Oncologic Outcomes of Interventions to Decrease Allograft Ischemia-Reperfusion Injury within Patients Undergoing Liver Transplantation for Hepatocellular Carcinoma: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
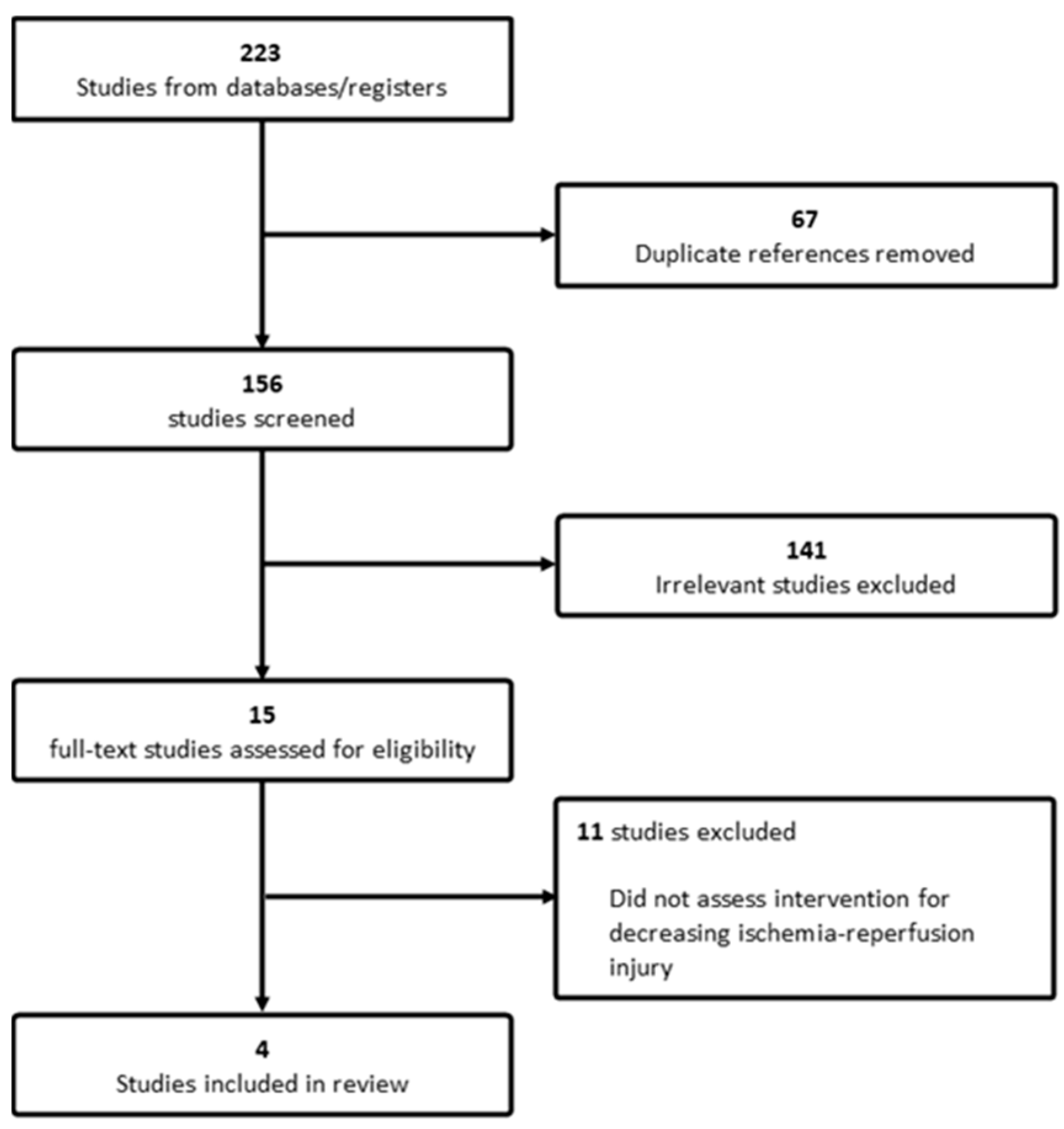
2.4. Screening and Data Collection
2.5. Qualitative Assessment
3. Results
3.1. Studies and Patient Characteristics
3.2. Interventions to Decrease Ischemia-Reperfusion Injury
3.3. Immunosuppressive Regimen and Rejection Incidence
3.4. Post-Operative Outcomes
3.5. Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.D.; Larson, J.J.; Watt, K.D.; Allen, A.M.; Wiesner, R.H.; Gores, G.J.; Roberts, L.R.; Heimbach, J.A.; Leise, M.D. Hepatocellular Carcinoma Is the Most Common Indication for Liver Transplantation and Placement on the Waitlist in the United States. Clin. Gastroenterol. Hepatol. 2017, 15, 767–775. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Mazzaferro, V.M.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N. Engl. J. Med. 1996, 334, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Yao, F. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology 2001, 33, 1394–1403. [Google Scholar] [CrossRef]
- Yao, F. Liver transplantation for hepatocellular carcinoma: Comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transplant. 2002, 8, 765–774. [Google Scholar] [CrossRef]
- Yao, F.Y.; Xiao, L.; Bass, N.M.; Kerlan, R.; Ascher, N.L.; Roberts, J.P. Liver Transplantation for Hepatocellular Carcinoma: Validation of the UCSF-Expanded Criteria Based on Preoperative Imaging. Am. J. Transplant. 2007, 7, 2587–2596. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Åberg, F.; Abrahamsson, J.; Schult, A.; Bennet, W.; Rizell, M.; Sternby-Eilard, M. The RETREAT score provides valid predictions regarding hepatocellular carcinoma recurrence after liver transplantation. Transpl. Int. 2021, 34, 2869–2874. [Google Scholar] [CrossRef] [PubMed]
- Nevola, R.; Ruocco, R.; Criscuolo, L.; Villani, A.; Alfano, M.; Beccia, D.; Imbriani, S.; Claar, E.; Cozzolino, D.; Sasso, F.C.; et al. Predictors of early and late hepatocellular carcinoma recurrence. World J. Gastroenterol. 2023, 29, 1243–1260. [Google Scholar] [CrossRef]
- Grat, M.; Krawczyk, M.; Wronka, K.M.; Stypulkowski, J.; Lewandowski, Z.; Wasilewicz, M.; Krawczyk, P.; Grat, K.; Patkowski, W.; Zieniewicz, K. Ischemia-reperfusion injury and the risk of hepatocellular carcinoma recurrence after deceased donor liver transplantation. Sci. Rep. 2018, 8, 8935. [Google Scholar] [CrossRef]
- Kornberg, A.; Witt, U.; Kornberg, J.; Friess, H.; Thrum, K. Extended Ischemia Times Promote Risk of HCC Recurrence in Liver Transplant Patients. Dig. Dis. Sci. 2015, 60, 2832–2839. