Abstract
Over half of all new cancer cases in Alberta are diagnosed among people aged 65+ years, a group that encompasses vast variation. Patient-reported experience measures are routinely collected within Cancer Care Alberta; however, the specific consideration of the needs and concerns of older Albertans with cancer is lacking. In 2021, 2204 adults who had received treatment at a cancer centre in Alberta completed the Ambulatory Oncology Patient Satisfaction Survey (AOPSS). In this study, we explored the age differences in satisfaction across six dimensions of person-centred care and in the proportions of unmet needs across eight types of issues, with specific attention to older adults. Using three age groups (18–39, 40–64, 65+), only the physical comfort dimension showed significantly lower satisfaction among those aged 65+ years. Using five age groups (18–39, 40–64, 65–74, 75–84, 85+), significantly lower levels of satisfaction were found related to ‘physical comfort’ for those aged 65–74 and 75–84, ‘coordination and continuity of care’ for those aged 75–84 and 85+, and ‘information, communication, and education’ for those aged 85+. Therefore, grouping together all older adults aged 65+ years obscured lower levels of satisfaction with some dimensions of person-centred care among those aged 75–84 and 85+ years. Unmet needs generally increased with age for all types of issues, with significant differences across age groups for emotional, financial, social/family, and sexual health issues. The lower levels of satisfaction and higher proportions of unmet needs call for tailored interventions to promote optimal care experiences and outcomes among older adults receiving cancer care in Alberta and their families.
1. Introduction
In Alberta, Canada, 57% of new cancer cases were diagnosed among people aged 65+ years in 2021 [1]. Age-related health, functional, psychosocial, and existential changes [2] impact cancer care experiences, preferences, and outcomes [3,4,5]; however, little programmatic attention has been given to the unique concerns of this population in Alberta. Documented disparities among older adults with cancer, including over- and under-treatment [6,7], slower improvements in survival [8,9], unmet needs [10,11,12], and a lack of research [13,14,15], suggest that age-related changes may not be adequately addressed in cancer care. Given that the number of older Albertans has more than doubled in the past 20 years and is expected to nearly double again in the next 20 years [16], Cancer Care Alberta must strategically prepare to address older adults’ particular care needs.
Patient-reported experience measures (PREMs) provide insights into patients’ perceptions of their personal care experiences. They play a critical role in health system quality improvement through the production of knowledge to inform the development of health practices and policies that align with patient experiences and needs [17]. Specifically, PREMs can be used to address inequalities in care experiences, identifying groups of patients who report poorer experiences of care to prioritize initiatives that optimize experiences and outcomes [18,19].
The Ambulatory Oncology Patient Satisfaction Survey (AOPSS) [20,21] is a PREM used across many Canadian provinces to assess patients’ cancer care experiences. Since 2004, the AOPSS has been distributed every two years to people receiving cancer care in Alberta. Analysis has provided important insights to inform quality improvement initiatives and cancer care innovations in Alberta [22,23,24] and in other provinces [25,26,27]; however, little consideration has been given to the age-specific experiences of older adults with cancer.
In AOPSS analyses, adults aged 65+ are often grouped together, creating a single group that typically includes more than half of all respondents [21,22]. However, the life stage and experiences of a 65-year-old may differ greatly from those of a 75- or 85-year-old. Therefore, sociologists break down the older adult population into life-stage subgroups, often identified as the young–old (65–74 years), the middle–old (75–84 years), and the old–old (85+ years) [28]. Although age-related changes happen at different times and in different ways for different people [2], resulting in vast heterogeneity in health and functional status among older adults within each of these chronological groups [29], the consideration of the differences among these age groups begins to recognize the heterogeneity among older adults. Studies of patient satisfaction with cancer care in other jurisdictions that break down the older adult population into subgroups have shown lower levels of satisfaction in very old adults [18]. However, differences in healthcare context and available resources can have an important impact on satisfaction. Little is known about cancer care satisfaction among subgroups of older adults in the Canadian—and specifically the Alberta—context.
Therefore, the purpose of this study was to better understand the concerns and needs of older Albertans with cancer through a retrospective age analysis of the 2021 Alberta AOPSS. Specifically, we explored age differences in satisfaction across six dimensions of person-centred care and in the proportion of unmet needs across eight types of issues among adults receiving cancer care in Alberta, with particular attention to older adults. The findings may be used to inform the implementation of health system innovations that improve the experiences and outcomes of older people with cancer in Alberta and their families.
2. Materials and Methods
2.1. Design
We used a retrospective exploratory design to conduct a secondary age analysis of the 2021 AOPSS Alberta dataset.
2.2. Procedure
In Alberta, the AOPSS was distributed from February to May 2021. In February, potential respondents received a package in the mail containing an information sheet; a paper copy of the survey; a self-addressed, stamped return envelope; and a link to an online version of the survey with a unique patient identifier code. In March, a reminder was sent to those who had not yet returned the survey.
2.3. Respondents
A total of 4000 survey packages were mailed to patients with a cancer diagnosis who had received at least one systemic (intravenous or oral route) or radiation treatment at one of the 17 ambulatory cancer centres in Alberta in the previous 6 months. Some participants may have received both types of treatment. Eligible patients were identified from the Alberta Cancer Registry. A random sample of eligible patients was taken for the 2 metro cancer centres in Calgary and Edmonton. To ensure adequate representation of those living in smaller urban, rural, and remote areas, a census sample of patients was taken for the 4 regional and 11 community cancer centres.
2.4. Measure
The AOPSS was developed and nationally validated by the National Research Corporation (NRC) in 2003 [20]. After minor changes, the revised tool was again validated in 2012 [21]. Administered across many jurisdictions in Canada, the NRC managed the survey and maintained a national dashboard of results until 2023.
The AOPSS contains 97 questions [20,21,30]. Of these, 82 questions address experiences across the trajectory of cancer care. From these, the NRC identified 44 core questions to construct six validated dimensions of person-centred care, including (1) respect for patient preferences; (2) physical comfort; (3) access to care; (4) coordination and continuity of care; (5) information, communication, and education; and (6) emotional support [20,21]. The AOPSS also includes a question asking, ‘Did you get all of the help you wanted to cope with the following? (a) Practical issues (e.g., transportation, accommodation), (b) Financial issues (e.g., costs of treatments), (c) Social/family issues (e.g., worry about friends and family), (d) Emotional issues (e.g., fears and worries, sadness), (e) Spiritual issues (meaning/purpose of life, faith), (f) Informational issues (e.g., understanding your illness, talking with the healthcare team), (g) Physical issues (e.g., pain, fatigue), (h) Sexual health issues’ (No; Yes, somewhat; Yes, mostly; and Yes, definitely). In addition, a question about the overall rating of care asked, ‘Overall, how would you rate the quality of care at [cancer centre name] in the past 6 months?’ (Excellent, Very Good, Good, Fair, Poor) [21]. In the 2021 AOPSS, five Alberta-specific questions addressed experiences and satisfaction with virtual care, the goal of treatment, prognosis and advance care planning, and the involvement of the family physician. The survey ends with seven sociodemographic questions and an open-ended question for any other comments the respondent would like to make about their cancer care services.
Respondents chose to complete and return a paper copy of the survey or to complete the survey online. A contact phone number was provided to answer any survey-related questions.
In addition to the AOPSS survey data, the following associated cancer registry data were used: age (at survey distribution), tumor group, type of treatment received, cancer centre, and the first three digits of each respondent’s home postal code (forward sortation area, FSA).
2.5. Analysis
We selected the age groups for our analysis based on accepted definitions, ensuring that all groups included 50 or more respondents. In Alberta, young adults with cancer are defined as those aged 15–39 years [31] and older adults are typically defined as those aged 65+ years [32,33]. We used established sociological definitions to further categorize older adults as young–old (65–74 years), middle–old (75–84 years), and old–old (85+ years) [28].
