Real-World Evidence Study of Patients with KRAS-Mutated NSCLC in Finland
Abstract
1. Introduction
2. Materials and Methods
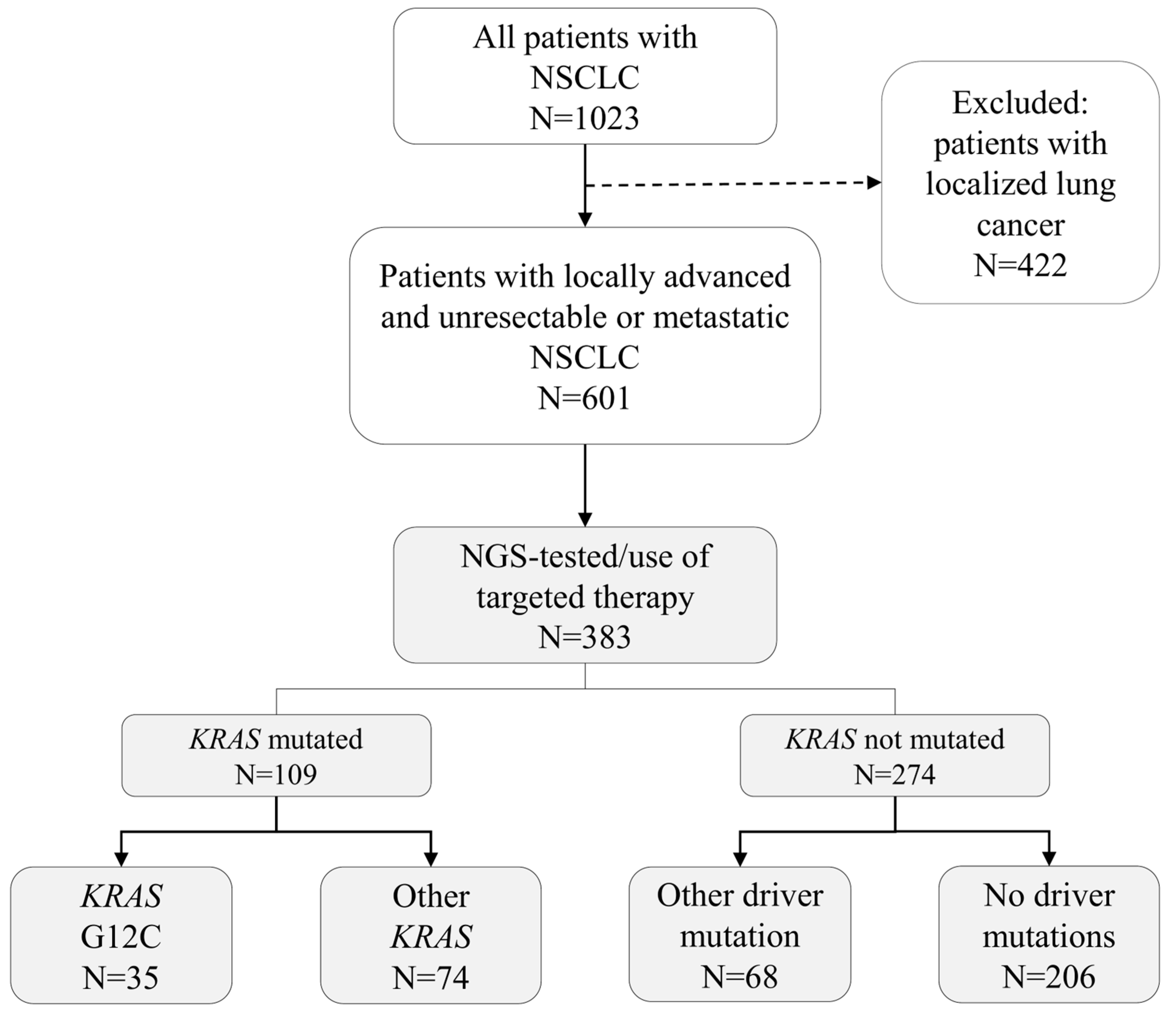
2.1. Cohort Formation
2.2. Patient Stratification
2.3. Patient Characteristics
2.4. Treatment Lines
