Wedge Resection versus Stereotactic Body Radiation Therapy for Non-Small Cell Lung Cancer Tumors ≤8 mm
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Study Design
2.3. Statistical Analysis
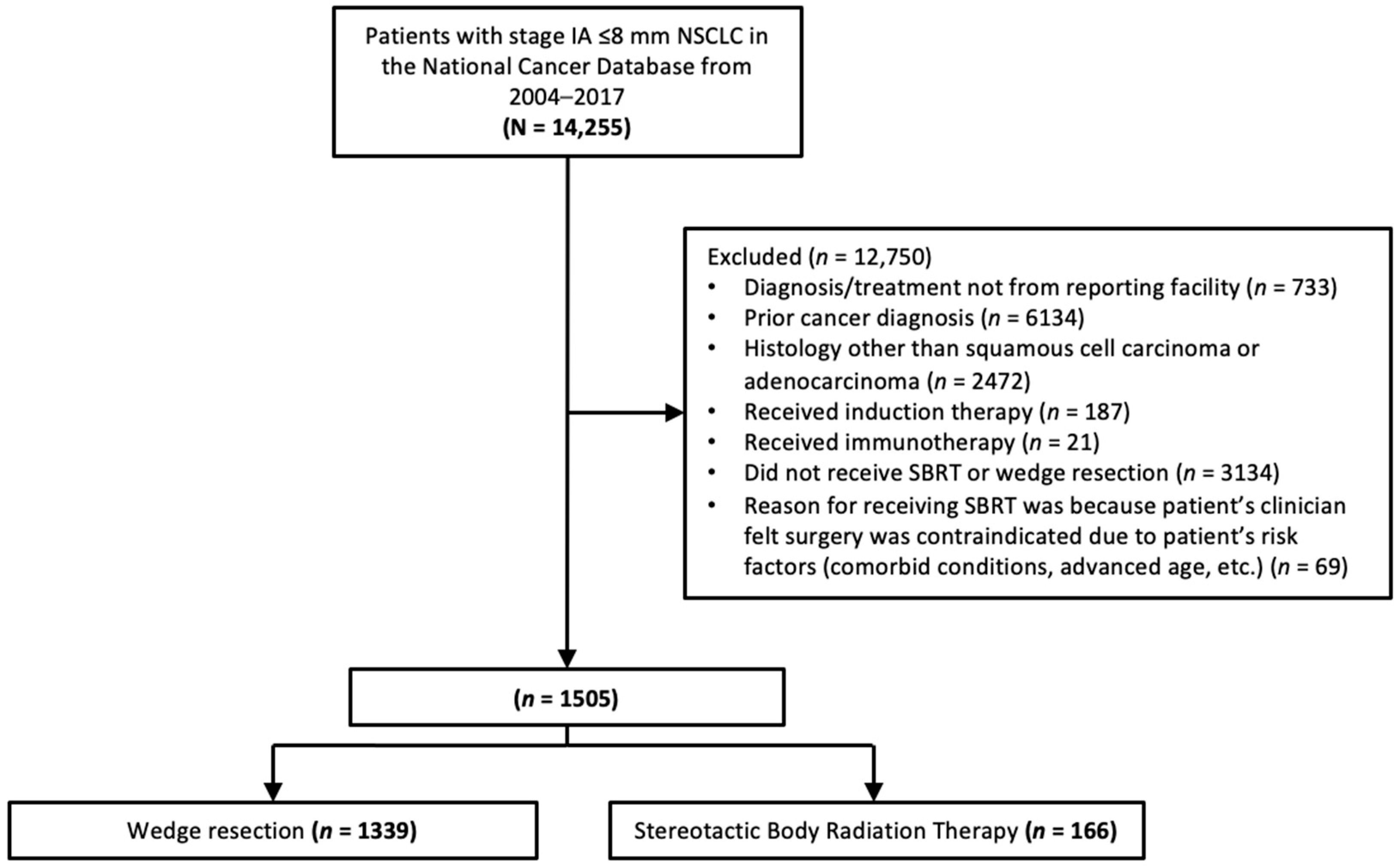