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S.; Yoshida, A.; Facciuto, M.; Moonka, D.; Abouljoud, M.S.; Schwartz, M.E.; Florman, S.S. Ischemia time impacts recurrence of hepatocellular carcinoma after liver transplantation. Hepatology 2015, 61, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, A.; Witt, U.; Kornberg, J.; Friess, H.; Thrum, K. Treating ischaemia-reperfusion injury with prostaglandin E1 reduces the risk of early hepatocellular carcinoma recurrence following liver transplantation. Aliment. Pharmacol. Ther. 2015, 42, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Kalisvaart, M.; O'Rourke, J.; Shetty, S.; Parente, A.; Muller, X.; Isaac, J.; Muellhaupt, B.; Muiesan, P.; Shah, T.; et al. Hypothermic Oxygenated Liver Perfusion (HOPE) Prevents Tumor Recurrence in Liver Transplantation From Donation After Circulatory Death. Ann. Surg. 2020, 272, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, T.; Ju, W.; Li, F.; Zhang, Q.; Chen, Z.; Gong, J.; Zhao, Q.; Wang, D.; Chen, M.; et al. Ischemic-Free Liver Transplantation Reduces the Recurrence of Hepatocellular Carcinoma After Liver Transplantation. Front. Oncol. 2021, 11, 773535. [Google Scholar] [CrossRef] [PubMed]
- Rigo, F.; De Stefano, N.; Patrono, D.; De Donato, V.; Campi, L.; Turturica, D.; Doria, T.; Sciannameo, V.; Berchialla, P.; Tandoi, F.; et al. Impact of Hypothermic Oxygenated Machine Perfusion on Hepatocellular Carcinoma Recurrence after Liver Transplantation. J. Pers. Med. 2023, 13, 703. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhao, Q.; Jia, Z.; Huang, C.; Wang, D.; Ju, W.; Zhang, J.; Yang, L.; Huang, S.; Chen, M.; et al. A randomized-controlled trial of ischemia-free liver transplantation for end-stage liver disease. J. Hepatol. 2023, 79, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Rampes, S.; Ma, D. Hepatic ischemia-reperfusion injury in liver transplant setting: Mechanisms and protective strategies. J. Biomed. Res. 2019, 33, 221. [Google Scholar] [CrossRef]
- Jiménez-Castro, M.B.; Cornide-Petronio, M.E.; Gracia-Sancho, J.; Peralta, C. Inflammasome-Mediated Inflammation in Liver Ischemia-Reperfusion Injury. Cells 2019, 8, 1131. [Google Scholar] [CrossRef]
- Orci, L.A.; Lacotte, S.; Delaune, V.; Slits, F.; Oldani, G.; Lazarevic, V.; Rossetti, C.; Rubbia-Brandt, L.; Morel, P.; Toso, C. Effects of the gut–liver axis on ischaemia-mediated hepatocellular carcinoma recurrence in the mouse liver. J. Hepatol. 2018, 68, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Maspero, M.; Yilmaz, S.; Cazzaniga, B.; Raj, R.; Ali, K.; Mazzaferro, V.; Schlegel, A. The role of ischaemia-reperfusion injury and liver regeneration in hepatic tumour recurrence. JHEP Rep. 2023, 5, 100846. [Google Scholar] [CrossRef] [PubMed]
- Saidi, R.F.; Kenari, S.K.H. Liver Ischemia/Reperfusion Injury: An Overview. J. Investig. Surg. 2014, 27, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Czigany, Z.; Pratschke, J.; Froněk, J.; Guba, M.; Schöning, W.; Raptis, D.A.M.; Andrassy, J.; Kramer, M.; Strnad, P.; Tolba, R.H.; et al. Hypothermic Oxygenated Machine Perfusion Reduces Early Allograft Injury and Improves Post-transplant Outcomes in Extended Criteria Donation Liver Transplantation From Donation After Brain Death: Results From a Multicenter Randomized Controlled Trial (HOPE ECD-DBD). Ann. Surg. 2021, 274, 705–712. [Google Scholar] [CrossRef]