The location data available were limited to the FSA for each respondent’s home address. With the assistance of a data and geospatial resources specialist, we used Geographic Information System (GIS) software, ArcGIS Pro version 2.9, to determine rurality by associating each FSA with the largest overlapping Alberta Health Services 2018 Official Local Geographic Area (LGA) and its corresponding classification on the Alberta Health Services Rural–Urban Continuum [34]. The following groupings were used for the rural–urban continuum classifications: ‘metro’ included metro centres (Edmonton, Calgary) and metro-influenced areas; ‘urban’ included only urban centres (Grand Prairie, Fort McMurray, Red Deer, Lethbridge, Medicine Hat); ‘rural and remote’ included moderately urban-influenced areas, large rural centres and surrounding areas, rural areas, and rural remote areas [34].
The sociodemographic, health, and clinical characteristics of the patients were analyzed using descriptive statistics. We tested for significant differences across age groups using Pearson’s chi-square tests for nominal variables and independent-samples Kruskal–Wallis tests for ordinal variables. For these tests, we used pairwise (test-by-test) deletion of respondents with missing data. For variables tested with Pearson chi-square tests, if the cells had an expected count of 5 or less, the response levels were combined or Monte Carlo estimates of exact p-values were used. For Pearson’s chi-square tests, we performed post hoc tests using z tests for independent proportions. For independent-samples Kruskal–Wallis tests, we performed post hoc tests using pairwise comparisons. In both cases, p-values were adjusted using the Bonferroni correction for multiple tests.
The primary outcome of interest was person-centred care, assessed using the six dimensions listed above. We treated the scores for each dimension as continuous variables. To investigate the impact of the patients’ age group on perceived person-centred care, we conducted a one-way multivariate analysis of variance (MANOVA). The MANOVA model allowed for a single statistical test across all six dimensions of person-centred care, reducing the risk of false positive results, which can occur when conducting separate tests for each dimension. For the MANOVA, we used listwise deletion of respondents with missing data on any dimension. The equality of covariance matrices was tested using Box’s test and the equality of error variances was tested using Levene’s test based on the median, which is more robust than the mean for skewed data [35]. If a significant main effect of the age group was observed, further post hoc tests were performed using Fisher’s least significant difference test to determine the specific differences for each dimension between age groups. The first one-way MANOVA investigated the impact of using a three-level age grouping (18–39, 40–64, and 65+) as the independent variable on the six person-centred dimension scores, which served as the dependent variables. The second one-way MANOVA investigated the impact of using a five-level age grouping (18–39, 40–64, 65–74. 75–84, and 85+) as the independent variable on the six person-centred dimension scores, which served as the dependent variables.
We used independent-samples Kruskal–Wallis tests to compare unmet needs and the overall rating of satisfaction with cancer centre care, which were constructed as ordinal variables using all response categories, across age groups. For these tests, we used pairwise (test-by-test) deletion of respondents with missing data. Post hoc pairwise comparisons were conducted when we found a significant difference across age groups, with p-values adjusted using the Bonferroni correction for multiple tests.
Initial data cleaning, exploration, and figure creation were done using Microsoft Excel for Microsoft 365 MSO (Version 2302). For statistical tests, the data were exported to IBM SPSS Statistics (Version 25 or 27) for analysis, and a predetermined level of statistical significance was set at p < 0.05.
3. Results
Of the 4000 surveys distributed, 39 (1.0%) were undeliverable and 2204 were returned, giving a 55.6% response rate.
3.1. Sociodemographic, Health, and Clinical Characteristics
3.1.1. Age Distribution of AOPSS Respondents
The age distribution of the survey respondents (Figure 1a) reflects the age distribution of new cancer diagnoses in Alberta (Figure 1b) and of people with cancer who attended a Cancer Care Alberta facility (Figure 1c) in 2021, with a notable over-representation of those aged 65–74 years and under-representation of the youngest and oldest groups.
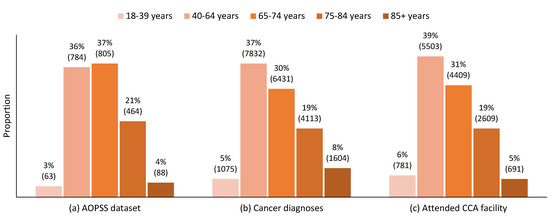
Figure 1.
The 2021 comparative age distributions of (a) Ambulatory Oncology Patient Satisfaction Survey (AOPSS) respondents, (b) new cancer diagnoses in Alberta, and (c) people diagnosed with cancer that attended a Cancer Care Alberta facility. Note: n for each group is indicated in brackets.
3.1.2. Sociodemographic and Health Characteristics
There were significant differences across age groups for sex, education, the person who completed the survey, and self-rated health (Table 1). Post hoc pairwise comparisons between age groups are presented in Appendix A, Table A1. The oldest age group (85+ years) had the highest proportion of male respondents (n = 51, 58.0%) and of respondents with less than high school education (n = 35, 39.8%). Although not significantly different across age groups, the highest proportion of those living in rural and remote areas was among those aged 65–74 years (n = 404. 50.2%), closely followed by those aged 85+ years (n = 44, 50.0%) and 75–84 years (n = 227, 48.9%).

Table 1.
Distribution of sociodemographic and health characteristics by age group.
The proportion of surveys completed with help or by someone other than the patient in the 85+ age group (n = 36, 36.4%) was significantly higher than for all other age groups and that in the 75–84 age group (n = 82, 17.7%) was significantly higher than the 40–64 and 65–74 age groups (Table 1 and Table A1).
Generally, the proportion of those reporting excellent or very good health decreased with age, while the proportion of those reporting fair or poor health increased with age (Table 1). Specifically, those aged 85+ reported significantly poorer health than those aged 18–39, 40–64, and 65–74 years (Table A1). Notably, the proportion of those reporting good health remained relatively constant across age groups (Table 1).
3.1.3. Clinical Characteristics
There were statistically significant differences across age groups with respect to the tumor site, time since diagnosis, type of cancer centre, involvement of the family doctor, treatment intent, and type of treatment(s) received (for IV and oral chemotherapy) (Table 2). Post hoc pairwise comparisons between age groups are presented in Appendix A, Table A2. In the younger age groups (18–39 years and 40–64 years), the highest proportion of respondents had breast cancers (n = 29, 46.0% and n = 248, 31.6%, respectively). In the older age groups (65–74, 75–84, and 85+ years), the highest proportion of respondents had hematological cancers and the proportion increased with age (n = 214, 26.6%; n = 153, 33.0%; and n = 39, 44.3%, respectively). This was also reflected in the post hoc comparisons (Table A2). Among the oldest respondents (85+ years), most had been living with cancer for two or more years (n = 58, 65.9%), whereas, among the youngest respondents (18–39 years), most had received their diagnosis within the past year (n = 35, 55.6%). Accordingly, those aged 18–39 years had a significantly shorter time since diagnosis than all other age groups and those aged 85+ years had a significantly longer time since diagnosis than all other age groups (Table A2).

Table 2.
Distribution of clinical characteristics by age group.
The young adult group (18–39 years) had the highest proportion of respondents receiving care at a metropolitan cancer centre (Table 2); however, the post hoc comparisons did not show any significant differences between age groups (Table A2). The proportions of those receiving care at regional cancer centres were, however, significantly higher among those aged 65–74 years (n = 246, 30.6%) and 75–84 years (n = 154, 33.2%) than those aged 40–64 years (n = 181, 23.1%) (Table 1 and Table A1). The highest proportion of those receiving care at community (rural) cancer centres was in the middle-aged group (40–64 years; n = 122, 15.6%); however, the post hoc comparisons again did not show any significant differences across age groups (Table A2).
