Peritoneal Metastatic Gastric Cancer: Local Treatment Options and Recommendations
Abstract
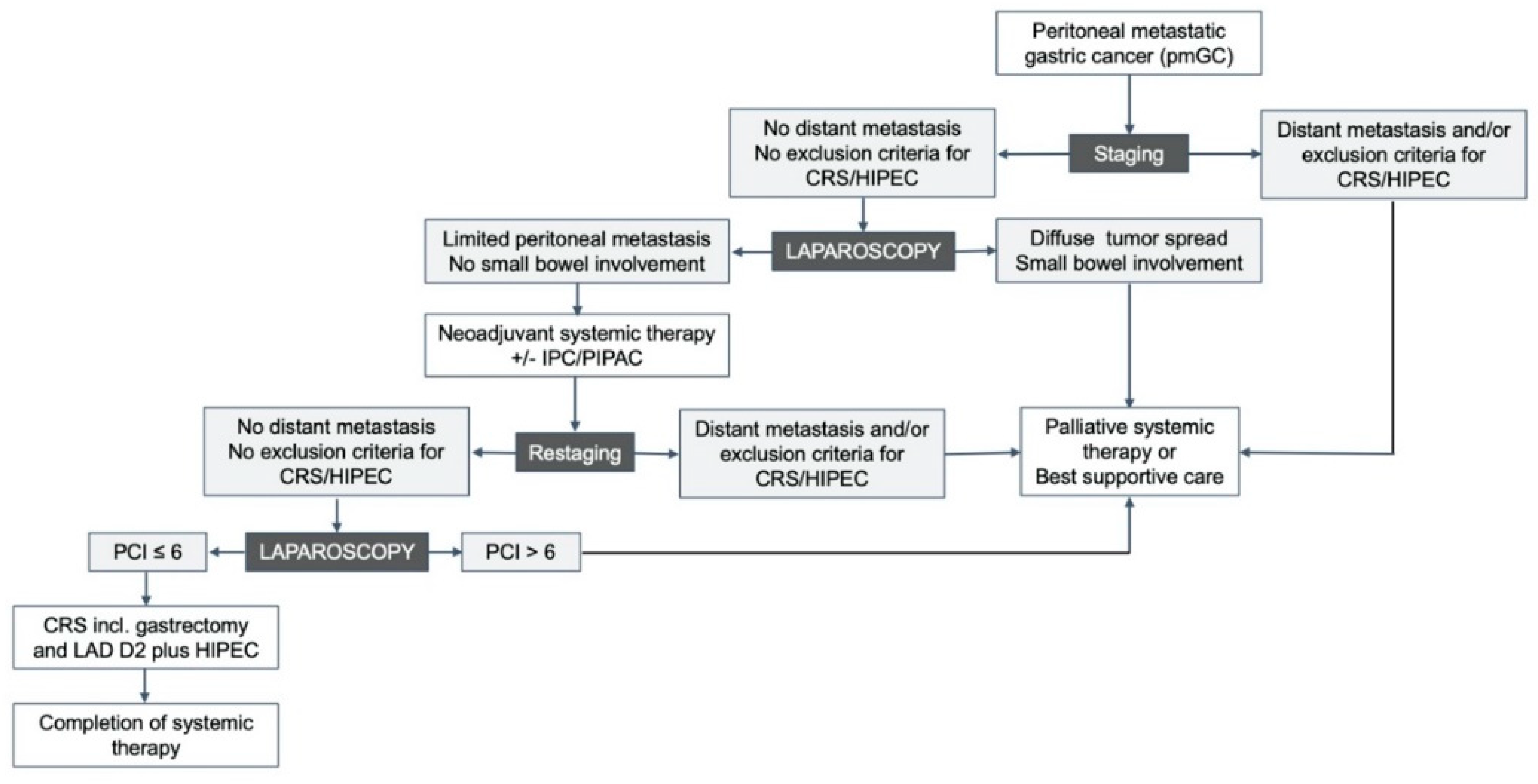
1. Introduction
2. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC)
3. Neoadjuvant Treatment: Intraperitoneal Systemic Chemotherapy (NIPS)
4. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC)
5. Laparoscopic HIPEC with Palliative Intent
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CC | completeness of cytoreduction score |
| CRS | cytoreductive surgery |
| CTCAE | Common Terminology Criteria for Adverse Events |
| ESMO | European Society for Medical Oncology |
| FA | folinic acid |
| FLOT | 5-FU/leucovorin/oxaliplatin/docetaxel |
| 5-FU | 5- fluorouracil |
| HER2 | human epidermal growth factor receptor 2 |
| HIPEC | hyperthermic intraperitoneal chemotherapy |
| IPC | intraperitoneal chemotherapy |
| IPTW | inverse probability of treatment weighting |
| LAD | lymphadenectomy |
| MFS | metastasis-free survival |
| MMC | mitomycin C |
| MSI | microsatellite instability |
| NCCN | National Comprehensive Cancer Network |
| NICE | National Institute for Health and Care Excellence |
| NIPS | neoadjuvant intraperitoneal systemic chemotherapy |
| RCT | randomized controlled trial |
| NRCT | non-randomized controlled trial |
| OS | overall survival |
| PCI | Peritoneal Cancer Index |
| PFS | progression-free survival |
| PIPAC | pressurized intraperitoneal aerosol chemotherapy |
| pmGC | peritoneal metastatic gastric cancer |
| PR | partial response |
| RCT | randomized controlled trial |
| TR | total response |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ikeguchi, M.; Oka, A.; Tsujitani, S.; Maeta, M.; Kaibara, N. Relationship between area of serosal invasion and intraperitoneal free cancer cells in patients with gastric cancer. Anticancer Res. 1994, 14, 2131–2134. [Google Scholar] [PubMed]