2.5. Treatment Outcomes
2.6. Healthcare Resource Utilization
3. Results
3.1. Patient Characteristics and Treatments
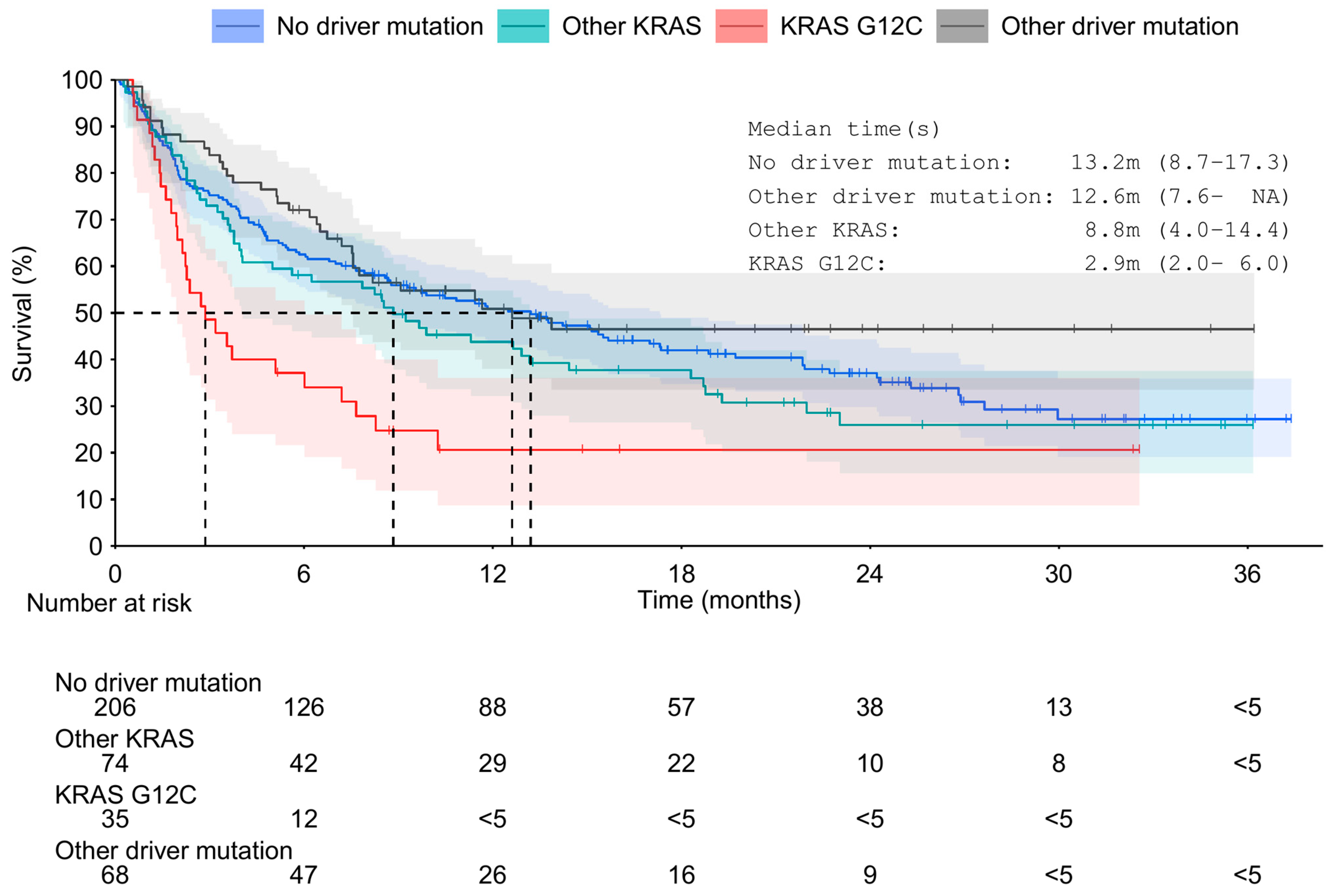
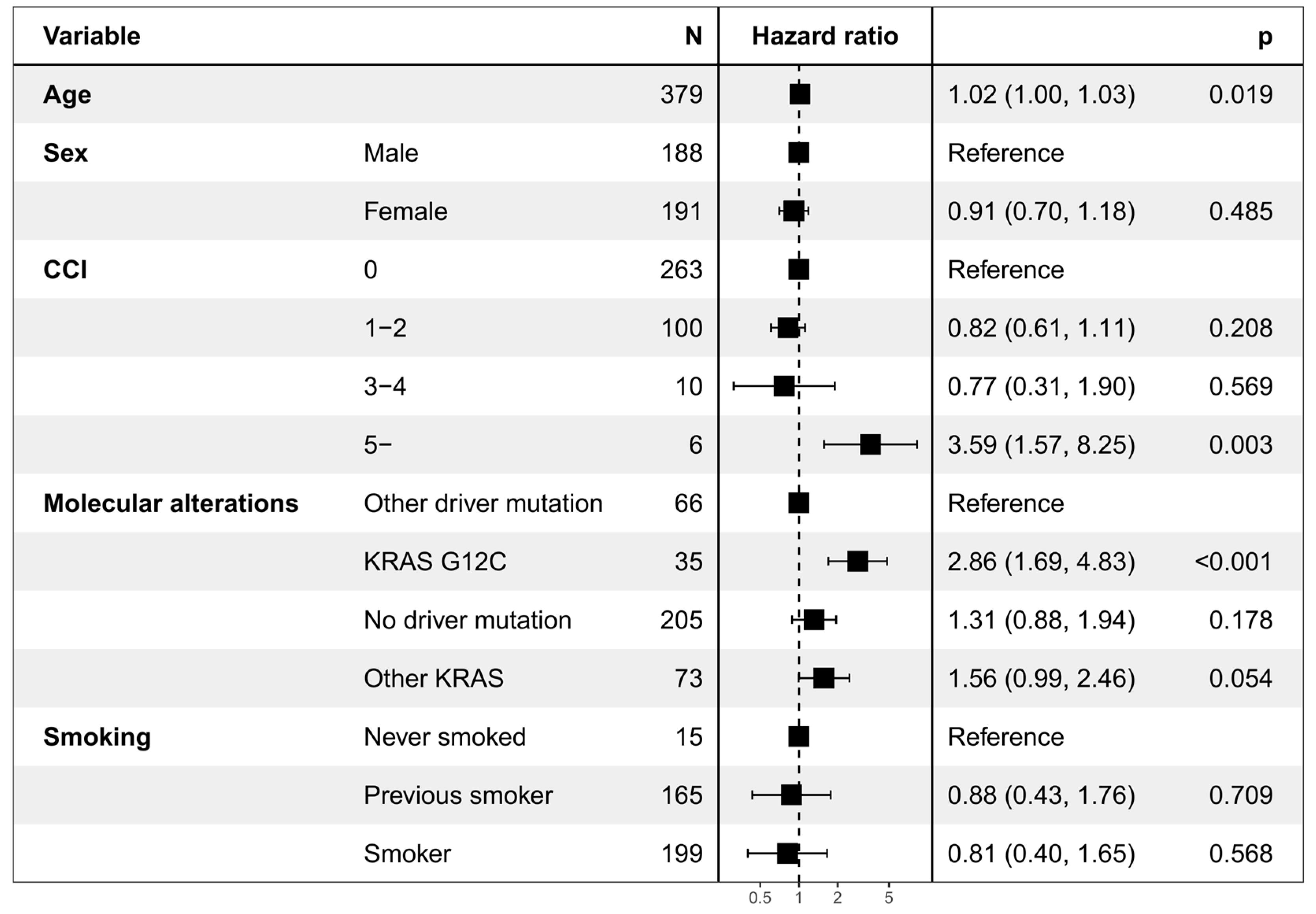
3.2. Overall Survival and Time to Next Treatment