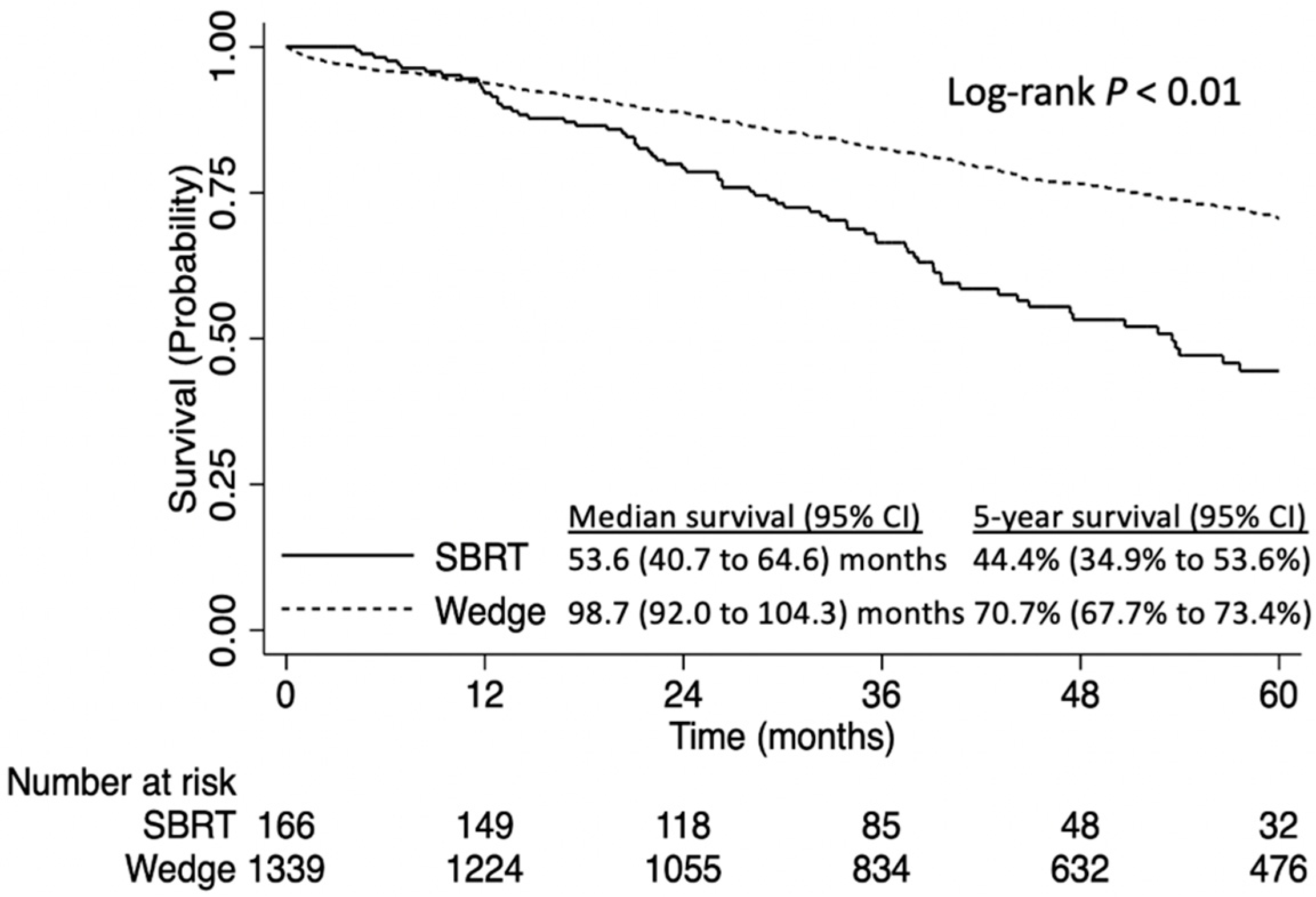
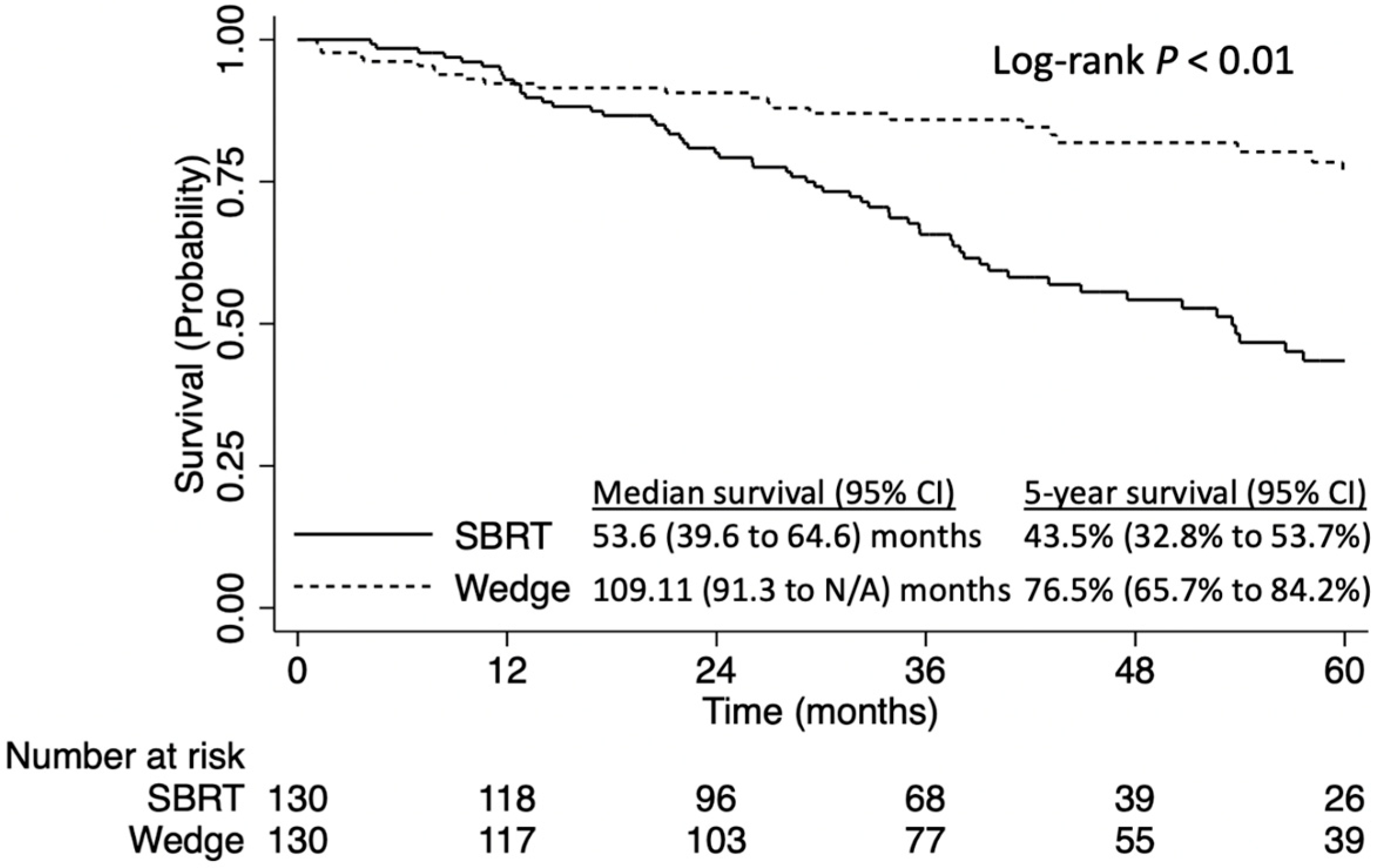
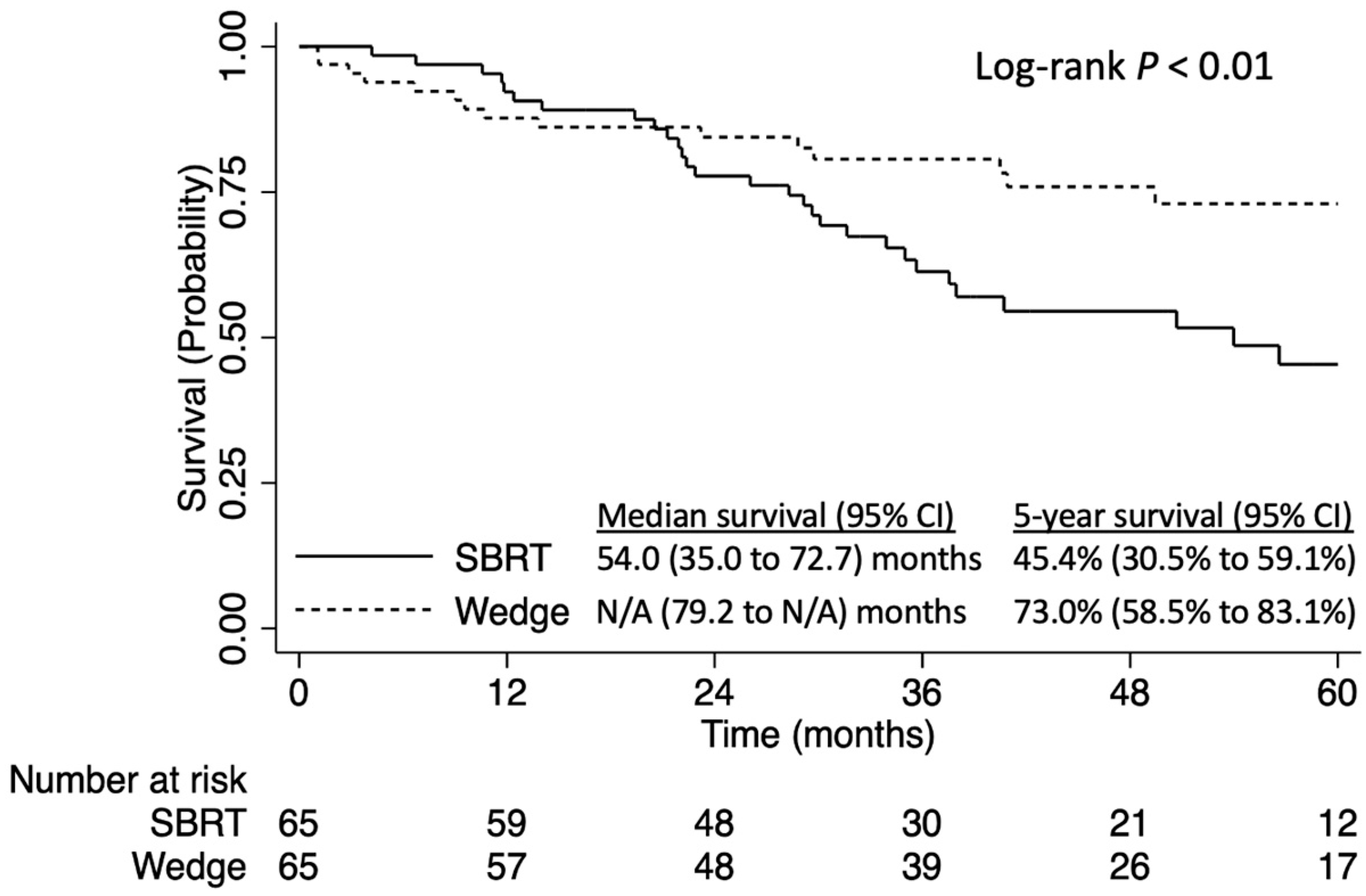
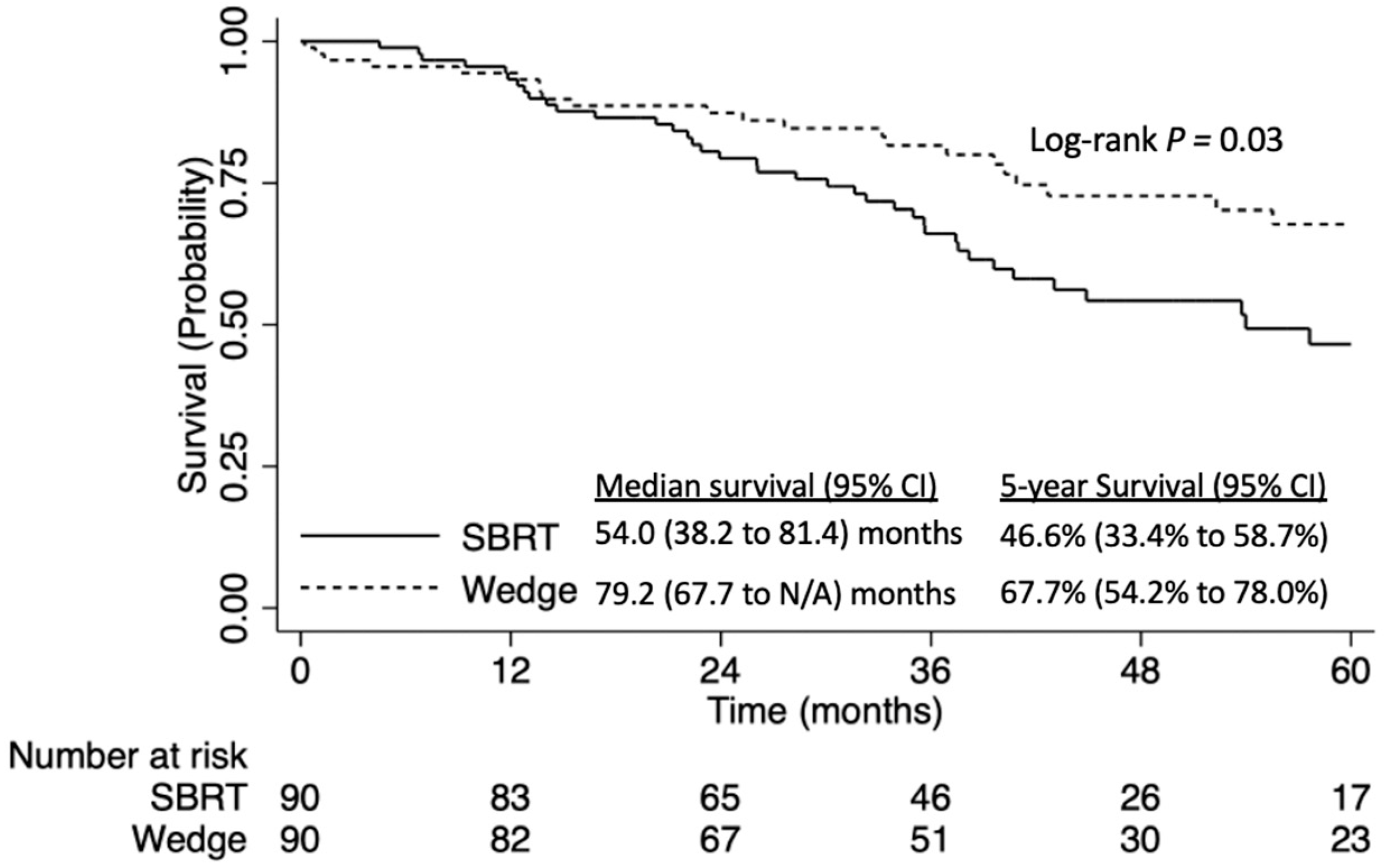
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Library of Medicine (U.S.). (2016-Present). Veterans Affairs Lung Cancer Surgery or StereotacticRadiotherapy (VALOR). Identifier: NCT02984761. Available online: https://clinicaltrials.gov/ct2/show/NCT02984761 (accessed on 19 April 2022).