There was an increase with age in the proportion of respondents who reported that their family doctor was very involved and the proportion of respondents unsure about their family doctor’s involvement also increased with age (Table 2). However, the only significant differences were in those aged 40–64 years having significantly less family doctor involvement than those aged 75–84 and 85+ years (Table A2).The proportion of respondents reporting a treatment intent of control, rather than cure, also increased with age, with almost two thirds (n = 54, 61.4%) of those in the oldest group (85+ years) reporting control as the treatment intent (Table 2), a significantly greater proportion than all other age groups (Table A2). Interestingly, the oldest age group also showed the highest proportion of respondents who were unsure about their treatment intent (n = 6, 6.8%) or left the question unanswered (n = 12, 13.6%).
3.2. Dimensions of Person-Centred Care
3.2.1. Three-Level Age Grouping
For the six dimensions of person-centred care, when three age groups were used, the differences across age groups primarily pointed towards lower satisfaction for young adults (18–39 years, Figure 2). For the MANOVA, we included 781 respondents who had complete data across all dimensions (18–39 years, n = 39; 40–64 years, n = 361; 65+ years, n = 381). The MANOVA results showed a statistically significant difference in perceived person-centred care based on patients’ three-level age groups, Pillai’s trace = 0.032, F(12, 1548) = 2.104, p = 0.014. The assumption of equality of covariance matrices in MANOVA was not violated (Box’s M = 51.3, p = 0.198). Although Levene’s median-based test for equal variances suggested that the assumption of homogeneity of variances was met in five dimensions, it was violated for the ‘physical comfort’ dimension, F(2, 778) = 5.50, p = 0.004. Nevertheless, we proceeded with the MANOVA, considering its robustness against slight violations of this assumption [36] and reporting Pillai’s trace, which is the most robust test statistic when assumptions are violated [36,37].
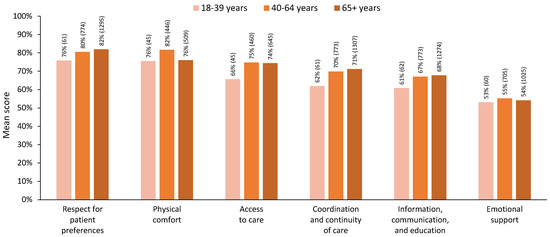
Figure 2.
Mean scores for each dimension of person-centred care by age group, using three age groups. Note: All respondents with valid dimension scores are included in this figure; n for each group is indicated in brackets.
Post hoc comparisons showed significantly lower levels of satisfaction for younger adults in the ‘access to care’ and ‘coordination and continuity of care’ dimensions (p < 0.05). Satisfaction for older adults aged 65+ was similar to or higher than satisfaction in the younger age groups for all dimensions except physical comfort, in which satisfaction for those 65+ years was significantly lower than for those aged 40–64 years (p < 0.05). Detailed statistics are presented in Table 3.

Table 3.
MANOVA results for dimensions of person-centred care using three age groups.
3.2.2. Five-Level Age Grouping
When five age groups were used to explore the age differences among the dimensions of person-centred care, a different story emerged. Decreasing patterns of satisfaction for older adults aged 75–84 years were evident across most dimensions, and those aged 85+ years showed levels of satisfaction lower than those aged 65–74 years on all dimensions of person-centred care (Figure 3). For the MANOVA, we included 781 respondents who had complete data across all dimensions (18–39 years, n = 39; 40–64 years, n = 361; 65–74 years, n = 264; 75–84 years, n = 105; 85+ years, n = 12). The MANOVA results showed a statistically significant difference in perceived person-centred care based on a patient’s five-level age group, Pillai’s trace = 0.059, F(24, 3096) = 1.94, p = 0.004. The assumption of equality of covariance matrices in the MANOVA was met (Box’s M = 95.2, p = 0.373). Although Levene’s median-based test for equal variances suggested that the assumption of homogeneity of variances was met in four dimensions, it was violated for the ‘physical comfort’ dimension, F(4, 776) = 2.98 p = 0.019, and the ‘emotional support’ dimension, F(4, 776) = 2.78, p = 0.026. Nevertheless, we proceeded with the MANOVA, considering its robustness against slight violations of this assumption [36] and reporting Pillai’s trace, which is the most robust test statistic when assumptions are violated [36,37].
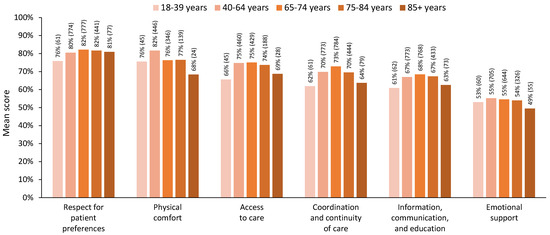
Figure 3.
Mean score for each dimension of person-centred care scores by age group, using five age groups. Note: All respondents with valid dimension scores are included in this figure; n for each group is indicated in brackets.
Post hoc comparisons showed significantly lower satisfaction for those aged 75–84 and 85+ years on several dimensions. Specifically, older adults aged 75–84 years and 85+ years showed significantly lower satisfaction in the ‘coordination and continuity of care’ dimension than adults aged 40–64 years (p < 0.05). Older adults aged 85+ years also showed significantly lower satisfaction in this dimension than adults aged 65–74 years (p < 0.05). Moreover, adults aged 85+ years showed significantly lower satisfaction than those aged 40–64, 65–74, and 75–84 years on the ‘information, communication, and education’ dimension (p < 0.05). Consistent with the three-level age group analysis, older adults aged 65–74 years and 75–84 years showed significantly lower levels of satisfaction on the ‘physical comfort’ dimension than adults aged 40–64 (p < 0.05). Adults aged 85+ had the lowest level of satisfaction on the ‘physical comfort’ dimension; however, this did not show significance in the post hoc tests due to the smaller sample size. Detailed statistics are presented in Table 4.

Table 4.
MANOVA results for dimensions of person-centred care using five age groups.
3.3. Unmet Needs
When respondents were asked if they had received the help that they wanted related to eight types of issues, the proportion of respondents who answered ‘no’ generally increased with age across all types of issues (Figure 4). The highest proportion of unmet needs was found among those aged 75–84 and/or 85+ years across all types of issues (Figure 4). The differences in responses across age groups were significant for emotional, financial, social/family, and sexual health issues (p < 0.05). The differences across age groups for practical and spiritual issues were also nearing statistical significance (p = 0.099 and p = 0.069, respectively). Detailed statistics for the significance testing, including all response categories, and pairwise comparisons are reported in Table 5.
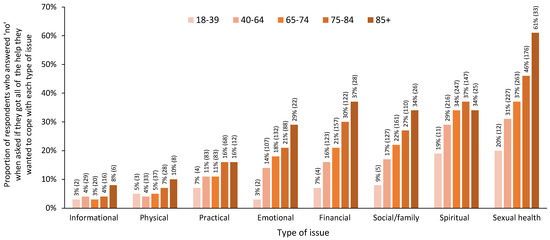
Figure 4.
Proportion of respondents reporting that they did not receive the help that they wanted across eight types of issues by age group, using five age groups. Note: For simplicity, only the proportion of ‘no’ responses for each age group is represented in this figure; n for each group is indicated in brackets. Detailed statistics for significance testing, including all response categories, are presented in Table 5.

Table 5.
Independent-samples Kruskal–Wallis test results for receiving the help that respondents wanted across eight types of issues by age group, using five age groups.
3.4. Overall Ratings of Care across Five Age Groups
There was a statistically significant difference across age groups when respondents were asked about their overall quality of care, H(4) = 12.84, p = 0.012. The post hoc pairwise comparisons demonstrated that those aged 85+ years reported significantly lower satisfaction with their overall quality of care than those aged 65–74 years (p < 0.05). Detailed statistics are presented in Table 6.

Table 6.
Independent-samples Kruskal–Wallis test results and mean ranks for overall ratings of care by age group, using five age groups.