- Riihimaki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. Metastatic spread in patients with gastric cancer. Oncotarget 2016, 7, 52307–52316. [Google Scholar] [CrossRef]
- D’Angelica, M.; Gonen, M.; Brennan, M.F.; Turnbull, A.D.; Bains, M.; Karpeh, M.S. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann. Surg. 2004, 240, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Sasako, M.; Sano, T.; Yamamoto, S.; Kurokawa, Y.; Nashimoto, A.; Kurita, A.; Hiratsuka, M.; Tsujinaka, T.; Kinoshita, T.; Arai, K.; et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N. Engl. J. Med. 2008, 359, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, I.; van Gestel, Y.R.; van Ramshorst, B.; Luyer, M.D.; Bosscha, K.; Nienhuijs, S.W.; Lemmens, V.E.; de Hingh, I.H. Peritoneal carcinomatosis of gastric origin: A population-based study on incidence, survival and risk factors. Int. J. Cancer 2014, 134, 622–628. [Google Scholar] [CrossRef]
- Monig, S.; Ott, K.; Gockel, I.; Lorenz, D.; Ludwig, K.; Messmann, H.; Moehler, M.; Piso, P.; Weimann, A.; Meyer, H.J. S3 guidelines on gastric cancer-diagnosis and treatment of adenocarcinoma of the stomach and esophagogastric junction: Version 2.0-August 2019. AWMF register number: 032/009OL. Chirurg 2020, 91, 37–40. [Google Scholar] [CrossRef]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E.C.; on behalf of theESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef]
- Zaanan, A.; Bouche, O.; Benhaim, L.; Buecher, B.; Chapelle, N.; Dubreuil, O.; Fares, N.; Granger, V.; Lefort, C.; Gagniere, J.; et al. Gastric cancer: French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO). Dig. Liver Dis. 2018, 50, 768–779. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Gastric Cancer, Version 1.2023, NCCN Clinical Practice Guidelines in Ocology. 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed on 20 December 2023).