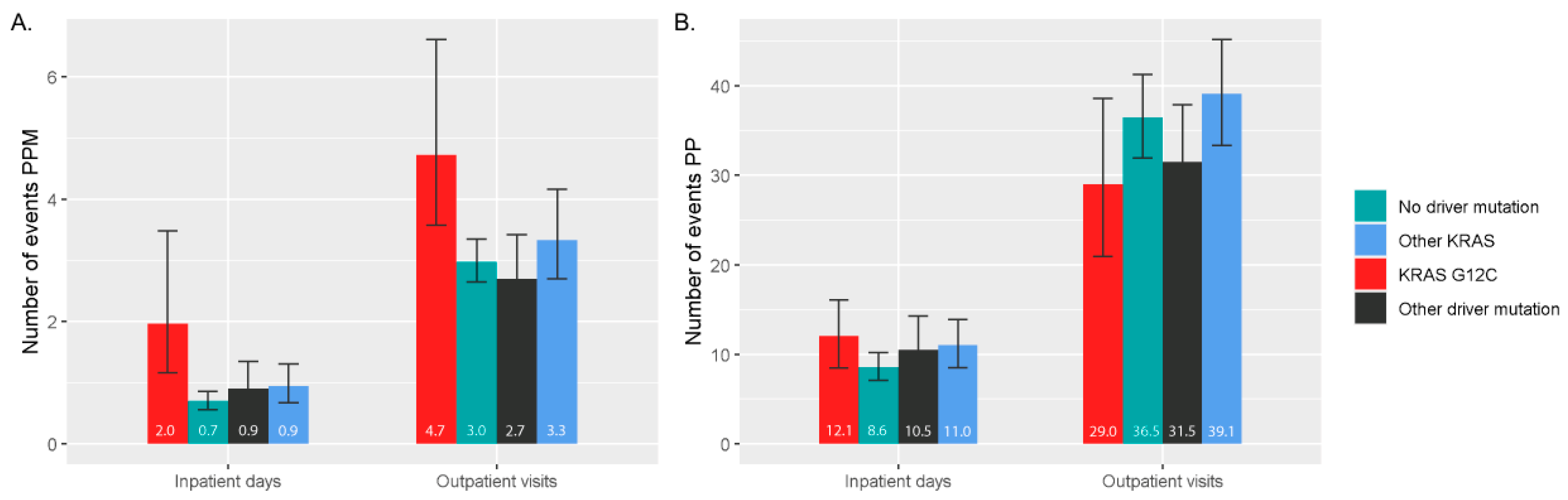
3.3. Healthcare Resource Utilization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- SEER*Explorer Application. Available online: https://seer.cancer.gov/statistics-network/explorer/application.html?site=47&data_type=1&graph_type=2&compareBy=subtype&chk_subtype_612=612&chk_subtype_613=613&chk_subtype_611=611&chk_subtype_610=610&hdn_rate_type=1&sex=1&race=1&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&advopt_display=1 (accessed on 17 March 2023).