- National Library of Medicine (U.S.). (2015-Present). SBRT (Stereotactic Body Radiation Therapy) vs. Surgery in High Risk Patients with Early Stage Lung Cancer. Identifier: NCT02562027. Available online: https://clinicaltrials.gov/ct2/show/NCT02562027 (accessed on 19 April 2022).
- National Library of Medicine (U.S.). (2015-Present). JoLT-Ca Sublobar Resection (SR) Versus Stereotactic Ablative Radiotherapy (SAbR) for Lung Cancer (STABLE-MATES). Identifier: NCT02468024. Available online: https://clinicaltrials.gov/ct2/show/NCT02468024 (accessed on 19 April 2022).
- National Library of Medicine (U.S.). (2012-Present). Radical Resection vs. Ablative Stereotactic Radiotherapy in Patients with Operable Stage I NSCLC (POSTILV). Identifier: NCT01753414. Available online: https://clinicaltrials.gov/ct2/show/NCT01753414 (accessed on 19 April 2022).
- Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; Sicks, J.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Peng, L.; Deng, H.Y.; Liu, Z.K.; Shang, Q.W.; Huang, K.L.; Zheng, Q.Q.; Li, W.; Wang, Y. Wedge Resection vs. Stereotactic Body Radiation Therapy for Clinical Stage I Non-small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Front. Surg. 2022, 9, 850276. [Google Scholar] [CrossRef]
- Shen, J.; Zhuang, W.; Xu, C.; Jin, K.; Chen, B.; Tian, D.; Hiley, C.; Onishi, H.; Zhu, C.; Qiao, G. Surgery or Non-surgical Treatment of ≤8 mm Non-small Cell Lung Cancer: A Population-Based Study. Front. Surg. 2021, 8, 632561. [Google Scholar] [CrossRef]
- Network, N.C.C. Non-Small Cell Lung Cancer (Version 1.2022). Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 2 November 2021).
- Lee, S.M.; Park, C.M.; Goo, J.M.; Lee, H.J.; Wi, J.Y.; Kang, C.H. Invasive pulmonary adenocarcinomas versus preinvasive lesions appearing as ground-glass nodules: Differentiation by using CT features. Radiology 2013, 268, 265–273. [Google Scholar] [CrossRef]
- Wallace, M.J.; Krishnamurthy, S.; Broemeling, L.D.; Gupta, S.; Ahrar, K.; Morello, F.A., Jr.; Hicks, M.E. CT-guided percutaneous fine-needle aspiration biopsy of small (< or =1-cm) pulmonary lesions. Radiology 2002, 225, 823–828. [Google Scholar] [CrossRef]
- Mazzone, P.J.; Lam, L. Evaluating the Patient with a Pulmonary Nodule: A Review. Jama 2022, 327, 264–273. [Google Scholar] [CrossRef]
- Nakayama, H.; Yamada, K.; Saito, H.; Oshita, F.; Ito, H.; Kameda, Y.; Noda, K. Sublobar resection for patients with peripheral small adenocarcinomas of the lung: Surgical outcome is associated with features on computed tomographic imaging. Ann. Thorac. Surg. 2007, 84, 1675–1679. [Google Scholar] [CrossRef]
- Kato, H.; Oizumi, H.; Suzuki, J.; Hamada, A.; Watarai, H.; Nakahashi, K.; Sadahiro, M. Thoracoscopic wedge resection and segmentectomy for small-sized pulmonary nodules. J. Vis. Surg. 2017, 3, 66. [Google Scholar] [CrossRef]
- Pan, H.; Rose, B.S.; Simpson, D.R.; Mell, L.K.; Mundt, A.J.; Lawson, J.D. Clinical practice patterns of lung stereotactic body radiation therapy in the United States: A secondary analysis. Am. J. Clin. Oncol. 2013, 36, 269–272. [Google Scholar] [CrossRef]
- Howington, J.A.; Blum, M.G.; Chang, A.C.; Balekian, A.A.; Murthy, S.C. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e278S–e313S. [Google Scholar] [CrossRef]
- Mallin, K.; Browner, A.; Palis, B.; Gay, G.; McCabe, R.; Nogueira, L.; Yabroff, R.; Shulman, L.; Facktor, M.; Winchester, D.P.; et al. Incident Cases Captured in the National Cancer Database Compared with Those in U.S. Population Based Central Cancer Registries in 2012–2014. Ann. Surg. Oncol. 2019, 26, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Stewart, A.K.; Winchester, D.P.; Ko, C.Y. The National Cancer Data Base: A powerful initiative to improve cancer care in the United States. Ann. Surg. Oncol. 2008, 15, 683–690. [Google Scholar] [CrossRef]
- Yang, C.F.; Kumar, A.; Gulack, B.C.; Mulvihill, M.S.; Hartwig, M.G.; Wang, X.; D’Amico, T.A.; Berry, M.F. Long-term outcomes after lobectomy for non-small cell lung cancer when unsuspected pN2 disease is found: A National Cancer Data Base analysis. J. Thorac. Cardiovasc. Surg. 2016, 151, 1380–1388. [Google Scholar] [CrossRef]