- Fujitani, K.; Yang, H.K.; Mizusawa, J.; Kim, Y.W.; Terashima, M.; Han, S.U.; Iwasaki, Y.; Hyung, W.J.; Takagane, A.; Park, D.J.; et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): A phase 3, randomised controlled trial. Lancet Oncol. 2016, 17, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Song, Y.; Wang, Z.; Gao, P.; Chen, X.; Xu, Y.; Liang, J.; Xu, H. Benefits of hyperthermic intraperitoneal chemotherapy for patients with serosal invasion in gastric cancer: A meta-analysis of the randomized controlled trials. BMC Cancer 2012, 12, 526. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H.; Jablonski, K.A. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann. Surg. 1995, 221, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H.; Cunliffe, W.J.; Belliveau, J.; de Bruijn, E.A.; Graves, T.; Mullins, R.E.; Schlag, P. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin. Oncol. 1989, 16, 83–97. [Google Scholar] [PubMed]
- Sugarbaker, P.H. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol. 2006, 7, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Peritonectomy procedures. Ann. Surg. 1995, 221, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat. Res. 1996, 82, 359–374. [Google Scholar]
- Zhang, J.F.; Lv, L.; Zhao, S.; Zhou, Q.; Jiang, C.G. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) Combined with Surgery: A 12-Year Meta-Analysis of this Promising Treatment Strategy for Advanced Gastric Cancer at Different Stages. Ann. Surg. Oncol. 2022, 29, 3170–3186. [Google Scholar] [CrossRef]
- Chen, Z.; Ali, M.; Kai, Z.; Wang, Y.; Wang, C. HIPEC with CRS versus cytoreductive surgery (CRS) for the gastric cancer metastasis to peritoneum. Clin. Transl. Oncol. 2023, 25, 1011–1016. [Google Scholar] [CrossRef]
- Desiderio, J.; Chao, J.; Melstrom, L.; Warner, S.; Tozzi, F.; Fong, Y.; Parisi, A.; Woo, Y. The 30-year experience-A meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur. J. Cancer 2017, 79, 1–14. [Google Scholar] [CrossRef]
- Bonnot, P.E.; Piessen, G.; Kepenekian, V.; Decullier, E.; Pocard, M.; Meunier, B.; Bereder, J.M.; Abboud, K.; Marchal, F.; Quenet, F.; et al. Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J. Clin. Oncol. 2019, 37, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Manzanedo, I.; Pereira, F.; Rihuete Caro, C.; Perez-Viejo, E.; Serrano, A.; Gutierrez Calvo, A.; Regueira, F.M.; Casado-Adam, A.; Cascales-Campos, P.A.; Arteaga, X.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Gastric Cancer with Peritoneal Carcinomatosis: Multicenter Study of Spanish Group of Peritoneal Oncologic Surgery (GECOP). Ann. Surg. Oncol. 2019, 26, 2615–2621. [Google Scholar] [CrossRef] [PubMed]
- Rau, B.; Brandl, A.; Piso, P.; Pelz, J.; Busch, P.; Demtroder, C.; Schule, S.; Schlitt, H.J.; Roitman, M.; Tepel, J.; et al. Peritoneal metastasis in gastric cancer: Results from the German database. Gastric Cancer 2020, 23, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Marano, L.; Marrelli, D.; Sammartino, P.; Biacchi, D.; Graziosi, L.; Marino, E.; Coccolini, F.; Fugazzola, P.; Valle, M.; Federici, O.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Synchronous Peritoneal Metastases: Multicenter Study of ‘Italian Peritoneal Surface Malignancies Oncoteam-S.I.C.O.’. Ann. Surg. Oncol. 2021, 28, 9060–9070. [Google Scholar] [CrossRef] [PubMed]
