Pan-Canadian Analysis of Practice Patterns in Small Cell Carcinoma of the Cervix: Insights from a Multidisciplinary Survey
Abstract
1. Introduction
2. Materials and Methods
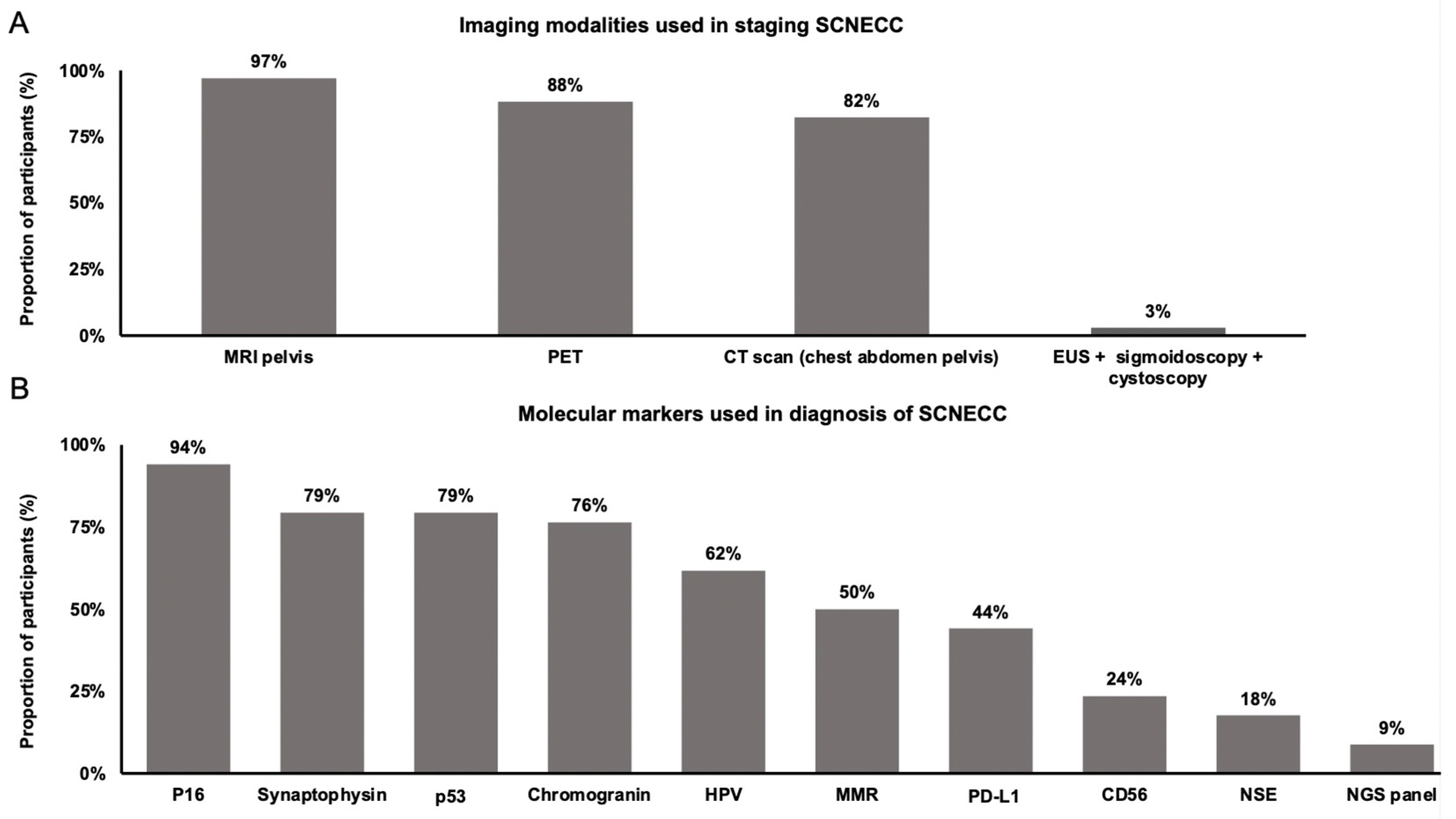
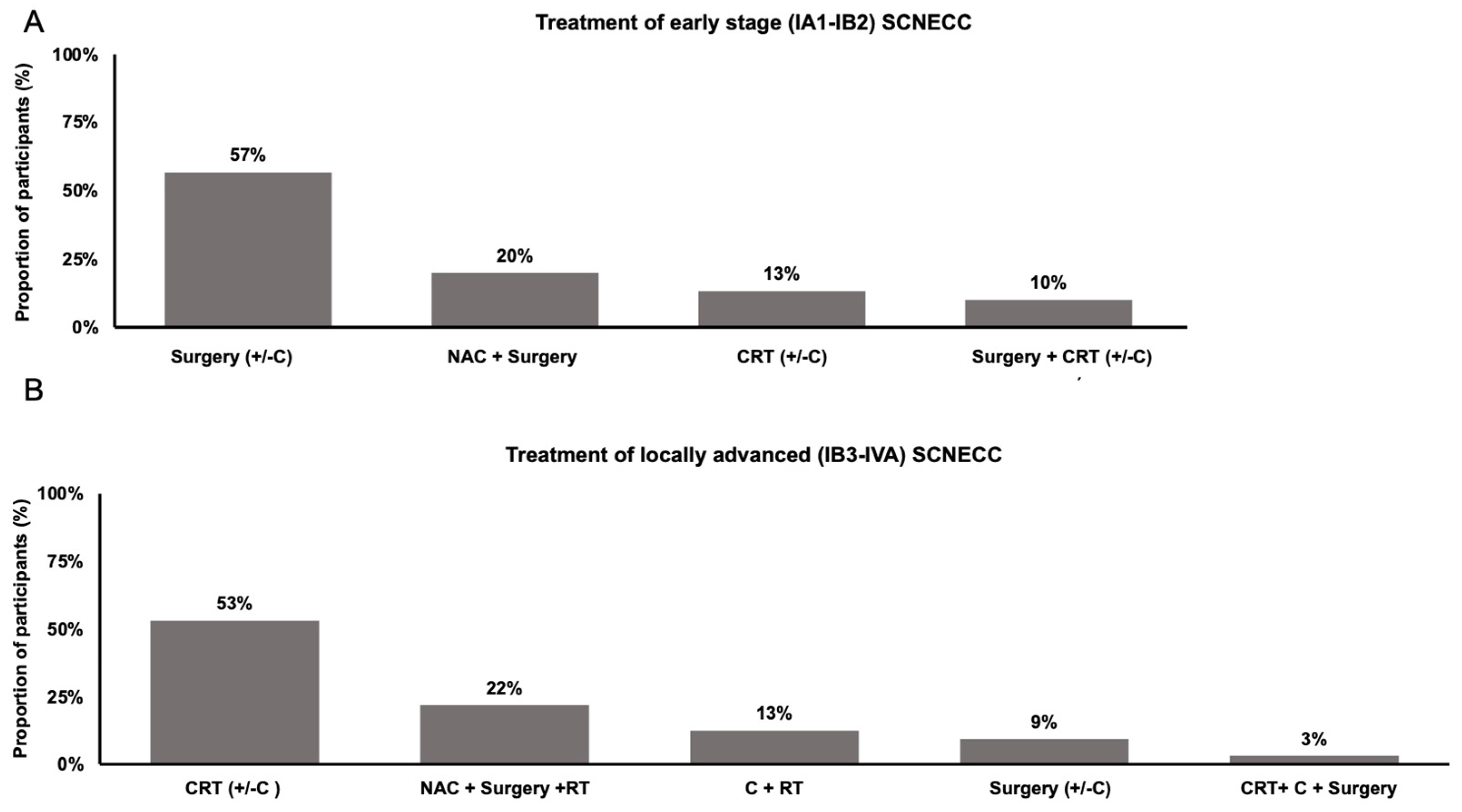