- Tian, S.; Higgins, K.A.; Curran, W.J.; Cassidy, R.J. Stereotactic body radiation therapy vs. surgery in early-stage non-small cell lung cancer: Lessons learned, current recommendations, future directions. J. Thorac. Dis. 2018, 10, 1201–1204. [Google Scholar] [CrossRef]
- Yerokun, B.A.; Yang, C.J.; Gulack, B.C.; Li, X.; Mulvihill, M.S.; Gu, L.; Wang, X.; Harpole, D.H.; D’Amico, T.A.; Berry, M.F.; et al. A national analysis of wedge resection versus stereotactic body radiation therapy for stage IA non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2017, 154, 675–686.e674. [Google Scholar] [CrossRef] [PubMed]
- Ackerson, B.G.; Tong, B.C.; Hong, J.C.; Gu, L.; Chino, J.; Trotter, J.W.; D’Amico, T.A.; Torok, J.A.; Lafata, K.; Chang, C.; et al. Stereotactic body radiation therapy versus sublobar resection for stage I NSCLC. Lung. Cancer 2018, 125, 185–191. [Google Scholar] [CrossRef]
- Albano, D.; Bilfinger, T.; Nemesure, B. 1-, 3-, and 5-year survival among early-stage lung cancer patients treated with lobectomy vs SBRT. Lung Cancer 2018, 9, 65–71. [Google Scholar] [CrossRef]
- Chi, A.; Fang, W.; Sun, Y.; Wen, S. Comparison of Long-term Survival of Patients with Early-Stage Non-Small Cell Lung Cancer after Surgery vs Stereotactic Body Radiotherapy. JAMA Netw. Open. 2019, 2, e1915724. [Google Scholar] [CrossRef]
- Lagerwaard, F.J.; Verstegen, N.E.; Haasbeek, C.J.; Slotman, B.J.; Paul, M.A.; Smit, E.F.; Senan, S. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 348–353. [Google Scholar] [CrossRef]
- Lin, Q.; Sun, X.; Zhou, N.; Wang, Z.; Xu, Y.; Wang, Y. Outcomes of stereotactic body radiotherapy versus lobectomy for stage I non-small cell lung cancer: A propensity score matching analysis. BMC Pulm. Med. 2019, 19, 98. [Google Scholar] [CrossRef]
- Mokhles, S.; Verstegen, N.; Maat, A.P.; Birim, Ö.; Bogers, A.J.; Mokhles, M.M.; Lagerwaard, F.J.; Senan, S.; Takkenberg, J.J. Comparison of clinical outcome of stage I non-small cell lung cancer treated surgically or with stereotactic radiotherapy: Results from propensity score analysis. Lung Cancer 2015, 87, 283–289. [Google Scholar] [CrossRef]
- Onishi, H.; Shirato, H.; Nagata, Y.; Hiraoka, M.; Fujino, M.; Gomi, K.; Karasawa, K.; Hayakawa, K.; Niibe, Y.; Takai, Y.; et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: Can SBRT be comparable to surgery? Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1352–1358. [Google Scholar] [CrossRef]
- Ostheimer, C.; Evers, C.; Palm, F.; Mikolajczyk, R.; Vordermark, D.; Medenwald, D. Mortality after radiotherapy or surgery in the treatment of early stage non-small-cell lung cancer: A population-based study on recent developments. J. Cancer Res. Clin. Oncol. 2019, 145, 2813–2822. [Google Scholar] [CrossRef]
- Shirvani, S.M.; Jiang, J.; Chang, J.Y.; Welsh, J.; Likhacheva, A.; Buchholz, T.A.; Swisher, S.G.; Smith, B.D. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg. 2014, 149, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Verstegen, N.E.; Oosterhuis, J.W.; Palma, D.A.; Rodrigues, G.; Lagerwaard, F.J.; van der Elst, A.; Mollema, R.; van Tets, W.F.; Warner, A.; Joosten, J.J.; et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): Outcomes of a propensity score-matched analysis. Ann. Oncol. 2013, 24, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Schipper, M.; Kidwell, K.; Lin, J.; Reddy, R.; Ren, Y.; Chang, A.; Lv, F.; Orringer, M.; Spring Kong, F.M. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: A meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Littau, M.J.; Freeman, R.; Vigneswaran, W.T.; Luchette, F.A.; Baker, M.S.; Raad, W.; Abdelsattar, Z.M. Comparative effectiveness of stereotactic body radiation therapy versus surgery for stage I lung cancer in otherwise healthy patients: An instrumental variable analysis. JTCVS Open 2022, 9, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Bai, H.X.; Chan, L.; Su, C.; Zhang, P.J.; Yang, L.; Zhang, Z. Sublobar resection compared with stereotactic body radiation therapy and ablation for early stage non-small cell lung cancer: A National Cancer Database study. J. Thorac. Cardiovasc. Surg. 2020, 160, 1350–1357.e1311. [Google Scholar] [CrossRef] [PubMed]