- Brandl, A.; Yonemura, Y.; Glehen, O.; Sugarbaker, P.; Rau, B. Long term survival in patients with peritoneal metastasised gastric cancer treated with cytoreductive surgery and HIPEC: A multi-institutional cohort from PSOGI. Eur. J. Surg. Oncol. 2021, 47, 172–180. [Google Scholar] [CrossRef]
- Badgwell, B.; Ikoma, N.; Murphy, M.B.; Wang, X.; Estrella, J.; Roy-Chowdhuri, S.; Das, P.; Minsky, B.D.; Lano, E.; Song, S.; et al. A Phase II Trial of Cytoreduction, Gastrectomy, and Hyperthermic Intraperitoneal Perfusion with Chemotherapy for Patients with Gastric Cancer and Carcinomatosis or Positive Cytology. Ann. Surg. Oncol. 2021, 28, 258–264. [Google Scholar] [CrossRef]
- Yang, X.J.; Huang, C.Q.; Suo, T.; Mei, L.J.; Yang, G.L.; Cheng, F.L.; Zhou, Y.F.; Xiong, B.; Yonemura, Y.; Li, Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: Final results of a phase III randomized clinical trial. Ann. Surg. Oncol. 2011, 18, 1575–1581. [Google Scholar] [CrossRef]
- Rudloff, U.; Langan, R.C.; Mullinax, J.E.; Beane, J.D.; Steinberg, S.M.; Beresnev, T.; Webb, C.C.; Walker, M.; Toomey, M.A.; Schrump, D.; et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: Results of the GYMSSA trial. J. Surg. Oncol. 2014, 110, 275–284. [Google Scholar] [CrossRef]
- Rau, B.; Lang, H.; Koenigsrainer, A.; Gockel, I.; Rau, H.G.; Seeliger, H.; Lerchenmueller, C.; Reim, D.; Wahba, R.; Angele, M.; et al. Effect of Hyperthermic Intraperitoneal Chemotherapy on Cytoreductive Surgery in Gastric Cancer With Synchronous Peritoneal Metastases: The Phase III GASTRIPEC-I Trial. J. Clin. Oncol. 2023, 42, 146–156. [Google Scholar] [CrossRef]
- NICE. Interventional Procedure Overview of Cytoreduction Surgery with Hyperthermic Intraoperative Peritoneal Chemotherapy for Peritoneal Carcinomatosis; National Institute of Health and Care Excellence: London, UK, 2021; IP 256/3 [IPG688]. [Google Scholar]
- Chicago Consensus Working Group. The Chicago Consensus on Peritoneal Surface Malignancies: Management of Gastric Metastases. Ann. Surg. Oncol. 2020, 27, 1768–1773. [Google Scholar] [CrossRef]
- Ukegjini, K.; Guidi, M.; Lehmann, K.; Suveg, K.; Putora, P.M.; Cihoric, N.; Steffen, T. Current Research and Development in Hyperthermic Intraperitoneal Chemotherapy (HIPEC)-A Cross-Sectional Analysis of Clinical Trials Registered on ClinicalTrials.gov. Cancers 2023, 15, 1926. [Google Scholar] [CrossRef]
- Gotze, T.O.; Piso, P.; Lorenzen, S.; Bankstahl, U.S.; Pauligk, C.; Elshafei, M.; Amato, G.; Reim, D.; Bechstein, W.O.; Konigsrainer, A.; et al. Preventive HIPEC in combination with perioperative FLOT versus FLOT alone for resectable diffuse type gastric and gastroesophageal junction type II/III adenocarcinoma—The phase III “PREVENT”-(FLOT9) trial of the AIO /CAOGI /ACO. BMC Cancer 2021, 21, 1158. [Google Scholar] [CrossRef]
- Di Giorgio, A.; Gerardi, C.; Abatini, C.; Melotti, G.; Bonavina, L.; Torri, V.; Santullo, F.; Garattini, S.; De Luca, M.; Rulli, E.; et al. Prophylactic surgery plus hyperthermic intraperitoneal chemotherapy (HIPEC CO2) versus standard surgery for gastric carcinoma at high risk of peritoneal carcinomatosis: Short and long-term outcomes (GOETH STUDY)-a collaborative randomized controlled trial by ACOI, FONDAZIONE AIOM, SIC, SICE, and SICO. Trials 2022, 23, 969. [Google Scholar] [CrossRef]