4. Discussion
In this study, we conducted an age analysis of the 2021 Alberta AOPSS survey data. Our primary outcome of interest was satisfaction across six dimensions of person-centred care. When we used three age groups, those aged 65+ years showed levels of satisfaction approximately equal to or greater than those aged 18–39 years and 40–64 years on all dimensions of person-centred care, except for physical comfort, for which satisfaction was significantly lower for those aged 65+ years than those aged 40–64 years. However, when we used five age groups, dividing the older adults into three groups, a decreasing pattern of satisfaction for those aged 75–84 years was evident on most dimensions, and those aged 85+ years showed levels of satisfaction lower than those aged 65–74 years on all dimensions of person-centred care.
The MANOVA results showed a statistically significant difference across age groups for both analyses. However, the post hoc comparisons with three age groups pointed towards lower levels of satisfaction primarily in the 18–39 years age group, except for the ‘physical comfort’ dimension, which was significantly lower in those aged 65+ years. With the five age groups, the post hoc analysis confirmed the significantly lower levels of satisfaction for older adults on the ‘physical comfort’ dimension, specifically among those aged 65–74 and 75–84. In addition, this analysis showed significantly lower levels of satisfaction among those aged 75+ on the ‘coordination and continuity of care’ dimension and for those aged 85+ on the ‘information, communication, and education’ dimension.
This analysis of a large sample of people receiving cancer care in Alberta highlights how using a single group for all older adults aged 65+ years can obscure the lower levels of satisfaction among those aged 75–84 and 85+ years, particularly on the ‘coordination and continuity of care’ and ‘information, communication, and education’ dimensions. Older adults are a vastly heterogeneous group. Although chronological age is only a rough proxy for the variation in health and functional status that occurs among older adults [29], dividing older adults into multiple age groups begins to acknowledge the variation in patient-reported experiences.
The dimensions that showed lower satisfaction among older adults are consistent with the existing understanding of age-related concerns. Multimorbidity increases with age, affecting only 13.3% of Canadians aged 20–64 but 32.8% of Canadians aged 65–74, 42.7% of Canadians aged 75–84, and 47.7% of Canadians aged 85+ years [38]. Multimorbidity may contribute to greater physical discomfort during cancer care, affecting the choice and completion of treatment [39]. In addition, the management of multiple morbidities calls for additional coordination of care among multiple medical specialists, primary care providers, and allied healthcare providers. Age-related health, functional, and social changes may also interact with cancer-related changes and require active coordination among cancer care providers and community health or social care services during and after cancer treatment. Furthermore, shifting values related to quality and quantity of life [5] and a lack of research to inform treatment decisions among older adults with cancer [13] can contribute to greater complexity in the treatment decision-making process, calling for greater coordination, as well as intentional information sharing and communication, among healthcare providers, patients, and families/caregivers. Communication challenges among older adults with cancer and their care providers are not new and may be impacted by age-related sensory, cognitive, and functional changes that impact interactions; the involvement of families/caregivers; and/or ageist attitudes among both care providers and patients [40,41]. Decreased satisfaction among older adults on these dimensions of person-centred care is critical given the potential impact on health outcomes.
In addition, the proportion of respondents who did not receive the help that they wanted generally increased with age across all types of issues. The difference across age groups was statistically significant for emotional, financial, social/family, and sexual health issues, and it neared statistical significance for practical and spiritual issues. This finding is consistent with previous studies that have identified unmet needs among older adults diagnosed with cancer [10,42], among older adults undergoing active cancer treatment [11,43], and among older cancer survivors [12]. The types of unmet needs highlighted, however, vary widely across studies, including medical issues [10]; informational issues [10,11,42,43,44]; practical issues, such as transportation or insurance [12,42]; financial issues [12,44]; psychological issues [11,12]; physical issues [11,12]; relational issues [12]; communication issues [42,43]; spiritual issues [44]; and issues relating to coordination among care providers, including primary care providers [42]. The differences in unmet needs across studies may reflect differences in the measures used, as well as variations in the health system context, specifically related to the available services and resources.
Notably, in a previous Canadian study of cancer survivors, researchers also found a high number of older adults expressing concern about sexual issues, with a high proportion reporting that they did not receive the help that they wanted [12], echoing the high proportion of unmet sexual health issues among older adults in this study. Communication by healthcare providers about sexual side effects has been found to decrease as patient age increases [41]. The use of sexual health assessment tools, and an awareness of the potential impact of cancer and cancer treatment on sexual health, may help to address the unmet needs related to sexual health among older adults with cancer [45].
Finally, the overall rating of the care at cancer centres also showed significant differences across age groups. The pairwise comparisons pointed towards lower levels of satisfaction with the quality of care among those aged 85+ years. Across all these analyses, it is important to note the overall pattern of lower satisfaction and unmet needs among those aged 85+ years. A strength of this study was having a sufficient sample size to detect significant differences for this group. In Alberta, in 2021, the number of cancer diagnoses among those aged 85+ years was about 50% higher than that among those aged 18–39 years, with both groups comprising similar proportions of those attending a cancer centre, 6% for those aged 18–39 years and 5% for those aged 85+ years (Figure 1). Current estimates suggest that the number of Canadians aged 85+ with cancer will more than double (increase by 130%) in the next 20 years [46]. In Alberta, previous AOPSS results have informed the development of programs and services tailored to the needs and concerns of young adults with cancer; the results of this age analysis clearly highlight the need for services and resources tailored to the needs and concerns of older adults with cancer and their families/caregivers.
4.1. Implications
Insights from this age analysis can inform the development of services and resources tailored to support older adults with cancer and their families, highlighting which groups to target with various interventions. Interventions and services addressing physical comfort should target older adults aged 65+ years; those addressing coordination and continuity of care would most benefit those aged 75+ years; and tailored information, communication, and education would most benefit those aged 85+ years. Resources to address unmet needs, particularly those related to emotional, financial, social/family, and sexual health issues, should be considered for all older adults receiving cancer care in Alberta. Geriatric assessment and management (GAM) and patient navigation are key interventions to address these areas of concern.
GAM is an effective approach to understanding variation, addressing age-related concerns, and improving outcomes in the care of older adults with cancer [47]. Geriatric assessment is the most commonly reported supportive intervention for older people having cancer treatment [48]. In the American Society of Clinical Oncology guidelines, experts recommend GAM for patients aged 65+ with identified vulnerabilities, to inform cancer treatment decision making and supportive interventions to optimize treatment outcomes [49]. Randomized controlled trials have demonstrated that, among older adults receiving cancer treatment, GAM can reduce toxicity and complications; promote treatment completion; improve quality of life and physical function/mobility; increase age-related conversations among oncologists and patients; and improve communication satisfaction for patients and families/caregivers [47,49,50]. Therefore, GAM holds evidence-based potential for positive impacts in at least two of the dimensions of person-centred care that showed lower levels of satisfaction among older adults with cancer in Alberta.
Navigation is supported by strong evidence for improvements in patient satisfaction with care and quality of life, with emerging evidence for improved communication [51]. Specifically, in Canada, patients treated for cancer who were assigned a nurse navigator reported higher satisfaction across all dimensions of person-centred care on the AOPSS [27]. Among older adults with cancer specifically, a systematic review of navigation also found a positive impact on satisfaction [52]. Within Cancer Care Alberta, the cancer patient navigator role was designed to address concerns related to continuity of care, including informational, management, and relational continuity [53]. New models of cancer care navigation, including generalist navigators in rural settings, and population-specific navigators for Indigenous persons and young adults, have been successfully implemented in Alberta, decreasing emergency visits and hospital admissions and increasing positive care experiences [53,54]. Therefore, to address the lower levels of satisfaction among older adults identified in this study, particularly with respect to the coordination and continuity of care, opportunities exist to educate generalist navigators about best practices in geriatric oncology and to develop a population-specific navigator for older adults with cancer.
The clear involvement of families/caregivers in completing the survey among older adults with cancer highlights the need to include and address family/caregiver concerns in interventions for older adults with cancer [48]. GAM and navigation interventions also show promise for family/caregiver communication and support [50,51].