- Weder, W.; Moghanaki, D.; Stiles, B.; Siva, S.; Rocco, G. The great debate flashes: Surgery versus stereotactic body radiotherapy as the primary treatment of early-stage lung cancer. Eur. J. Cardiothorac. Surg. 2018, 53, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Moghanaki, D.; Simone, C.B., 2nd; Rimner, A.; Karas, T.Z.; Donington, J.; Shirvani, S.M.; Daly, M.; Videtic, G.M.; Movsas, B. The value of collaboration between thoracic surgeons and radiation oncologists while awaiting evidence in operable stage i non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2018, 155, 429–431. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristic | SBRT (n = 166) | Wedge Resection (n = 1339) | p Value |
|---|---|---|---|
| Age (median, IQR), years | 71.0 (66.0, 78.0) | 68.0 (61.0, 73.0) | <0.01 |
| Sex, n (%) | 0.24 | ||
| Male | 70 (42.2%) | 502 (37.5%) | |
| Female | 96 (57.8%) | 837 (62.5%) | |
| Race, n (%) | 0.04 | ||
| White | 153 (92.2%) | 1156 (86.3%) | |
| Black | 10 (6.0%) | 117 (8.7%) | |
| Other | 1 (0.6%) | 53 (4.0%) | |
| Unknown | 2 (1.2%) | 13 (1.0%) | |
| CDCC Score, n (%) | 0.09 | ||
| 0 | 86 (51.8%) | 603 (45.0%) | |
| 1 | 43 (25.9%) | 481 (35.9%) | |
| 2 | 27 (16.3%) | 187 (14.0%) | |
| 3+ | 10 (6.0%) | 68 (5.1%) | |
| Year of Diagnosis (median, IQR) | 2015 (2012, 2016) | 2013 (2010, 2016) | <0.01 |
| Tumor Size (median, IQR), mm | 7 (4, 8) | 7 (5, 8) | 0.69 |
| Tumor Location, n (%) | 0.07 | ||
| RUL | 60 (36.1%) | 486 (36.3%) | |
| RML | 8 (4.8%) | 48 (3.6%) | |
| RLL | 28 (16.9%) | 228 (17.0%) | |
| LUL | 53 (31.9%) | 350 (26.1%) | |
| LLL | 13 (7.8%) | 201 (15.0%) | |
| Unknown | 4 (2.4%) | 26 (1.9%) | |
| Histology, n (%) | <0.01 | ||
| Squamous cell carcinoma | 57 (34.3%) | 261 (19.5%) | |
| Adenocarcinoma | 109 (65.7%) | 1078 (80.5%) | |
| Insurance Status, n (%) | <0.01 | ||
| Uninsured | 3 (1.8%) | 19 (1.4%) | |
| Private | 18 (10.8%) | 386 (28.8%) | |
| Medicaid | 8 (4.8%) | 84 (6.3%) | |
| Medicare | 131 (78.9%) | 827 (61.8%) | |
| Other | 4 (2.4%) | 11 (0.8%) | |
| Unknown | 2 (1.2%) | 12 (0.9%) | |
| Facility Type, n (%) | 0.31 | ||
| Community cancer program | 7 (4.2%) | 52 (3.9%) | |
| Comprehensive community | 68 (41.0%) | 471 (35.2%) | |
| Academic/research program | 60 (36.1%) | 582 (43.5%) | |
| Integrated network cancer program | 31 (18.7%) | 225 (16.8%) | |
| Unknown | 0 (0.0%) | 9 (0.7%) | |
| Median Household Income, n (%) | 0.03 | ||
| First quartile | 30 (18.1%) | 192 (14.3) | |
| Second quartile | 30 (18.1%) | 234 (17.5%) | |
| Third quartile | 40 (24.1%) | 257 (19.2%) | |
| Fourth quartile | 44 (26.5%) | 514 (38.4%) | |
| Unknown | 22 (13.3%) | 142 (10.6%) |
| Patient Characteristic | Hazard Ratio | 95% CI | p Value |
|---|---|---|---|
| Age (per year) | 1.03 | 1.02, 1.04 | <0.01 |
| Female vs. male | 0.64 | 0.53, 0.78 | <0.01 |
| Race (ref = white) | |||
| Black | 0.95 | 0.66, 1.37 | 0.80 |
| Other | 0.80 | 0.39, 1.63 | 0.54 |
| Year of diagnosis (per year) | 0.94 | 0.91, 0.97 | <0.01 |
| Median household income (ref = quartile 1) | |||
| Second quartile | 0.92 | 0.68, 1.23 | 0.56 |
| Third quartile | 0.96 | 0.71, 1.29 | 0.79 |
| Fourth quartile | 0.64 | 0.48, 0.85 | <0.01 |
| Insurance type (ref = uninsured) | |||
| Private | 1.03 | 0.43, 2.46 | 0.94 |
| Medicaid | 1.17 | 0.46, 2.99 | 0.74 |
| Medicare | 1.10 | 0.46, 2.61 | 0.82 |
| Other | 0.53 | 0.13, 2.19 | 0.38 |
| Distance from facility (per mile) | 0.7 (0.4, 0.8) | 0.7 (0.5, 0.8) | 0.69 |
| Facility type (ref = community cancer program) | |||
| Comprehensive community clinic | 1.48 | 0.90, 2.43 | 0.12 |
| Academic/research program | 1.22 | 0.74, 2.00 | 0.44 |
| Integrated network cancer program | 1.30 | 0.77, 2.19 | 0.32 |
| CDCC score (ref = 0) | |||
| 1 | 1.28 | 1.03, 1.59 | 0.02 |
| 2 | 1.36 | 1.04, 1.78 | 0.03 |
| 3+ | 1.71 | 1.11, 2.63 | 0.02 |
| Squamous cell carcinoma v adenocarcinoma | 1.09 | 0.88, 1.35 | 0.43 |
| Tumor size (per cm) | 1.00 | 0.96, 1.05 | 0.95 |
| Tumor location (ref = RUL) | <0.01 | ||
| RML | 0.86 | 0.50, 1.46 | 0.56 |
