min, H.S.; Herrel, L.A.; Dupree, J.M.; Ellimoottil, C.; Miller, D.C. Costs of Cancer Care Across the Disease Continuum. Oncologist 2018, 23, 798–805. [Google Scholar] [CrossRef]
- Atkins, M.; Coutinho, A.D.; Nunna, S.; Gupte-Singh, K.; Eaddy, M. Confirming the timing of phase-based costing in oncology studies: A case example in advanced melanoma. J. Med. Econ. 2018, 21, 212–217. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Bentley, J.P.; Ramachandran, S.; Chang, Y.; Banahan, B.F.; Shah, R.; Bhakta, N.; Yang, Y. Phase-Specific and Lifetime Costs of Multiple Myeloma among Older Adults in the US. JAMA Netw. Open 2021, 4, e2116357. [Google Scholar] [CrossRef] [PubMed]
- Schulthess, D.; Gassull, D.; Makady, A.; Ludlow, A.; Rothman, B.; Have, P.T.; Wu, Y.; Ekstrom, L.; Minnema, M.; Jagasia, M. Are CAR-T therapies living up to their hype? A study using real-world data in two cohorts to determine how well they are actually working in practice compared with bone marrow transplants. BMJ Evid.-Based Med. 2021, 26, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Bray, F. Evaluation of data quality in the cancer registry: Principles and methods Part II. Completeness. Eur. J. Cancer 2009, 45, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Prodhan, S.; King, M.J.; De, P.; Gilbert, J. Health Services Data: The Ontario Cancer Registry (a Unique, Linked, and Automated Population-Based Registry). In Data and Measures in Health Services Research; Springer: Boston, MA, USA, 2016; pp. 1–27. [Google Scholar] [CrossRef]
- Schneeweiss, S.; Avorn, J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J. Clin. Epidemiol. 2005, 58, 323–337. [Google Scholar] [CrossRef]

| Characteristic | Lymphoma | Leukemia | ||
|---|---|---|---|---|
| Number | Percent | Number | Percent | |
| Overall sample | 47,255 | 100 | 26,846 | 100 |
| Type of cancer | ||||
| Hodgkin lymphoma | 5233 | 11.1 | ||
| Non-Hodgkin lymphoma | 42,022 | 88.9 | ||
| Acute lymphocytic leukemia | 2694 | 10.0 | ||
| Chronic lymphocytic leukemia | 10,830 | 40.3 | ||
| Acute myeloid leukemia | 6553 | 24.4 | ||
| Chronic myeloid leukemia | 3405 | 12.7 | ||
| Other leukemias | 3364 | 12.5 | ||
| Median age at diagnosis in years (Q1, Q3) | 64.0 (51.0, 75.0) | 66.0 (53.0, 77.0) | ||
| Sex | ||||
| Male | 25,805 | 54.6 | 15,531 | 57.9 |
| Female | 21,450 | 45.4 | 11,315 | 42.1 |
| Neighbourhood income quintile | ||||
| 1—Low | 8682 | 18.5 | 5242 | 19.6 |
| 2 | 9456 | 20.1 | 5394 | 20.2 |
| 3 | 9224 | 19.6 | 5199 | 19.4 |
| 4 | 9491 | 20.2 | 5421 | 20.2 |
| 5—High | 10,188 | 21.7 | 5497 | 20.6 |
| Rural residence | 5916 | 12.5 | 3614 | 13.5 |
| Died during follow-up | 18,759 | 39.7 | 12,916 | 48.1 |
| Median follow-up time in days (Q1, Q3) | 47,255 | 1603.0 (582.0, 3061.0) | 26,846 | 1327.5 (383.0, 2837.0) |
| Year of diagnosis | ||||
| 2005 | 2424 | 5.1 | 1480 | 5.5 |
| 2006 | 2461 | 5.2 | 1526 | 5.7 |
| 2007 | 2612 | 5.5 | 1683 | 6.3 |
| 2008 | 2633 | 5.6 | 1651 | 6.1 |
| 2009 | 2608 | 5.5 | 1701 | 6.3 |
| 2010 | 2983 | 6.3 | 1946 | 7.2 |
| 2011 | 3019 | 6.4 | 1793 | 6.7 |
| 2012 | 3286 | 7.0 | 1740 | 6.5 |
| 2013 | 3440 | 7.3 | 1888 | 7.0 |