4.2. Future Directions
As the AOPSS is a bi-annual survey in Alberta, future analyses may consider longitudinal changes over time related to age differences in satisfaction, supporting the evaluation of interventions addressing age-related concerns. In this study, we used univariate analyses to explore the patterns and significant differences in satisfaction across dimensions of person-centred care, unmet needs, and the overall rating of cancer centre care across age groups. Future research could incorporate multivariate analyses to explore and provide a greater understanding of these relationships. However, given that health records often contain limited information concerning other sociodemographic characteristics and age is readily available, age may remain a valuable proxy to identify those requiring additional support.
The respondents for this survey were sampled from people who had received systemic or radiation treatment at a cancer centre in Alberta within the 6 months prior to survey distribution. Among adults aged 75+ years, and particularly among those aged 85+ years, those receiving systemic or radiation treatment may be an increasingly select sub-group of those who have been diagnosed with cancer. To fully understand care satisfaction and unmet needs among older adults with cancer, future research may seek to also understand the experiences of those not receiving active treatment, as well as those with suspected or clinical diagnoses for whom further diagnostic investigations are not pursued, providing a more comprehensive understanding of the supportive services and resources needed.
4.3. Limitations
Significantly lower levels of satisfaction were identified among the youngest (18–39 years) and oldest (85+ years) age groups. However, due to missing data and smaller sample sizes limiting the power to detect significant differences for these groups, we chose to use a more liberal and powerful post hoc test for the MANOVA—Fisher’s least significant difference test. This test is not typically recommended because it does not adjust the significance for multiple comparisons, raising the risk of a Type I error [55]. It does, however, decrease the risk of Type II errors, giving a sense of where there may be significant results if more conservative tests were used with larger sample sizes. In addition, although the proportion of unmet needs for several types of issues was highest for those aged 85+ years, we did not find significant differences for this group using the more conservative Bonferroni test for post hoc analysis. Therefore, approaches to increase the number of responses for the oldest and youngest groups and reduce missing data in future studies would strengthen the power and ability to use more conservative statistical analyses.
The distribution of the 2021 AOPSS survey coincided with the third wave of COVID-19 in Alberta [56]. During this time, many cancer care visits were conducted virtually, in-person supportive care activities were limited, and the presence of families/caregivers was restricted. Lower satisfaction with cancer care was noted in Alberta during the COVID-19 pandemic [57]; however, susceptibility to stress for cancer patients during the COVID-19 pandemic was not associated with age [58]. Therefore, it would not be reasonable to attribute the age differences in satisfaction found in this study to the COVID-19 pandemic alone. Age analysis of future patient-reported experience data collected after the COVID-19 pandemic will lead to a greater understanding of the ongoing age differences in care satisfaction.
The dataset used for this retrospective analysis did not include information about the health or functional status of respondents beyond self-rated health. Given the vast variation among older adults, challenges related to multimorbidity, activities of daily living, cognitive status, or mood may impact satisfaction with care and unmet needs [10,18,59,60]; however, data related to these domains are currently limited for older adults with cancer in Alberta. The greater integration of GAM into cancer care could provide opportunities to explore the relationships between care satisfaction and domains of geriatric concern, further informing targeted interventions to strengthen care experiences and outcomes.
Patients who experience sensory or cognitive deficits, have lower levels of education, lack the active involvement of their family/caregivers, or have higher levels of physical discomfort or fatigue may also be less able or willing to complete the lengthy AOPSS. In this study, many of these characteristics increased with age. Among older adults, we saw a higher proportion of surveys completed by, or with the help of, someone else and of missing data. Therefore, the respondents who chose, and were able, to complete and return the survey may have been different from those who were unable or chose not to do so, particularly among older adults. In future studies, the greater integration of interviews and/or telephone survey completion may facilitate the involvement of those facing barriers to survey completion [61], strengthening the representativeness of the results and increasing the responses.
As noted, among older adults, there was a higher proportion of AOPSS completed by, or with the help of, someone else. Therefore, the responses in the older age groups may reflect a greater proportion of family/caregiver perspectives, in addition to patient perspectives. Previous studies have found lower levels of satisfaction among families/caregivers as compared to patients in cancer care [62]. These family/caregiver perspectives, however, are also critical in informing quality improvements [63], suggesting a need for further research that considers both patient and family/caregiver satisfaction.
5. Conclusions
Grouping together all older adults aged 65+ years when analyzing data from patient experience measures can obscure the lower levels of satisfaction among those aged 75+ and 85+ years, resulting in important and nuanced age-related concerns in these older age groups being overlooked. The significantly lower levels of satisfaction among older adults in the dimensions of ‘physical comfort’, ‘coordination and continuity of care’, and ‘information, communication, and education’, as well as increasing unmet needs, significant for emotional, financial, social/family, and sexual health issues, highlight the need for programmatic attention, with tailored services and resources, to address the needs and concerns of older adults with cancer and their families in Alberta.
Author Contributions
This manuscript is the result of collaborative work among the authors, with all authors contributing substantially to the work reported. Conceptualization, F.J.S., L.W. and S.D.; methodology, F.J.S., S.Q., C.L. and L.W.; software, F.J.S. and S.Q.; validation, F.J.S. and S.Q.; formal analysis, F.J.S. and S.Q.; investigation, F.J.S.; resources, L.W. and S.D.; data curation, F.J.S. and S.Q.; writing—original draft preparation, F.J.S.; writing—review and editing, all authors; visualization, F.J.S.; supervision, S.D. and L.W.; project administration, F.J.S.; funding acquisition, F.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a University of Calgary Eyes High Postdoctoral Match Funding Award and a Canadian Institutes of Health Research Health System Impact Postdoctoral Fellowship in collaboration with the Cancer Strategic Clinical Network, Alberta Health Services, held by Fay J. Strohschein.
Institutional Review Board Statement
The 2021 AOPSS was delivered in accordance with the Declaration of Helsinki and the Alberta Research Ethics Community Consensus Initiative (ARECCI) guidelines for quality improvement initiatives. The protocol for this retrospective age analysis was reviewed and approved by the Health Research Ethics Board of Alberta (HREBA)—Cancer Committee (IRB#00009687) on 9 June 2021, approval number HREBA.CC-21-0190.
Informed Consent Statement
With HREBA approval, patient consent was waived as this study involved the secondary use of de-identified data originally collected for quality improvement purposes. The use of the data for the purpose of this study was consistent with the conditions presented to AOPSS respondents in the introductory information for the survey during data collection. De-identified data are presented only in aggregate. Obtaining consent would have been impracticable or impossible, given the number of patients, with whom we would otherwise have had no contact and where, in some cases, the patient may have been deceased. The collection and use of these data are governed by Alberta’s Freedom of Information and Protection of Privacy Act.
Data Availability Statement
Restrictions apply to the availability of these data, according to the Alberta Health Information Act (https://www.alberta.ca/health-information-act) and associated regulations. Data were obtained from Alberta Health Services and are only available with appropriate ethical and operational approval.
Acknowledgments
Thank you to Renna Truong, Data & Geospatial Resources Specialist within the Spatial and Numeric Data Services of the University of Calgary, who conducted the rurality determination with ArcGIS software, version 2.9. Thank you to Allison Scott, who provided valuable feedback and the 2021 Alberta cancer statistics.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A
This appendix contains tables with the results of the post hoc comparisons between age groups for sociodemographic and health characteristics (Table A1), which complement the results in Table 1, and clinical characteristics (Table A2), which complement Table 2. Please see Section 2.5 for a description of the analyses.

Table A1.
Post hoc comparisons between age groups for sociodemographic and health characteristics showing a significant difference across age groups.
Table A1.