| RLL | 1.25 | 0.95, 1.63 | 0.11 |
| LUL | 1.11 | 0.88, 1.39 | 0.36 |
| LLL | 1.03 | 0.75, 1.40 | 0.84 |
| Wedge Resection vs. SBRT | 0.55 | 0.42, 0.73 | <0.01 |
| Patient Characteristic | SBRT (n = 130) | Wedge Resection (n = 130) | Absolute Standardized Difference (%) | p Value |
|---|---|---|---|---|
| Age (years), median (IQR) | 71.5 (11) | 71 (11) | 6.7 | 0.64 |
| Sex, n (%) | 0.80 | |||
| Male | 56 (43.1%) | 58 (44.6%) | 3.1 | |
| Female | 74 (56.9%) | 72 (55.4%) | 3.1 | |
| Race, n (%) | 0.53 | |||
| White | 120 (92.3%) | 123 (94.6%) | 7.8 | |
| Black | 9 (6.9%) | 7 (5.4%) | 5.9 | |
| Other | 1 (0.8%) | 0 (0%) | 5.1 | |
| CDCC Score, n (%) | 0.69 | |||
| 0 | 67 (51.5%) | 76 (58.5%) | 14.5 | |
| 1 | 36 (27.7%) | 33 (25.4%) | 5.0 | |
| 2 | 20 (15.4%) | 16 (12.3%) | 8.6 | |
| 3+ | 7 (5.4%) | 5 (3.8%) | 6.6 | |
| Year of Diagnosis (median, IQR) | 2015 (2012, 2016) | 2015 (2013, 2016) | 11.5 | 0.28 |
| Tumor Size (median, IQR), mm | 7 (3) | 7 (3) | 1.6 | 0.35 |
| Tumor Location, n (%) | 0.76 | |||
| RUL | 50 (38.5%) | 52 (49.0%) | 3.2 | |
| RML | 6 (4.6%) | 10 (7.7%) | 15.1 | |
| RLL | 21 (16.2%) | 23 (17.7%) | 4.0 | |
| LUL | 42 (32.3%) | 37 (28.5%) | 8.4 | |
| LLL | 11 (8.5%) | 8 (6.2%) | 7.2 | |
| Histology, n (%) | 0.51 | |||
| Squamous cell carcinoma | 39 (30.0%) | 44 (33.8%) | 8.8 | |
| Adenocarcinoma | 91 (70.0%) | 86 (66.2%) | 8.8 | |
| Insurance Status, n (%) | 0.95 | |||
| Uninsured | <10 | <10 | 1.8 | |
| Private | 14 (10.8%) | 15 (11.5%) | 2.0 | |
| Medicaid | <10 | <10 | 6.7 | |
| Medicare | 103 (79.2%) | 102 (78.5%) | 1.7 | |
| Other | <10 | <10 | 6.0 | |
| Facility Type, n (%) | 0.78 | |||
| Community cancer program | 5 (3.8%) | 3 (2.3%) | 7.7 | |
| Comprehensive community | 51 (39.2%) | 55 (42.3%) | 6.3 | |
| Academic/research program | 52 (40.0%) | 54 (41.5%) | 3.1 | |
| Integrated network cancer program | 22 (16.9%) | 18 (13.8%) | 8.1 | |
| Median Household Income, n (%) | 0.50 | |||
| First quartile | 26 (20.0%) | 30 (23.1%) | 8.4 | |
| Second quartile | 27 (20.8%) | 19 (14.6%) | 15.3 | |
| Third quartile | 36 (27.7%) | 33 (25.4%) | 5.4 | |
| Fourth quartile | 41 (31.5%) | 48 (36.9%) | 11.2 |
| Patient Characteristic | Hazard Ratio | 95% CI | p Value |
|---|---|---|---|
| Age (per year) | 1.03 | 1.00, 1.05 | 0.02 |
| Female vs. male | 0.59 | 0.43, 0.81 | <0.01 |
| Race (ref = white) | |||
| Black | 0.80 | 0.41, 1.56 | 0.52 |
| Other | 0.63 | 0.19, 2.07 | 0.45 |
| Year of diagnosis (per year) | 0.96 | 0.91, 1.01 | 0.08 |
| Median household income (ref = quartile 1) | |||
| Second quartile | 0.72 | 0.42, 1.22 | 0.23 |
| Third quartile | 0.86 | 0.53, 1.39 | 0.54 |
| Fourth quartile | 0.52 | 0.32, 0.84 | <0.01 |
| Insurance type (ref = uninsured) | |||
| Private | 1.93 | 0.26, 14.60 | 0.52 |
| Medicaid | 1.94 | 0.22, 17.01 | 0.55 |
| Medicare | 2.70 | 0.35, 20.77 | 0.34 |
| Other | 1.88 | 0.16, 21.62 | 0.61 |
| Distance from facility (per mile) | 1.00 | 1.00, 1.00 | 0.62 |
| Facility type (ref = community cancer program) | |||
| Comprehensive community clinic | 2.20 | 1.00, 4.86 | 0.05 |
| Academic/research program | 1.77 | 0.79, 3.94 | 0.17 |
| Integrated network cancer program | 2.12 | 0.93, 4.86 | 0.07 |
| Squamous cell carcinoma vs. adenocarcinoma | 1.15 | 0.81, 1.64 | 0.44 |
| Tumor size (per mm) | 1.03 | 0.96, 1.11 | 0.38 |
| Tumor location (ref = RUL) | |||
| RML | 1.59 | 0.79, 3.19 | 0.19 |
| RLL | 1.18 | 0.76, 1.84 | 0.47 |
| LUL | 1.18 | 0.80, 1.72 | 0.40 |
| LLL | 1.33 | 0.82, 2.14 | 0.23 |
| Wedge Resection vs. SBRT | 0.51 | 0.34, 0.77 | <0.01 |
| Patient Characteristic | SBRT (n = 65) | Wedge Resection (n = 65) | Absolute Standardized Difference (%) | p Value |
|---|---|---|---|---|
| Age (years), median (IQR) | 74 (12) | 75 (9) | 16.1 | 0.31 |
| Sex, n (%) | 0.60 | |||
| Male | 27 (42.0%) | 30 (46.0%) | 9.6 | |
| Female | 38 (58.0%) | 35 (54.0%) | 9.6 | |
| Race, n (%) | 0.93 | |||
| White | 61 (94.0%) | 60 (92.0%) | 5.4 | |
| Black | 3 (5.0%) | 4 (6.0%) | 6.5 | |
| Other | 1 (2.0%) | 1 (2.0%) | 0.0 | |
| Year of Diagnosis (median, IQR) | 2014 (2012, 2016) | 2015 (2012, 2016) | 5.8 | 0.91 |
| Tumor Size (median, IQR), mm | 7 (3) | 7 (2) | 14.7 | 0.97 |