- Detelich, D.; Markmann, J.F. The dawn of liver perfusion machines. Curr. Opin. Organ. Transplant. 2018, 23, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Jakubauskas, M.; Jakubauskiene, L.; Leber, B.; Strupas, K.; Stiegler, P.; Schemmer, P. Machine Perfusion in Liver Transplantation: A Systematic Review and Meta-Analysis. Visc. Med. 2022, 38, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, J.V.; Henry, S.D.; Chen, S.W.; Brown, T.; Nachber, E.; Arrington, B.; Boykin, J.; Samstein, B.; Brown, R.S.; Emond, J.C.; et al. Hypothermic Machine Preservation Attenuates Ischemia/Reperfusion Markers After Liver Transplantation: Preliminary Results. J. Surg. Res. 2011, 167, E365–E373. [Google Scholar] [CrossRef]
- Patrono, D.M.; Catalano, G.; Rizza, G.; Lavorato, N.; Berchialla, P.; Gambella, A.; Caropreso, P.; Mengozzi, G.; Romagnoli, R.P. Perfusate Analysis during Dual Hypothermic Oxygenated Machine Perfusion of Liver Grafts: Correlations with Donor Factors and Early Outcomes. Transplantation 2020, 104, 1929–1942. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Wang, S.; Li, S.; Yang, Y.; Fang, Z.; Huang, H.; Wang, Y.; Fan, X.; Ye, Q. Hypothermic oxygenated machine perfusion alleviates liver injury in donation after circulatory death through activating autophagy in mice. Artif. Organs 2019, 43, E320–E332. [Google Scholar] [CrossRef]
- Azizieh, Y.; Westhaver, L.P.; Badrudin, D.; Boudreau, J.E.; Gala-Lopez, B.L. Changing liver utilization and discard rates in clinical transplantation in the ex-vivo machine preservation era. Front. Med. Technol. 2023, 5, 1079003. [Google Scholar] [CrossRef]
- Eshmuminov, D.; Becker, D.; Borrego, L.B.; Hefti, M.; Schuler, M.J.; Hagedorn, C.; Muller, X.; Mueller, M.; Onder, C.; Graf, R.; et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat. Biotechnol. 2020, 38, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Mugaanyi, J.; Dai, L.; Lu, C.; Mao, S.; Huang, J.; Lu, C. A Meta-Analysis and Systematic Review of Normothermic and Hypothermic Machine Perfusion in Liver Transplantation. J. Clin. Med. 2022, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, J.; Prieto, J. Liver cytoprotection by prostaglandins. Pharmacol. Ther. 1993, 58, 67–92. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Wakabayashi, H.; Izuishi, K.; Okano, K.; Yachida, S.; Maeta, H. The Role of Prostaglandins in Liver Ischemia-Reperfusion Injury. Curr. Pharm. Des. 2006, 12, 2935–2951. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, R.; Grąt, M. Direct, remote and combined ischemic conditioning in liver surgery. World J. Hepatol. 2021, 13, 533–542. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; Leuschner, S.; McNally, S.J.; Garden, O.J.; Wigmore, S.J.; Harrison, E.M. Meta-analysis of ischaemic preconditioning for liver resections. Br. J. Surg. 2013, 100, 1689–1700. [Google Scholar] [CrossRef]