Post hoc comparisons between age groups for sociodemographic and health characteristics showing a significant difference across age groups.
| Post Hoc Comparisons | Adj p 3 | |
|---|---|---|
| Sex 1 | ||
| Female | 18–39 > 65–74 | 0.022 |
| 18–39 > 85+ | 0.007 | |
| 40–64 > 65–74 | <0.001 | |
| 40–64 > 75–84 | 0.005 | |
| 40–64 > 85+ | 0.002 | |
| Male | 18–39 < 65–74 | 0.022 |
| 18–39 < 85+ | 0.007 | |
| 40–64 < 65–74 | <0.001 | |
| 40–64 < 75–84 | 0.005 | |
| 40–64 < 85+ | 0.002 | |
| Education 2 | ||
| 18–39 > 65–74 | 0.001 | |
| 18–39 > 75–84 | <0.001 | |
| 18–39 > 85+ | <0.001 | |
| 40–64 > 65–74 | 0.011 | |
| 40–64 > 75–84 | <0.001 | |
| 40–64 > 85+ | <0.001 | |
| 65–74 > 75–84 | <0.001 | |
| 65–74 > 85+ | <0.001 | |
| 75–84 > 85+ | 0.048 | |
| Who completed survey 1 | ||
| Patient | 18–39 > 85+ | <0.001 |
| 40–64 > 75–84 | <0.001 | |
| 40–64 > 85+ | <0.001 | |
| 65–74 > 75–84 | 0.004 | |
| 65–74 > 85+ | <0.001 | |
| 75–84 > 85+ | <0.001 | |
| Patient with help/someone else | 18–39 < 85+ | <0.001 |
| 40–64 < 75–84 | <0.001 | |
| 40–64 < 85+ | <0.001 | |
| 65–74 < 75–84 | 0.004 | |
| 65–74 < 85+ | <0.001 | |
| 75–84 < 85+ | <0.001 | |
| Self-rated health 2 | ||
| 18–39 > 75–84 | 0.011 | |
| 18–39 > 85+ | <0.001 | |
| 40–64 > 85+ | 0.001 | |
| 65–74 > 85+ | 0.004 |
Note: Post hoc tests were only conducted for variables for which we found a significant difference across age groups. Only the results of significant post hoc comparisons are presented. 1 For nominal variables, only groups that had significant post hoc tests are included in the table; 2 for ordinal variables, the groups are not listed because post hoc tests were applied across groups; 3 significance values have been adjusted by the Bonferroni correction for multiple tests.

Table A2.
Post hoc comparisons between age groups for clinical characteristics showing a significant difference across age groups.
Table A2.
Post hoc comparisons between age groups for clinical characteristics showing a significant difference across age groups.
| Post Hoc Comparisons | Adj p 6 | |
|---|---|---|
| Tumor site 1 | ||
| Hematological | 40–64 < 65–74 | 0.037 |
| 40–64 < 75–84 | <0.001 | |
| 40–64 < 85+ | <0.001 | |
| 65–74 < 85+ | 0.005 | |
| Breast | 18–39 > 65–74 | <0.001 |
| 18–39 > 75–84 | <0.001 | |
| 18–39 > 85+ | <0.001 | |
| 40–64 > 65–74 | <0.001 | |
| 40–64 > 75–84 | <0.001 | |
| 40–64 > 85+ | <0.001 | |
| Gastrointestinal | 40–64 > 85+ | 0.046 |
| Genitourinary | 40–64 < 65–74 | <0.001 |
| 40–64 < 75–84 | <0.001 | |
| Skin 3 | 65–74 < 85+ | 0.047 |
| 75–84 < 85+ | 0.015 | |
| Central nervous system | 18–39 > 40–64 | 0.005 |
| 18–39 > 65–74 | <0.001 | |
| 18–39 > 75–84 | <0.001 | |
| Time since diagnosis 2 | ||
| 18–39 < 40–64 | 0.019 | |
| 18–39 < 65–74 | <0.001 | |
| 18–39 < 75–84 | <0.001 | |
| 18–39 < 85+ | <0.001 | |
| 40–64 < 75–84 | <0.001 | |
| 40–64 < 85+ | <0.001 | |
| 65–74 < 85+ | <0.001 | |
| 75–84 < 85+ | 0.014 | |
| Type of cancer centre where care was received 1 | ||
| Regional | 40–64 < 65–74 | 0.008 |
| 40–64 < 75–84 | 0.001 | |
| Involvement of family doctor in care while on cancer treatment 2,4 | ||
| 40–64 < 75–84 | 0.015 | |
| 40–64 < 85+ | 0.032 | |
| Treatment intent 1 | ||
| Cure | 18–39 > 40–64 | 0.005 |
| 18–39 > 65–74 | <0.001 | |
| 18–39 > 75–84 | <0.001 | |
| 18–39 > 85+ | <0.001 | |
| 40–64 > 75–84 | 0.024 | |
| 40–64 > 85+ | <0.001 | |
| 65–74 > 85+ | <0.001 | |
| 75–84 > 85+ | 0.003 | |
| Control | 18–39 < 40–64 | 0.014 |
| 18–39 < 65–74 | 0.001 | |
| 18–39 < 75–84 | <0.001 | |
| 18–39 < 85+ | <0.001 | |
| 40–64 < 75–84 | 0.046 | |
| 40–64 < 85+ | <0.001 | |
| 65–74 < 85+ | 0.009 | |
| Unsure | 40–64 < 85+ | 0.021 |
| 65–74 < 85+ | 0.019 | |
| Type of cancer treatment(s) received (not mutually exclusive) 1,5 | ||
| IV chemotherapy | 18–39 > 85+ | 0.001 |
| 40–64 > 75–84 | < 0.001 | |
| 40–64 > 85+ | < 0.001 | |
| 65–74 > 75–84 | 0.022 | |
| 65–74 > 85+ | < 0.001 | |
| 75–84 > 85+ | 0.028 | |
| Oral chemotherapy | 18–39 < 85+ | 0.023 |
| 40–64 < 85+ | 0.017 |
Note: Post hoc tests were only conducted for variables for which we found a significant difference across age groups. Only results of significant post hoc comparisons are presented. 1 For nominal variables, only groups that had significant post hoc tests are included in the table; 2 for ordinal variables, the groups are not listed because post hoc tests were applied across groups; 3 skin includes melanoma and non-melanoma skin cancers; 4 for family doctor involvement, ‘do not have’ and ‘unsure’ were considered not applicable responses and were not included in the post hoc testing; 5 based on tumor registry data—some respondents received more than one type of treatment; 6 significance values have been adjusted by the Bonferroni correction for multiple tests.
References
- Cancer Research & Analytics—Cancer Care Alberta. Alberta Cancer Registry; Alberta Health Services: AB, Canada, 2023. [Google Scholar]
- World Health Organization. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2015; Available online: https://apps.who.int/iris/handle/10665/186463 (accessed on 4 May 2018).
- Soto-Perez-de-Celis, E.; Li, D.; Yuan, Y.; Lau, Y.M.; Hurria, A. Functional versus chronological age: Geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018, 19, e305–e316. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Older Adult Oncology (Version 1.2024—8 December 2023). 2024. Available online: https://www.nccn.org/ (accessed on 25 January 2024).
- Shrestha, A.; Martin, C.; Burton, M.; Walters, S.; Collins, K.; Wyld, L. Quality of life versus length of life considerations in cancer patients: A systematic literature review. Psycho-Oncol. 2019, 28, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Canadian Partnership against Cancer. Cancer System Performance: 2017 Report. 2017. Available online: https://www.partnershipagainstcancer.ca/topics/2017-cancer-system-performance-report/ (accessed on 17 October 2017).