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non–Small Cell Lung Cancer: Epidemiology, Risk Factors, Treatment, and Survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The Biology and Management of Non-Small Cell Lung Cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- El Osta, B.E.; Behera, M.; Kim, S.; Berry, L.D.; Sica, G.; Pillai, R.N.; Owonikoko, T.K.; Kris, M.G.; Johnson, B.E.; Kwiatkowski, D.J.; et al. Characteristics and Outcomes of Patients (Pts) with Metastatic KRAS Mutant Lung Adenocarcinomas: Lung Cancer Mutation Consortium (LCMC) Database. J. Clin. Oncol. 2017, 35 (Suppl. S15), 9021. [Google Scholar] [CrossRef]
- Shepherd, F.A.; Domerg, C.; Hainaut, P.; Jänne, P.A.; Pignon, J.-P.; Graziano, S.; Douillard, J.-Y.; Brambilla, E.; Le Chevalier, T.; Seymour, L.; et al. Pooled Analysis of the Prognostic and Predictive Effects of KRAS Mutation Status and KRAS Mutation Subtype in Early-Stage Resected Non–Small-Cell Lung Cancer in Four Trials of Adjuvant Chemotherapy. J. Clin. Oncol. 2013, 31, 2173–2181. [Google Scholar] [CrossRef]
- Terrenato, I.; Ercolani, C.; Di Benedetto, A.; Gallo, E.; Melucci, E.; Casini, B.; Rollo, F.; Palange, A.; Visca, P.; Pescarmona, E.; et al. Real-World Systematic Analysis of Driver Mutations’ Prevalence in Early- and Advanced-Stage NSCLC: Implications for Targeted Therapies in the Adjuvant Setting. Cancers 2022, 14, 2971. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.; Shen, R.; Ang, D.C.; Johnson, M.L.; D’Angelo, S.P.; Paik, P.K.; Brzostowski, E.B.; Riely, G.J.; Kris, M.G.; Zakowski, M.F.; et al. Molecular Epidemiology of EGFR and KRAS Mutations in 3026 Lung Adenocarcinomas: Higher Susceptibility of Women to Smoking-Related KRAS-Mutant Cancers. Clin. Cancer Res. 2012, 18, 6169–6177. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.; Bortolini, M.; Calleja, A.; Munro, R.; Kong, S.; Daumont, M.J.; Penrod, J.R.; Lakhdari, K.; Lacoin, L.; Cheung, W.Y. Trends in Treatment Patterns and Survival Outcomes in Advanced Non-Small Cell Lung Cancer: A Canadian Population-Based Real-World Analysis. BMC Cancer 2022, 22, 255. [Google Scholar] [CrossRef]
- Marabese, M.; Ganzinelli, M.; Garassino, M.C.; Shepherd, F.A.; Piva, S.; Caiola, E.; Macerelli, M.; Bettini, A.; Lauricella, C.; Floriani, I.; et al. KRAS Mutations Affect Prognosis of Non-Small-Cell Lung Cancer Patients Treated with First-Line Platinum Containing Chemotherapy. Oncotarget 2015, 6, 34014–34022. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Sima, C.S.; Chaft, J.; Paik, P.K.; Pao, W.; Kris, M.G.; Ladanyi, M.; Riely, G.J. Association of KRAS and EGFR Mutations with Survival in Patients with Advanced Lung Adenocarcinomas. Cancer 2013, 119, 356–362. [Google Scholar] [CrossRef]
- Cascetta, P.; Marinello, A.; Lazzari, C.; Gregorc, V.; Planchard, D.; Bianco, R.; Normanno, N.; Morabito, A. KRAS in NSCLC: State of the Art and Future Perspectives. Cancers 2022, 14, 5430. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- de Langen, A.J.; Johnson, M.L.; Mazieres, J.; Dingemans, A.-M.C.; Mountzios, G.; Pless, M.; Wolf, J.; Schuler, M.; Lena, H.; Skoulidis, F.; et al. Sotorasib versus Docetaxel for Previously Treated Non-Small-Cell Lung Cancer with KRASG12C Mutation: A Randomised, Open-Label, Phase 3 Trial. Lancet 2023, 401, 733–746. [Google Scholar] [CrossRef]