- Parente, A.; Tirotta, F.; Pini, A.; Eden, J.; Dondossola, D.; Manzia, T.M.; Dutkowski, P.; Schlegel, A. Machine perfusion techniques for liver transplantation–A meta-analysis of the first seven randomized-controlled trials. J. Hepatol. 2023, 79, 1201–1213. [Google Scholar] [CrossRef]
| Author | Year | Country | Study Design | Recipient Transplant Criteria | Donor Type | Sample Size | Intervention to Decrease IRI | Perfusate Solution | Outcomes Assessed |
|---|---|---|---|---|---|---|---|---|---|
| Kornberg et al. [14] | 2015 | Germany | Single-centre, retrospective cohort | Milan | NR | 106 patients 59 intervention 47 control | Prostaglandin analogue (Alprostadil) | - | Overall survival Recurrence-free survival |
| Mueller et al. [15] | 2020 | Switzerland UK | Multi-centre, retrospective cohort | Milan UCSF Metroticket 2.0 | DBD DCD | 280 patients 70 intervention 210 control | Hypothermic machine perfusion | - | Recurrence-free survival Peri-operative complications Graft and patient survival Biopsy-proven acute rejection De novo tumour growth |
| Tang et al. [16] | 2021 | China | Single-centre, retrospective cohort | Milan | DBD | 226 patients 30 intervention 196 control | Normothermic machine perfusion | The perfusate contained approximately 1.3 L cross-matched leucocyte-depleted washed red cells, 1.4 L Succinylated gelatinor, 30 mL 5% sodium bicarbonate, 0.5 g metronidazole, 37,500 U heparin, 1.5 g cefoperazone sodium and sulbactam sodium, 30 mL 10% calcium gluconate, 3 mL 25% magnesium sulfate and 250 mL compound amino acid injection. | Overall survival Recurrence-free survival |
| Rigo et al. [17] | 2023 | Italy | Single-centre, retrospective cohort | Metroticket 2.0 | DBD DCD | 326 patients 80 intervention 246 control | Hypothermic machine perfusion | Belzer MP solution (BridgeToLife, Northbrook, IL, USA) | Recurrence-free survival Peri-operative complications Biopsy-proven acute rejection |
| Author | Ischemia-Reperfusion Injury | Overall Survival | Recurrence-Free Survival |
|---|---|---|---|
| Kornberg et al. [14] | Significantly lower mean AST peak level post-transplant 581.7 vs. 780.7 IU/mL Significantly lower mean ALT peak level post-transplant 559.6 vs. 701.4 IU/mL Significantly lower mean CRP peak level post-transplant 3.2 vs. 4.6 mg/dL | No significant difference with Alprostadil therapy 91.5% vs. 74.5% at 3 years 82.8% vs. 65.7% at 5 years | Significantly higher with Alprostadil therapy 87.9% vs. 65.3% at 3 years 85.7% vs. 63.1% at 5 years Significantly higher within Milan-Out subgroup of patients HR (95%CI) = 5.09 (1.64–15.76) |
| Mueller et al. [15] | Significantly higher median ALT day 1 level post-transplant 1305 vs. 893 U/L Significantly lower median CRP day level post-transplant 31 vs. 39 mg/L | NR | Significantly higher with hypothermic machine perfusion compared to untreated DBD liver recipients 92% vs. 73% at 5 years |