| Tumor Location, n (%) | 0.70 | |||
| RUL | 28 (43.0%) | 34 (52.0%) | 19.0 | |
| RML | 6 (9.0%) | 4 (6.0%) | 12.8 | |
| RLL | 9 (14.0%) | 10 (15.0%) | 4.1 | |
| LUL | 20 (31.0%) | 15 (23.0%) | 17.1 | |
| LLL | 2 (3.0%) | 2 (3.0%) | 0.0 | |
| Histology, n (%) | 0.85 | |||
| Squamous cell carcinoma | 21 (32.0%) | 22 (34.0%) | 3.6 | |
| Adenocarcinoma | 44 (68.0%) | 43 (66.0%) | 3.6 | |
| Insurance Status, n (%) | 0.99 | |||
| Uninsured | <10 | <10 | 3.4 | |
| Private | <10 | <10 | 3.7 | |
| Medicaid | 54 (83.0%) | 55 (85.0%) | 0.0 | |
| Medicare | <10 | <10 | 3.4 | |
| Other | 0.99 | |||
| Facility Type, n (%) | 0.67 | |||
| Community cancer program | 3 (5.0%) | 3 (5.0%) | 0.0 | |
| Comprehensive community | 24 (37.0%) | 18 (28.0%) | 19.6 | |
| Academic/research program | 27 (42.0%) | 29 (45.0%) | 6.2 | |
| Integrated network cancer program | 11 (17.0%) | 15 (23.0%) | 16.2 | |
| Median Household Income, n (%) | 0.82 | |||
| First quartile | 8 (12.0%) | 10 (15.0%) | 9.5 | |
| Second quartile | 14 (22.0%) | 11 (17.0%) | 11.6 | |
| Third quartile | 17 (26.0%) | 20 (31.0%) | 10.5 | |
| Fourth quartile | 26 (40.0%) | 24 (37.0%) | 6.3 |
| Patient Characteristic | Hazard Ratio | 95% CI | p Value |
|---|---|---|---|
| Age (per year) | 1.03 | 1.02, 1.04 | <0.01 |
| Female vs. male | 0.64 | 0.53, 0.78 | <0.01 |
| Race (ref = white) | |||
| Black | 0.95 | 0.66, 1.37 | 0.80 |
| Other | 0.80 | 0.39, 1.63 | 0.54 |
| Year of diagnosis (per year) | 0.94 | 0.91, 0.97 | <0.01 |
| CDCC score (ref = 0) | |||
| 1 | 1.28 | 1.03, 1.58 | 0.02 |
| 2 | 1.36 | 1.04, 1.78 | 0.03 |
| 3+ | 1.71 | 1.11, 2.64 | 0.02 |
| Median household income (ref = quartile 1) | |||
| Second quartile | 0.92 | 0.68, 1.23 | 0.56 |
| Third quartile | 0.96 | 0.71, 1.29 | 0.79 |
| Fourth quartile | 0.64 | 0.48, 0.85 | <0.01 |
| Insurance type (ref = uninsured) | |||
| Private | 1.03 | 0.43, 2.46 | 0.94 |
| Medicaid | 1.17 | 0.46, 2.99 | 0.74 |
| Medicare | 1.10 | 0.46, 2.61 | 0.83 |
| Other | 0.53 | 0.13, 2.19 | 0.38 |
| Distance from facility (per mile) | 1.00 | 1.00, 1.00 | 0.30 |
| Facility type (ref = community cancer program) | |||
| Comprehensive community clinic | 1.48 | 0.90, 2.43 | 0.12 |
| Academic/research program | 1.22 | 0.74, 2.01 | 0.44 |
| Integrated network cancer program | 1.30 | 0.77, 2.19 | 0.32 |
| Squamous cell carcinoma vs. adenocarcinoma | 1.09 | 0.88, 1.35 | 0.43 |
| Tumor size (per mm) | 1.00 | 0.96, 1.05 | 0.95 |
| Tumor location (ref = RUL) | |||
| RML | 0.86 | 0.50, 1.46 | 0.57 |
| RLL | 1.25 | 0.95, 1.64 | 0.11 |
| LUL | 1.11 | 0.88, 1.40 | 0.36 |
| LLL | 1.03 | 0.76, 1.41 | 0.84 |
| Wedge Resection vs. SBRT | 0.55 | 0.42, 0.73 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansur, A.; Saleem, Z.; Beqari, J.; Mathey-Andrews, C.; Potter, A.L.; Cranor, J.; Nees, A.T.; Srinivasan, D.; Yang, M.E.; Yang, C.-F.J.; et al. Wedge Resection versus Stereotactic Body Radiation Therapy for Non-Small Cell Lung Cancer Tumors ≤8 mm. Curr. Oncol. 2024, 31, 1529-1542. https://doi.org/10.3390/curroncol31030116
Mansur A, Saleem Z, Beqari J, Mathey-Andrews C, Potter AL, Cranor J, Nees AT, Srinivasan D, Yang ME, Yang C-FJ, et al. Wedge Resection versus Stereotactic Body Radiation Therapy for Non-Small Cell Lung Cancer Tumors ≤8 mm. Current Oncology. 2024; 31(3):1529-1542. https://doi.org/10.3390/curroncol31030116
Chicago/Turabian StyleMansur, Arian, Zain Saleem, Jorind Beqari, Camille Mathey-Andrews, Alexandra L. Potter, James Cranor, Alexandra T. Nees, Deepti Srinivasan, Margaret E. Yang, Chi-Fu Jeffrey Yang, and et al. 2024. "Wedge Resection versus Stereotactic Body Radiation Therapy for Non-Small Cell Lung Cancer Tumors ≤8 mm" Current Oncology 31, no. 3: 1529-1542. https://doi.org/10.3390/curroncol31030116
APA StyleMansur, A., Saleem, Z., Beqari, J., Mathey-Andrews, C., Potter, A. L., Cranor, J., Nees, A. T., Srinivasan, D., Yang, M. E., Yang, C.-F. J., & Auchincloss, H. G. (2024). Wedge Resection versus Stereotactic Body Radiation Therapy for Non-Small Cell Lung Cancer Tumors ≤8 mm. Current Oncology, 31(3), 1529-1542. https://doi.org/10.3390/curroncol31030116