- Dumontier, C.; Loh, K.P.; Bain, P.A.; Silliman, R.A.; Hshieh, T.; Abel, G.A.; Djulbegovic, B.; Driver, J.A.; Dale, W. Defining undertreatment and overtreatment in older adults with cancer: A scoping literature review. J. Clin. Oncol. 2020, 38, 2558. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Rutherford, M.J.; Bardot, A.; Ferlay, J.; Andersson, T.M.L.; Myklebust, T.Å.; Tervonen, H.; Thursfield, V.; Ransom, D.; Shack, L.; et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): A population-based study. Lancet Oncol. 2019, 20, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Wen, W.; Morgans, A.K.; Pao, W.; Shu, X.O.; Zheng, W. Disparities by race, age, and sex in the improvement of survival for major cancers: Results from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program in the United States, 1990 to 2010. JAMA Oncol. 2015, 1, 88–96. [Google Scholar] [CrossRef]
- Williams, G.R.; Pisu, M.; Rocque, G.B.; Williams, C.P.; Taylor, R.A.; Kvale, E.A.; Partridge, E.E.; Bhatia, S.; Kenzik, K.M. Unmet social support needs among older adults with cancer. Cancer 2019, 125, 473–481. [Google Scholar] [CrossRef]
- Puts, M.T.; Papoutsis, A.; Springall, E.; Tourangeau, A.E. A systematic review of unmet needs of newly diagnosed older cancer patients undergoing active cancer treatment. Support Care Cancer 2012, 20, 1377–1394. [Google Scholar] [CrossRef]
- Fitch, M.I.; Nicoll, I.; Lockwood, G.; Strohschein, F.J.; Newton, L. Cancer survivors 75 years and older: Physical, emotional and practical needs. BMJ Support Palliat. Care 2023, 13, e352–e360. [Google Scholar] [CrossRef]
- National Academies of Sciences Engineering Medicine. Improving the Evidence Base for Treatment Decision Making for Older Adults with Cancer: Proceedings of a Workshop—In Brief; Balogh, E., Johnson, A., Nass, S., Eds.; The National Academies Press: Washington, DC, USA, 2021; 10p. [Google Scholar]
- Outlaw, D.; Williams, G.R. Is the lack of evidence in older adults with cancer compromising safety? Expert Opin. Drug Saf. 2020, 19, 1059–1061. [Google Scholar] [CrossRef]
- VanderWalde, N.; Hurria, A.; Jagsi, R. Improving consistency and quality of care for older adults with cancer: The challenges of developing consensus guidelines for radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 721–725. [Google Scholar] [CrossRef]
- Statistics Canada. Age Pyramids: Historical Age and Sex at Birth Pyramid: Government of Canada. 2022. Available online: https://www12.statcan.gc.ca/census-recensement/2021/dp-pd/dv-vd/pyramid/index-en.htm (accessed on 19 December 2023).
- Casaca, P.; Schäfer, W.; Nunes, A.B.; Sousa, P. Using patient-reported outcome measures and patient-reported experience measures to elevate the quality of healthcare. Int. J. Qual. Health Care 2023, 35, mzad098. [Google Scholar] [CrossRef]
- Alessy, S.A.; Alhajji, M.; Rawlinson, J.; Baker, M.; Davies, E.A. Factors influencing cancer patients experiences of care in the USA, United Kingdom, and Canada: A systematic review. EClinicalMedicine 2022, 47, 101405. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.; Ochoa, C.; Chan, E.; Du, S.; Farias, A. Will improvements in patient experience with care impact clinical and quality of care outcomes?: A systematic review. Med. Care 2021, 59, 843–856. [Google Scholar] [CrossRef] [PubMed]
- National Research Corporation. Development and Validation of the Picker Ambulatory Oncology Survey Instrument in Canada; National Research Corporationl: Lincoln, NE, USA, 2003. [Google Scholar]
- Ferguson, D. Measuring the Patient’s Experience: Validation of the Ambulatory Oncology Patient Satisfaction Survey; National Research Corporation Picker Canada: ON, Canada, 2012. [Google Scholar]
- Watson, L.; Qi, S.; Photitai, E.; Deiure, A. A cross-sectional analysis of ambulatory oncology experience by treatment intent. Curr. Oncol. 2020, 28, 98–106. [Google Scholar] [CrossRef]
- Al-Rashdan, A.; Watson, L.; Yannitsos, D.; Qi, S.; Grendarova, P.; Barbera, L. Comparison of patient-reported experience of patients receiving radiotherapy measured by two validated surveys. Curr. Oncol. 2021, 28, 2180–2189. [Google Scholar] [CrossRef]
- Watson, L.; Link, C.; Qi, S.; Deiure, A. Quantifying the impact of family doctors on the care experiences of patients with cancer: Exploring evidence from the 2021 Ambulatory Oncology Patient Satisfaction Survey in Alberta, Canada. Curr. Oncol. 2023, 30, 641–652. [Google Scholar] [CrossRef]
- Coronado, A.C.; Tran, K.; Chadder, J.; Niu, J.; Fung, S.; Louzado, C.; Rahal, R. The experience of patients with cancer during diagnosis and treatment planning: A descriptive study of Canadian survey results. Curr. Oncol. 2017, 24, 332–337. [Google Scholar] [CrossRef]
- Bridge, E.; Conn, L.G.; Dhanju, S.; Singh, S.; Moody, L. The patient experience of ambulatory cancer treatment: A descriptive study. Curr. Oncol. 2019, 26, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Loiselle, C.G.; Attieh, S.; Cook, E.; Tardif, L.; Allard, M.; Rousseau, C.; Thomas, D.; Saha-Chaudhuri, P.; Talbot, D. The nurse pivot-navigator associated with more positive cancer care experiences and higher patient satisfaction. Can. Oncol. Nurs. J. 2020, 30, 48–53. [Google Scholar] [CrossRef]
- Little, W. Aging and the elderly. In Introduction to Sociology—2nd Canadian ed.; BCcampus: Victoria, BC, Canada, 2016; pp. 530–573. Available online: https://opentextbc.ca/introductiontosociology2ndedition/ (accessed on 14 February 2022).
- Lowsky, D.J.; Olshansky, S.J.; Bhattacharya, J.; Goldman, D.P. Heterogeneity in healthy aging. J. Gerontol. Series A Biol. Sci. Med. Sci. 2014, 69, 640–649. [Google Scholar] [CrossRef]
- Alberta Health Services and NRC Health. Ambulatory Oncology Patient Satisfaction Survey—Alberta; Alberta Health Services: AB, Canada, 2021. [Google Scholar]
- Guideline Resource Unit. Adolescent and Young Adult (AYA) Cancer: Clinical Practice Guideline SUPP-020; Cancer Control Alberta, Alberta Health Services: AB, Canada, 2020; Available online: https://www.albertahealthservices.ca/assets/info/hp/cancer/if-hp-cancer-guide-supp020-adolescent-young-adult.pdf (accessed on 14 March 2022).
- Fuss, J.; Hill, T. The Implications of an Aging Population for Government Finances in Alberta; Fraser Institute: Canada, 2021; Available online: https://www.fraserinstitute.org/studies/the-implications-of-an-aging-population-for-government-finances-in-alberta (accessed on 14 March 2022).
- Seniors Health Strategic Clinical Network Core Committee. Seniors Health Strategic Clinical Network: 2017–2020 Transformational Roadmap 2017. Available online: https://www.albertahealthservices.ca/assets/about/scn/ahs-scn-srs-roadmap.pdf (accessed on 24 February 2020).
- Alberta Health Services and Alberta Health. Official Standard Geographic Areas; Alberta Health Services/Alberta Health: AB, Canada, 2018; Available online: https://open.alberta.ca/dataset/a14b50c9-94b2-4024-8ee5-c13fb70abb4a/resource/70fd0f2c-5a7c-45a3-bdaa-e1b4f4c5d9a4/download/official-standard-geographic-area-document.pdf (accessed on 20 February 2023).
- Nordstokke, D.W.; Zumbo, B.D. A cautionary tale about Levene’s tests for equal variances. J. Educ. Res. Policy Stud. 2007, 7, 1–14. [Google Scholar]
- Monarota, M.S.; Farnisa, M.; Zauner, R. NRES 746: ANOVA/MANOVA 2021. Available online: https://kevintshoemaker.github.io/NRES-746/MANOVA.html (accessed on 11 December 2023).