| Tang et al. [16] | Significantly lower ALT day 1 level post-transplant 198.8 vs. 633.8 U/L Significantly lower AST day level post-transplant 437.1 vs. 1571.6 U/L | No significant difference with normothermic machine perfusion 96.7% vs. 90.2% at 1 year 90.6% vs. 68.1% at 3 years | Significantly higher with normothermic machine perfusion 92.2% vs. 73.0% at 1 year 86.7 vs. 46.3% at 3 years Normothermic machine perfusion independently associated with improved recurrence-free survival HR (95%CI) = 3.73 (1.17–11.9) |
| Rigo et al. [17] | Significantly lower AST peak level post-transplant 903.0 vs. 1140.0 Significantly lower ALT peak level post-transplant 496.5 vs. 742.0 | Similar estimated 5-year survival probability based on Metroticket 2.0 model for hypothermic perfusion vs. static cold storage grafts 0.9 vs. 0.9 | Comparable recurrence rates for hypothermic perfusion vs. static cold storage grafts 10% vs. 9% RFS HR (95%CI) = 1.34 (0.5–3.4) |
| Confounding | Selection of Participants | Classification of Intervention(s) | Deviation from Intended Intervention(s) | Missing Data | Outcome Measurement | Reported Result | Overall | |
|---|---|---|---|---|---|---|---|---|
| Kornberg et al. [14] | Moderate | Moderate | Moderate | Moderate | Low | Low | Low | Moderate |
| Mueller et al. [15] | Moderate | Moderate | Moderate | Low | Low | Low | Low | Moderate |
| Tang et al. [16] | Moderate | Moderate | Low | Low | Low | Low | Moderate | Moderate |
| Rigo et al. [17] | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faleiro, M.D.; Mir, Z.M.; Azizieh, Y.; Hiebert, S.E.; Livingstone, S.M.; Walsh, M.J.; Gala-Lopez, B.L. Oncologic Outcomes of Interventions to Decrease Allograft Ischemia-Reperfusion Injury within Patients Undergoing Liver Transplantation for Hepatocellular Carcinoma: A Systematic Review. Curr. Oncol. 2024, 31, 2895-2906. https://doi.org/10.3390/curroncol31060221
Faleiro MD, Mir ZM, Azizieh Y, Hiebert SE, Livingstone SM, Walsh MJ, Gala-Lopez BL. Oncologic Outcomes of Interventions to Decrease Allograft Ischemia-Reperfusion Injury within Patients Undergoing Liver Transplantation for Hepatocellular Carcinoma: A Systematic Review. Current Oncology. 2024; 31(6):2895-2906. https://doi.org/10.3390/curroncol31060221
Chicago/Turabian StyleFaleiro, Matheus D., Zuhaib M. Mir, Yara Azizieh, Stephanie E. Hiebert, Scott M. Livingstone, Mark J. Walsh, and Boris L. Gala-Lopez. 2024. "Oncologic Outcomes of Interventions to Decrease Allograft Ischemia-Reperfusion Injury within Patients Undergoing Liver Transplantation for Hepatocellular Carcinoma: A Systematic Review" Current Oncology 31, no. 6: 2895-2906. https://doi.org/10.3390/curroncol31060221
APA StyleFaleiro, M. D., Mir, Z. M., Azizieh, Y., Hiebert, S. E., Livingstone, S. M., Walsh, M. J., & Gala-Lopez, B. L. (2024). Oncologic Outcomes of Interventions to Decrease Allograft Ischemia-Reperfusion Injury within Patients Undergoing Liver Transplantation for Hepatocellular Carcinoma: A Systematic Review. Current Oncology, 31(6), 2895-2906. https://doi.org/10.3390/curroncol31060221