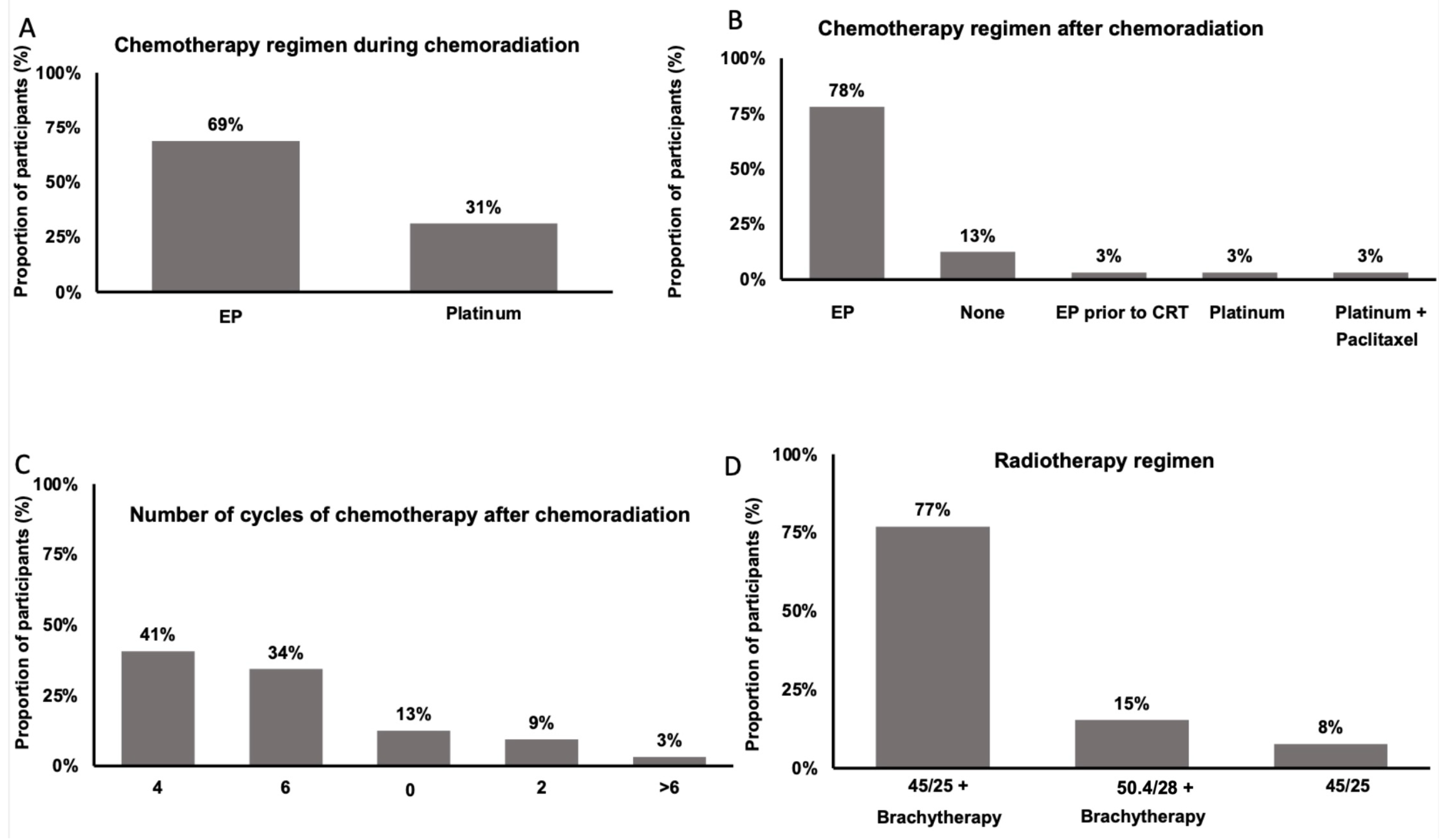
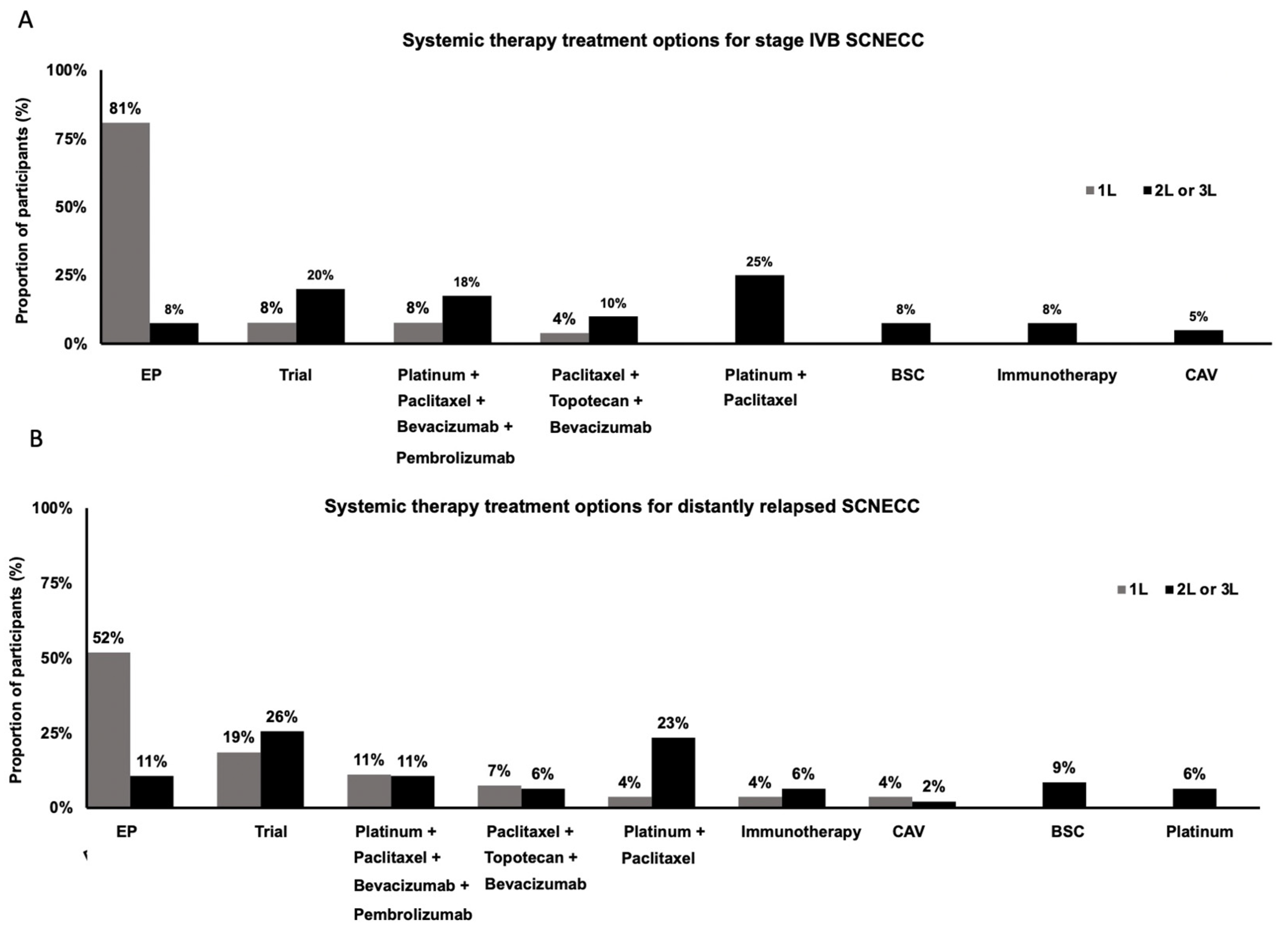
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Classification of Tumours of Female Reproductive Organs; International Agency for Research on Cancer: Lyon, France, 2014.