- Jänne, P.A.; Riely, G.J.; Gadgeel, S.M.; Heist, R.S.; Ou, S.-H.I.; Pacheco, J.M.; Johnson, M.L.; Sabari, J.K.; Leventakos, K.; Yau, E.; et al. Adagrasib in Non–Small-Cell Lung Cancer Harboring a KRASG12C Mutation. N. Engl. J. Med. 2022, 387, 120–131. [Google Scholar] [CrossRef]
- Soerjomataram, I.; Lortet-Tieulent, J.; Parkin, D.M.; Ferlay, J.; Mathers, C.; Forman, D.; Bray, F. Global Burden of Cancer in 2008: A Systematic Analysis of Disability-Adjusted Life-Years in 12 World Regions. Lancet 2012, 380, 1840–1850. [Google Scholar] [CrossRef]
- Hanly, P.; Soerjomataram, I.; Sharp, L. Measuring the Societal Burden of Cancer: The Cost of Lost Productivity Due to Premature Cancer-Related Mortality in Europe. Int. J. Cancer 2015, 136, E136–E145. [Google Scholar] [CrossRef]
- Wood, R.; Taylor-Stokes, G. Cost Burden Associated with Advanced Non-Small Cell Lung Cancer in Europe and Influence of Disease Stage. BMC Cancer 2019, 19, 214. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.-M.; Sundararajan, V. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data from 6 Countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Mäklin, S. Terveyden- ja Sosiaalihuollon Yksikkökustannukset Suomessa Vuonna 2017. 2017. Available online: https://www.julkari.fi/bitstream/handle/10024/142882/URN_ISBN_978-952-343-493-6.pdf?sequence=1&isAllowed=y (accessed on 17 March 2023).
- Loponen, H.; Paajanen, J.; Vihervaara, V.; Ylä-Viteli, S.; Tamminen, K.; Torvinen4, S.; Jokelainen, J.; Ylisaukko-oja, T.; Silvoniemi, M. Treatment Patterns and Outcomes in a Cohort of Finnish NSCLC Patients with ALK Rearrangement Reflect Rapid Evolution in Treatment Practices. Int. J. Clin. Exp. Med. Sci. 2021, 7, 200. [Google Scholar] [CrossRef]
- Leskelä, R.-L.; Peltonen, E.; Haavisto, I.; Herse, F.; Korhonen, S.; Nolvi, K.; Käkelä, S.; Autere, A.-M.; Tiainen, S.; Silvoniemi, M.; et al. Trends in Treatment of Non-Small Cell Lung Cancer in Finland 2014–2019. Acta Oncol. 2022, 61, 641–648. [Google Scholar] [CrossRef]
- Talvitie, E.-M.; Liljeroos, L.; Vilhonen, H.; Orte, K.; Leivo, I.; Kallajoki, M.; Taimen, P. Comprehensive Genomic Profiling of Finnish Lung Adenocarcinoma Cohort Reveals High Clinical Actionability and SMARCA4 Altered Tumors with Variable Histology and Poor Prognosis. Neoplasia 2022, 32, 100832. [Google Scholar] [CrossRef]
- Frost, M.G.; Jensen, K.J.; Gotfredsen, D.R.; Sørensen, A.M.S.; Ankarfeldt, M.Z.; Louie, K.S.; Sroczynski, N.; Jakobsen, E.; Andersen, J.L.; Jimenez-Solem, E.; et al. KRAS G12C Mutated Advanced Non-Small Cell Lung Cancer (NSCLC): Characteristics, Treatment Patterns and Overall Survival from a Danish Nationwide Observational Register Study. Lung Cancer 2023, 178, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef] [PubMed]
- Syöpätilastosovellus, Syöpärekisteri. Available online: https://syoparekisteri.fi/tilastot/tautitilastot/ (accessed on 20 March 2023).