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 5th ed.; Pearson Education: Boston, MA, USA, 2007. [Google Scholar]
- Public Health Agency of Canada. Aging and Chronic Diseases: A Profile of Canadian Seniors; HP35-137/1-2020E-PDF; Government of Canada: Ottawa, ON, Canada, 2020. Available online: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/aging-chronic-diseases-profile-canadian-seniors-report.html (accessed on 19 December 2023).
- George, M.; Smith, A.; Sabesan, S.; Ranmuthugala, G. Physical comorbidities and their relationship with cancer treatment and its outcomes in older adult populations: Systematic review. JMIR Cancer 2021, 7, e26425. [Google Scholar] [CrossRef]
- Greene, M.G.; Adelman, R.D. Physician–older patient communication about cancer. Patient Educ. Couns. 2003, 50, 55–60. [Google Scholar] [CrossRef]
- Schroyen, S.; Adam, S.; Marquet, M.; Jerusalem, G.; Thiel, S.; Giraudet, A.-L.; Missotten, P. Communication of healthcare professionals: Is there ageism? Eur. J. Cancer Care 2018, 27, e12780. [Google Scholar] [CrossRef]
- Tjong, M.C.; Menjak, I.; Trudeau, M.; Mehta, R.; Wright, F.; Leahey, A.; Ellis, J.; Gallagher, D.; Gibson, L.; Bristow, B.; et al. The perceptions and expectations of older women in the establishment of the Senior Women’s Breast Cancer Clinic (SWBCC): A needs assessment study. J. Cancer Educ. 2017, 32, 850–857. [Google Scholar] [CrossRef]
- van Weert, J.C.M.; Bolle, S.; van Dulmen, S.; Jansen, J. Older cancer patients’ information and communication needs: What they want is what they get? Patient Educ. Couns. 2013, 92, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J.; Jones, R.A.; Klimmek, R.; Krumm, S.; Darrell, L.P.; Song, D.; Stearns, V.; Ford, J.G. Cancer support and resource needs among African American older adults. Clin. J. Oncol. Nurs. 2012, 16, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Arthur, E.K.; Worly, B.; Carpenter, K.M.; Postl, C.; Rosko, A.E.; Krok-Schoen, J.L.; Quick, A.M.; Jenkins, L.C. Let’s get it on: Addressing sex and intimacy in older cancer survivors. J. Geriatr. Oncol. 2020, 12, 312–315. [Google Scholar] [CrossRef]
- Ferlay, J.; Laversanne, M.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Tomorrow; International Agency for Research on Cancer, World Health Organization: Lyon, France, 2020; Available online: https://gco.iarc.fr/tomorrow/en (accessed on 19 December 2023).
- Hamaker, M.; Lund, C.; Te Molder, M.; Soubeyran, P.; Wildiers, H.; Van Huis, L.; Rostoft, S. Geriatric assessment in the management of older patients with cancer—A systematic review (update). J. Geriatr. Oncol. 2022, 13, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Farrington, N.; Richardson, A.; Bridges, J. Interventions for older people having cancer treatment: A scoping review. J. Geriatr. Oncol. 2020, 11, 769–783. [Google Scholar] [CrossRef]
- Dale, W.; Klepin, H.D.; Williams, G.R.; Alibhai, S.M.H.; Bergerot, C.; Brintzenhofeszoc, K.; Hopkins, J.O.; Jhawer, M.P.; Katheria, V.; Loh, K.P.; et al. Practical assessment and management of vulnerabilities in older patients receiving systemic cancer therapy: ASCO guideline update. J. Clin. Oncol. 2023, 41, 4293–4312. [Google Scholar] [CrossRef] [PubMed]
- Mohile, S.G.; Epstein, R.M.; Hurria, A.; Heckler, C.E.; Canin, B.; Culakova, E.; Duberstein, P.; Gilmore, N.; Xu, H.; Plumb, S.; et al. Communication with older patients with cancer using geriatric assessment: A cluster-randomized clinical trial from the National Cancer Institute Community Oncology Research Program. JAMA Oncol. 2020, 6, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.J.; Milch, V.E.; Crawford-Williams, F.; Agbejule, O.A.; Joseph, R.; Johal, J.; Dick, N.; Wallen, M.P.; Ratcliffe, J.; Agarwal, A.; et al. Patient navigation across the cancer care continuum: An overview of systematic reviews and emerging literature. CA Cancer J. Clin. 2023, 73, 565–589. [Google Scholar] [CrossRef] [PubMed]
- van Ee, I.B.; Hagedoorn, M.; Slaets, J.P.J.; Smits, C.H.M. Patient navigation and activation interventions for elderly patients with cancer: A systematic review. Eur. J. Cancer Care 2017, 26, e12621. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Champ, S.; Vimy, K.; Delure, A.; Watson, L. Developing a provincial cancer patient navigation program utilizing a quality improvement approach Part one: Designing and implementing. Can. Oncol. Nurs. J. 2016, 26, 122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Watson, L.C.; Vimy, K.; Anderson, J.; Champ, S.; Deiure, A. Developing a provincial cancer patient navigation program utilizing a quality improvement approach part three: Evaluation and outcomes. Can. Oncol. Nurs. J. 2016, 26, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, M. A Protable Introduction to Data Analysis. The University of Queensland. 2024. Available online: https://uq.pressbooks.pub/portable-introduction-data-analysis/ (accessed on 28 February 2024).
- Government of Alberta. COVID-19 Alberta Statistics: Interactive Aggregate Data on COVID-19 Cases in Alberta 2022. Available online: https://www.alberta.ca/stats/covid-19-alberta-statistics.htm#total-cases (accessed on 28 June 2022).
- Watson, L.; Link, C.; Qi, S.; Photitai, E.; Chmielewski, L.; Fode, D.; DeIure, A. Understanding patient experiences before and during the COVID-19 pandemic: A quasi-experimental comparison of in-person and virtual cancer care. Patient Exp. J. 2022, 9, 95–102. [Google Scholar] [CrossRef]
- Bartmann, C.; Fischer, L.-M.; Hübner, T.; Müller-Reiter, M.; Wöckel, A.; McNeill, R.V.; Schlaiss, T.; Kittel-Schneider, S.; Kämmerer, U.; Diessner, J. The effects of the COVID-19 pandemic on psychological stress in breast cancer patients. BMC Cancer 2021, 21, 1–13. [Google Scholar] [CrossRef]
- Lines, L.M.; Barch, D.H.; Zabala, D.; Halpern, M.T.; Jacobsen, P.; Mollica, M. Associations between perceptions of care experiences and receipt of mental health care among older adults with cancer. J. Clin. Oncol. 2020, 38 (Suppl. S29), 175. [Google Scholar] [CrossRef]
- Mariano, C.; Hanson, L.C.; Deal, A.M.; Yang, H.; Bensen, J.; Hendrix, L.; Muss, H.B. Healthcare satisfaction in older and younger patients with cancer. J. Geriatr. Oncol. 2016, 7, 32–38. [Google Scholar] [CrossRef]
- Flannery, M.A.; Mohile, S.; Culakova, E.; Norton, S.; Kamen, C.; Dionne-Odom, J.N.; DiGiovanni, G.; Griggs, L.; Bradley, T.; Hopkins, J.O.; et al. Completion of patient-reported outcome questionnaires among older adults with advanced cancer. J. Pain Symptom Manag. 2022, 63, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Hannon, B.; Swami, N.; Krzyzanowska, M.K.; Leighl, N.; Rodin, G.; Le, L.W.; Zimmermann, C. Satisfaction with oncology care among patients with advanced cancer and their caregivers. Qual. Life Res. 2013, 22, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Bergerød, I.J.; Dalen, I.; Braut, G.S.; Gilje, B.; Wiig, S. Measuring next of kin satisfaction with hospital cancer care: Using a mixed-method approach as basis for improving quality and safety. J. Adv. Nurs. 2020, 76, 1232–1246. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).