- Winer, I.; Kim, C.; Gehrig, P. Neuroendocrine tumors of the gynecologic tract update. Gynecol. Oncol. 2021, 162, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Takei, Y.; Treilleux, I.; Devouassoux-Shisheboran, M.; Ledermann, J.; Viswanathan, A.N.; Mahner, S.; Provencher, D.M.; Mileshkin, L.; Åvall-Lundqvist, E.; et al. Gynecologic Cancer InterGroup (GCIG) Consensus Review for Small Cell Carcinoma of the Cervix. Int. J. Gynecol. Cancer 2014, 24, S102–S108. [Google Scholar] [CrossRef] [PubMed]
- Salvo, G.; Martin, A.G.; Gonzales, N.R.; Frumovitz, M. Updates and management algorithm for neuroendocrine tumors of the uterine cervix. Int. J. Gynecol. Cancer 2019, 29, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N.; on behalf of the ESMO Guidelines Committee. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv72–iv83. [Google Scholar] [CrossRef]
- Huang, R.; Yu, L.; Zheng, C.; Liang, Q.; Suye, S.; Yang, X.; Yin, H.; Ren, Z.; Shi, L.; Zhang, Z.; et al. Diagnostic value of four neuroendocrine markers in small cell neuroendocrine carcinomas of the cervix: A meta-analysis. Sci. Rep. 2020, 10, 14975. [Google Scholar] [CrossRef] [PubMed]
- Castle, P.E.; Pierz, A.; Stoler, M.H. A systematic review and meta-analysis on the attribution of human papillomavirus (HPV) in neuroendocrine cancers of the cervix. Gynecol. Oncol. 2018, 148, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Tempfer, C.B.; Tischoff, I.; Dogan, A.; Hilal, Z.; Schultheis, B.; Kern, P.; Rezniczek, G.A. Neuroendocrine carcinoma of the cervix: A systematic review of the literature. BMC Cancer 2018, 18, 530. [Google Scholar] [CrossRef] [PubMed]
- Alberta Innovates. ARECCI Ethics Screening Tool (A pRoject Ethics Community Consensus Initiative); Alberta Innovates: Edmonton, AB, Canada, 2017. [Google Scholar]
- NCCN. National Comprehensive Cancer Network. Guidelines Version 1.2024 Cervical Cancer; NCCN National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2024. [Google Scholar]
- Carroll, M.R.; Ramalingam, P.; Salvo, G.; Fujimoto, J.; Soto, L.M.S.; Phoolcharoen, N.; Hillman, R.T.; Cardnell, R.; Byers, L.; Frumovitz, M. Evaluation of PARP and PDL-1 as potential therapeutic targets for women with high-grade neuroendocrine carcinomas of the cervix. Int. J. Gynecol. Cancer 2020, 30, 1303–1307. [Google Scholar] [CrossRef]
- Wang, R.; Xiao, Y.; Ma, L.; Wu, Z.; Xia, H. Exploring a Better Adjuvant Treatment for Surgically Treated High-Grade Neuroendocrine Carcinoma of the Cervix. Gynecol. Obstet. Invest. 2022, 87, 398–405. [Google Scholar] [CrossRef]
- Boruta, D.M.; O Schorge, J.; A Duska, L.; Crum, C.P.; Castrillon, D.H.; E Sheets, E. Multimodality Therapy in Early-Stage Neuroendocrine Carcinoma of the Uterine Cervix. Gynecol. Oncol. 2001, 81, 82–87. [Google Scholar] [CrossRef]