- Isaksson, J.; Berglund, A.; Louie, K.; Willén, L.; Hamidian, A.; Edsjö, A.; Enlund, F.; Planck, M.; Vikström, A.; Johansson, M.; et al. KRAS G12C Mutant Non–Small Cell Lung Cancer Linked to Female Sex and High Risk of CNS Metastasis: Population-Based Demographics and Survival Data From the National Swedish Lung Cancer Registry. Clin. Lung Cancer 2023, 24, 507–518. [Google Scholar] [CrossRef]
- Sebastian, M.; Eberhardt, W.E.E.; Hoffknecht, P.; Metzenmacher, M.; Wehler, T.; Kokowski, K.; Alt, J.; Schütte, W.; Büttner, R.; Heukamp, L.C.; et al. KRAS G12C-Mutated Advanced Non-Small Cell Lung Cancer: A Real-World Cohort from the German Prospective, Observational, Nation-Wide CRISP Registry (AIO-TRK-0315). Lung Cancer 2021, 154, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Noordhof, A.L.; Swart, E.M.; Damhuis, R.A.M.; Hendriks, L.E.L.; Kunst, P.W.A.; Aarts, M.J.; van Geffen, W.H. Prognostic Implication of KRAS G12C Mutation in a Real-World KRAS-Mutated Stage IV NSCLC Cohort Treated with Immunotherapy in The Netherlands. JTO Clin. Res. Rep. 2023, 4, 100543. [Google Scholar] [CrossRef] [PubMed]
- Kyrölä, A.; Järvelin, J. Somaattinen Erikoissairaanhoito 2021. THL Tilastoraportti 38/2022; 2021. Available online: https://www.julkari.fi/bitstream/handle/10024/145419/TR38_2022_somaattinen_erikoissairaanhoito_2021.pdf?sequence=1&isAllowed=y (accessed on 17 March 2023).
- Kapiainen, S.; Eskelinen, J. Miesten Ja Naisten Terveysmenot Ikäryhmittäin 2011; Terveyden ja hyvinvoinnin laitos (THL), 2014. Available online: https://www.julkari.fi/bitstream/handle/10024/116156/URN_ISBN_978-952-302-192-1.pdf (accessed on 17 March 2023).
- Peters, S.; Bexelius, C.; Munk, V.; Leighl, N. The Impact of Brain Metastasis on Quality of Life, Resource Utilization and Survival in Patients with Non-Small-Cell Lung Cancer. Cancer Treat. Rev. 2016, 45, 139–162. [Google Scholar] [CrossRef]
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Oncogene-Addicted Metastatic Non-Small-Cell Lung Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 339–357. [Google Scholar] [CrossRef]
| Estimate/Level | Overall | KRAS G12C | Other KRAS | Other Driver Mutation | No Driver Mutation | p Value | Missing % b | |
|---|---|---|---|---|---|---|---|---|
| n | 383 | 35 | 74 | 68 | 206 | |||
| Age in years | median [IQR] | 69.0 [62.0, 75.0] | 68.0 [59.5, 72.0] | 67.5 [61.0, 73.0] | 72.0 [66.0, 77.0] | 69.0 [61.0, 74.0] | 0.033 | 0 |
| Age group, years, N (%) | ≤59 | 83 (21.7) | 8–11 | 13–16 | 8 (11.8) | 51 (24.8) | 0.143 | 0 |
| 60–79 | 254 (66.3) | 23 (65.7) | 51 (68.9) | 47 (69.1) | 133 (64.6) | |||
| ≥80 | 46 (12.0) | <5 | 7–10 | 13 (19.1) | 22 (10.7) | |||
| Sex, N (%) | Female | 193 (50.4) | 18 (51.4) | 33 (44.6) | 44 (64.7) | 98 (47.6) | 0.065 | 0 |
| Male | 190 (49.6) | 17 (48.6) | 41 (55.4) | 24 (35.3) | 108 (52.4) | |||