| 2014 | 3447 | 7.3 | 1934 | 7.2 |
| 2015 | 3405 | 7.2 | 1961 | 7.3 |
| 2016 | 3736 | 7.9 | 1813 | 6.8 |
| 2017 | 3741 | 7.9 | 1921 | 7.2 |
| 2018 | 3694 | 7.8 | 1909 | 7.1 |
| 2019 | 3766 | 8.0 | 1900 | 7.1 |
| Cancer Subtype | n | Number of Months in Phase | Cost by Phase | Lifetime Cost | ||||
|---|---|---|---|---|---|---|---|---|
| Initial | Continuing | Terminal | Initial | Continuing | Terminal | |||
| Hodgkin lymphoma | 5233 | 8.66 (8.62–8.70) | 84.49 (82.98–85.99) | 2.72 (2.68–2.77) | 53,832 (52,632–55,033) | 198,747 (185,322–212,172) | 15,604 (14,727–16,481) | 268,184 (252,681–283,687) |
| Non-Hodgkin lymphoma | 42,022 | 8.42 (8.40–8.44) | 67.54 (67.02–68.07) | 2.57 (2.56–2.58) | 55,233 (54,613–55,853) | 226,450 (221,942–230,958) | 40,151 (39,604–40,699) | 321,834 (316,159–327,510) |
| Acute lymphocytic leukemia | 2694 | 8.6 (8.54–8.67) | 80.06 (77.84–82.27) | 2.51 (2.44–2.58) | 157,173 (153,959–160,386) | 588,158 (551,397–624,918) | 33,464 (31,689–35,240) | 778,795 (737,045–820,544) |
| Chronic lymphocytic leukemia | 10,830 | 8.74 (8.71–8.76) | 71.43 (70.49–72.37) | 2.80 (2.78–2.82) | 19,075 (18,291–19,860) | 214,484 (208,500–220,469) | 29,023 (28,003–30,043) | 262,583 (254,794–270,372) |
| Acute myeloid leukemia | 6553 | 7.34 (7.26–7.43) | 47.13 (45.41–48.86) | 2.27 (2.24–2.30) | 98,752 (96,759–100,745) | 314,499 (299,884–329,115) | 65,264 (63,768–66,761) | 478,516 (460,411–496,620) |
| Chronic myeloid leukemia | 3405 | 8.4 (8.33–8.46) | 64.17 (62.28–66.05) | 2.69 (2.65–2.73) | 37,317 (35,588–39,046) | 371,618 (352,728–390,508) | 35,932 (34,168–37,697) | 444,867 (422,484–467,251) |
| Other leukemia | 3364 | 8.29 (8.21–8.37) | 65.41 (63.35–67.48) | 2.04 (1.98–2.09) | 41,867 (39,474–44,260) | 207,872 (191,328–224,416) | 43,189 (41,336–45,042) | 292,929 (272,139–313,719) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agarwal, A.; Kekre, N.; Atkins, H.; Imsirovic, H.; Hutton, B.; Coyle, D.; Thavorn, K. Phase-Based and Lifetime Health System Costs of Care for Patients Diagnosed with Leukemia and Lymphoma: A Population-Based Descriptive Study. Curr. Oncol. 2024, 31, 4192-4208. https://doi.org/10.3390/curroncol31080313
Agarwal A, Kekre N, Atkins H, Imsirovic H, Hutton B, Coyle D, Thavorn K. Phase-Based and Lifetime Health System Costs of Care for Patients Diagnosed with Leukemia and Lymphoma: A Population-Based Descriptive Study. Current Oncology. 2024; 31(8):4192-4208. https://doi.org/10.3390/curroncol31080313
Chicago/Turabian StyleAgarwal, Anubhav, Natasha Kekre, Harold Atkins, Haris Imsirovic, Brian Hutton, Doug Coyle, and Kednapa Thavorn. 2024. "Phase-Based and Lifetime Health System Costs of Care for Patients Diagnosed with Leukemia and Lymphoma: A Population-Based Descriptive Study" Current Oncology 31, no. 8: 4192-4208. https://doi.org/10.3390/curroncol31080313
APA StyleAgarwal, A., Kekre, N., Atkins, H., Imsirovic, H., Hutton, B., Coyle, D., & Thavorn, K. (2024). Phase-Based and Lifetime Health System Costs of Care for Patients Diagnosed with Leukemia and Lymphoma: A Population-Based Descriptive Study. Current Oncology, 31(8), 4192-4208. https://doi.org/10.3390/curroncol31080313