- Kim, C.; Salvo, G.; Ishikawa, M.; Chen, T.C.; Jhingran, A.; Bhosale, P.; Ramalingam, P.; Frumovitz, M. The role of postoperative radiation after radical hysterectomy for women with early-stage neuroendocrine carcinoma of the cervix: A meta-analysis. Gynecol. Oncol. 2023, 170, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.; Meng, Y.; Wu, P.; Li, Z.; Wen, H.; Ren, F.; Zou, D.; Lu, H.; Wu, L.; Zhou, S.; et al. The prognosis of patients with small cell carcinoma of the cervix: A retrospective study of the SEER database and a Chinese multicentre registry. Lancet Oncol. 2023, 24, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-C.; Huang, H.-J.; Wang, T.-Y.; Yang, L.-Y.; Chen, C.-H.; Cheng, Y.-M.; Liou, W.-H.; Hsu, S.-T.; Wen, K.-C.; Ou, Y.-C.; et al. Primary surgery versus primary radiation therapy for FIGO stages I–II small cell carcinoma of the uterine cervix: A retrospective Taiwanese Gynecologic Oncology Group study. Gynecol. Oncol. 2015, 137, 468–473. [Google Scholar] [CrossRef]
- Margolis, B.; Tergas, A.I.; Chen, L.; Hou, J.Y.; Burke, W.M.; Hu, J.C.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L.; Wright, J.D. Natural history and outcome of neuroendocrine carcinoma of the cervix. Gynecol. Oncol. 2016, 141, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.-H.; Schultheiss, T.E.; Wong, J.Y.; Wakabayashi, M.T.; Chen, Y.-J. Surgery Versus Radiation Treatment for High-Grade Neuroendocrine Cancer of Uterine Cervix: A Surveillance Epidemiology and End Results Database Analysis. Int. J. Gynecol. Cancer 2018, 28, 188–193. [Google Scholar] [CrossRef]
- Zivanovic, O.; Leitao, M.; Park, K.; Zhao, H.; Diaz, J.; Konner, J.; Alektiar, K.; Chi, D.; Abu-Rustum, N.; Aghajanian, C. Small cell neuroendocrine carcinoma of the cervix: Analysis of outcome, recurrence pattern and the impact of platinum-based combination chemotherapy. Gynecol. Oncol. 2009, 112, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Robin, T.P.; Amini, A.; Schefter, T.E.; Behbakht, K.; Fisher, C.M. Brachytherapy should not be omitted when treating locally advanced neuroendocrine cervical cancer with definitive chemoradiation therapy. Brachytherapy 2016, 15, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]
- Frumovitz, M.; Chisholm, G.B.; Jhingran, A.; Ramalingam, P.; Flores-Legarreta, A.; Bhosale, P.; Gonzales, N.R.; Hillman, R.T.; Salvo, G. Combination therapy with topotecan, paclitaxel, and bevacizumab improves progression-free survival in patients with recurrent high-grade neuroendocrine cervical cancer: A Neuroendocrine Cervical Tumor Registry (NeCTuR) study. Am. J. Obstet. Gynecol. 2023, 228, e1–e445. [Google Scholar] [CrossRef]
- Frumovitz, M.; Westin, S.N.; Salvo, G.; Zarifa, A.; Xu, M.; Yap, T.A.; Rodon, A.J.; Karp, D.D.; Abonofal, A.; Jazaeri, A.A.; et al. Phase II study of pembrolizumab efficacy and safety in women with recurrent small cell neuroendocrine carcinoma of the lower genital tract. Gynecol. Oncol. 2020, 158, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Frumovitz, M.; Burzawa, J.K.; Byers, L.A.; Lyons, Y.A.; Ramalingam, P.; Coleman, R.L.; Brown, J. Sequencing of mutational hotspots in cancer-related genes in small cell neuroendocrine cervical cancer. Gynecol. Oncol. 