| Length of follow-up in days | median [IQR] | 257.0 [87.5, 560.0] | 87.0 [51.5, 242.5] | 259.0 [83.5, 580.0] | 263.5 [156.8, 474.8] | 284.0 [101.0, 590.8] | 0.004 | 0 |
| Smoking status, N (%) | Never smoked | 15 (4.0) | 0 (0.0) | 0 (0.0) | 5 (7.6) | 10 (4.9) | <0.001 | 1 |
| Previous smoker a | 165 (43.5) | 8 (22.9) | 27 (37.0) | 40 (60.6) | 90 (43.9) | |||
| Smoker | 199 (52.5) | 27 (77.1) | 46 (63.0) | 21 (31.8) | 105 (51.2) | |||
| Performance status, ECOG, N (%) | 0–1 | 173 (66.5) | 14 (77.8) | 32 (66.7) | 35 (71.4) | 92 (63.4) | 0.543 | 32.1 |
| 2 | 52 (20.0) | <5 | 9 (18.8) | 7–10 | 35 (24.1) | |||
| 3–4 | 35 (13.5) | <5 | 7 (14.6) | 5–8 | 18 (12.4) | |||
| CCI score, N (%) | 0 | 265 (69.2) | 21 (60.0) | 54 (73.0) | 49 (71.1) | 141 (68.4) | 0.752 | 0.0 |
| 1–2 | 102 (28.5) | 10–13 | 16–19 | 15–18 | 55 (29.1) | |||
| 3+ | 16 (4.5) | <5 | <5 | <5 | 10 (5.3) | |||
| Histology, N (%) | Adenocarcinoma | 340 (93.9 | 31–34 | 70–73 | 64–67 | 170 (90.4) | 0.025 | 5.5 |
| Other NSCLC | 22 (6.1) | <5 | <5 | <5 | 18 (9.6) | |||
| Stage, N (%) | IA | <5 | 0 (0.0) | <5 | 0 (0.0) | <5 | 0.114 | 31.9 |
| IB | <5 | <5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| IIA | 15 (5.7) | <5 | 0 (0.0) | <6 | 6–9 | |||
| IIB | 8 (3.1) | 0 (0.0) | <5 | <5 | 6 (4.3) | |||
| IIIA | 73 (28.0) | 5 (23.8) | 10 (19.6) | 16 (31.4) | 42 (30.4) | |||
| IIIB | 35 (13.4) | <5 | 8 (15.7) | 7 (13.7) | 19 (13.8) | |||
| IV | 126 (48.3) | 12 (57.1) | 31 (60.8) | 22 (43.1) | 61 (44.2) | |||
| Metastasis index delay in days | median [IQR] | 7.0 [−4.0, 51.5] | 1.0 [−7.5, 26.0] | 2.0 [−9.8, 14.0] | 10.0 [−6.3, 49.3] | 15.5 [−1.0, 79.5] | <0.001 | 0 |
| Brain metastasis, N (%) | Yes | 59 (15.4) | <5 | 14 (18.9) | 7–10 | 34 (16.5) | 0.417 | 0 |
| No | 324 (84.6) | 31–34 | 60 (81.1) | 58–61 | 172 (83.5) | |||
| Received first-line therapy | Yes | 213 (55.6) | 17 (48.6) | 48 (64.9) | 42 (61.8) | 106 (51.5) | 0.124 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anttalainen, A.; Pietarinen, P.; Tuominen, S.; Mattila, R.; Mutka, A.; Knuuttila, A. Real-World Evidence Study of Patients with KRAS-Mutated NSCLC in Finland. Curr. Oncol. 2024, 31, 2700-2712. https://doi.org/10.3390/curroncol31050205
Anttalainen A, Pietarinen P, Tuominen S, Mattila R, Mutka A, Knuuttila A. Real-World Evidence Study of Patients with KRAS-Mutated NSCLC in Finland. Current Oncology. 2024; 31(5):2700-2712. https://doi.org/10.3390/curroncol31050205
Chicago/Turabian StyleAnttalainen, Anna, Paavo Pietarinen, Samuli Tuominen, Riikka Mattila, Aino Mutka, and Aija Knuuttila. 2024. "Real-World Evidence Study of Patients with KRAS-Mutated NSCLC in Finland" Current Oncology 31, no. 5: 2700-2712. https://doi.org/10.3390/curroncol31050205
APA StyleAnttalainen, A., Pietarinen, P., Tuominen, S., Mattila, R., Mutka, A., & Knuuttila, A. (2024). Real-World Evidence Study of Patients with KRAS-Mutated NSCLC in Finland. Current Oncology, 31(5), 2700-2712. https://doi.org/10.3390/curroncol31050205