2016, 141, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Cimic, A.; Vranic, S.; Arguello, D.; Contreras, E.; Gatalica, Z.; Swensen, J. Molecular Profiling Reveals Limited Targetable Biomarkers in Neuroendocrine Carcinoma of the Cervix. Appl. Immunohistochem. Mol. Morphol. AIMM 2021, 29, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Eskander, R.N.; Elvin, J.; Gay, L.; Ross, J.S.; Miller, V.A.; Kurzrock, R. Unique genomic landscape of high-grade neuroendocrine cervical carcinoma: Implications for rethinking current treatment paradigms. JCO Precis. Oncol. 2020, 4, 972–987. [Google Scholar] [CrossRef] [PubMed]
| Institution Characteristics | N | % | |
| Province | Ontario | 8 | 24 |
| Quebec | 6 | 18 | |
| British Columbia | 6 | 18 | |
| Alberta | 5 | 15 | |
| Manitoba | 3 | 9 | |
| Newfoundland | 2 | 6 | |
| Nova Scotia | 2 | 6 | |
| Saskatchewan | 2 | 6 | |
| Academic institution | Yes | 30 | 88 |
| No | 4 | 12 | |
| Specialties available | Gynecologic oncology | 32 | 94 |
| Radiation oncology | 34 | 100 | |
| Medical oncology | 33 | 97 | |
| Primary specialty that delivers systemic therapy | Gynecologic oncology | 19 | 56 |
| Medical oncology | 15 | 44 | |
| Radiation oncology | 0 | 0 | |
| Number of patients with SCNECC treated annually | 0 | 1 | 3 |
| 1 to 4 | 30 | 88 | |
| 5 to 10 | 2 | 6 | |
| >10 | 1 | 3 | |
| Practitioner Characteristics | N | % | |
| Primary specialty of practice | Gynecologic oncology | 17 | 50 |
| Medical oncology | 4 | 12 | |
| Radiation oncology | 13 | 38 | |
| Number of years in practice | ≤5 | 6 | 18 |
| 6 to 10 | 6 | 18 | |
| 11 to 15 | 11 | 32 | |
| ≥16 | 11 | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, K.Y.; Chehade, R.; Wang, A.Y.; Sachdeva, A.; MacKay, H.J.; Taggar, A.S. Pan-Canadian Analysis of Practice Patterns in Small Cell Carcinoma of the Cervix: Insights from a Multidisciplinary Survey. Curr. Oncol. 2024, 31, 2610-2619. https://doi.org/10.3390/curroncol31050196
Fan KY, Chehade R, Wang AY, Sachdeva A, MacKay HJ, Taggar AS. Pan-Canadian Analysis of Practice Patterns in Small Cell Carcinoma of the Cervix: Insights from a Multidisciplinary Survey. Current Oncology. 2024; 31(5):2610-2619. https://doi.org/10.3390/curroncol31050196
Chicago/Turabian StyleFan, Kevin Yijun, Rania Chehade, Andrew Yuanbo Wang, Anjali Sachdeva, Helen J. MacKay, and Amandeep S. Taggar. 2024. "Pan-Canadian Analysis of Practice Patterns in Small Cell Carcinoma of the Cervix: Insights from a Multidisciplinary Survey" Current Oncology 31, no. 5: 2610-2619. https://doi.org/10.3390/curroncol31050196
APA StyleFan, K. Y., Chehade, R., Wang, A. Y., Sachdeva, A., MacKay, H. J., & Taggar, A. S. (2024). Pan-Canadian Analysis of Practice Patterns in Small Cell Carcinoma of the Cervix: Insights from a Multidisciplinary Survey. Current Oncology, 31(5), 2610-2619. https://doi.org/10.3390/curroncol31050196