- Glehen, O.; Passot, G.; Villeneuve, L.; Vaudoyer, D.; Bin-Dorel, S.; Boschetti, G.; Piaton, E.; Garofalo, A. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: A randomized and multicenter phase III study. BMC Cancer 2014, 14, 183. [Google Scholar] [CrossRef]
- Koemans, W.J.; van der Kaaij, R.T.; Boot, H.; Buffart, T.; Veenhof, A.; Hartemink, K.J.; Grootscholten, C.; Snaebjornsson, P.; Retel, V.P.; van Tinteren, H.; et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy versus palliative systemic chemotherapy in stomach cancer patients with peritoneal dissemination, the study protocol of a multicentre randomised controlled trial (PERISCOPE II). BMC Cancer 2019, 19, 420. [Google Scholar] [CrossRef]
- Takahashi, N.; Kanda, M.; Yoshikawa, T.; Takiguchi, N.; Fujitani, K.; Miyamoto, K.; Ito, Y.; Takayama, O.; Imano, M.; Mitsumori, N.; et al. A randomized phase II multicenter trial to explore efficacy of weekly intraperitoneal in comparison with intravenous paclitaxel administered immediately after gastrectomy to the patients with high risk of peritoneal recurrence: Final results of the INPACT trial. Gastric Cancer 2018, 21, 1014–1023. [Google Scholar] [CrossRef]
- Ishigami, H.; Fujiwara, Y.; Fukushima, R.; Nashimoto, A.; Yabusaki, H.; Imano, M.; Imamoto, H.; Kodera, Y.; Uenosono, Y.; Amagai, K.; et al. Phase III Trial Comparing Intraperitoneal and Intravenous Paclitaxel Plus S-1 Versus Cisplatin Plus S-1 in Patients With Gastric Cancer With Peritoneal Metastasis: PHOENIX-GC Trial. J. Clin. Oncol. 2018, 36, 1922–1929. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kitayama, J.; Ishigami, H.; Emoto, S.; Yamashita, H.; Watanabe, T. A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer 2013, 119, 3354–3358. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Kodera, Y.; Fukushima, R.; Morita, M.; Fushida, S.; Yamashita, N.; Yoshikawa, K.; Ueda, S.; Yabusaki, H.; Kusumoto, T.; et al. Phase II Study of Intraperitoneal Administration of Paclitaxel Combined with S-1 and Cisplatin for Gastric Cancer with Peritoneal Metastasis. Ann. Surg. Oncol. 2024, 31, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, M.; Imano, M.; Chiba, Y.; Iwama, M.; Shiraisi, O.; Yasuda, A.; Tsubaki, M.; Nishida, S.; Kimura, Y.; Yasuda, T. Phase II trial of neoadjuvant chemotherapy with intraperitoneal paclitaxel, S-1, and intravenous cisplatin and paclitaxel for stage IIIA or IIIB gastric cancer. J. Surg. Oncol. 2019, 119, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Yamaguchi, H.; Ohzawa, H.; Miyato, H.; Kanamaru, R.; Kurashina, K.; Hosoya, Y.; Lefor, A.K.; Sata, N.; Kitayama, J. Intraperitoneal Administration of Paclitaxel Combined with S-1 Plus Oxaliplatin as Induction Therapy for Patients with Advanced Gastric Cancer with Peritoneal Metastases. Ann. Surg. Oncol. 2021, 28, 3863–3870. [Google Scholar] [CrossRef]
- Yonemura, Y.; Canbay, E.; Li, Y.; Coccolini, F.; Glehen, O.; Sugarbaker, P.H.; Morris, D.; Moran, B.; Gonzaletz-Moreno, S.; Deraco, M.; et al. A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent. Eur. J. Surg. Oncol. 2016, 42, 1123–1131. [Google Scholar] [CrossRef]
- Yonemura, Y.; Prabhu, A.; Sako, S.; Ishibashi, H.; Mizumoto, A.; Takao, N.; Ichinose, M.; Motoi, S.; Liu, Y.; Nishihara, K.; et al. Long Term Survival after Cytoreductive Surgery Combined with Perioperative Chemotherapy in Gastric Cancer Patients with Peritoneal Metastasis. Cancers 2020, 12, 116. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Yuan, F.; Lu, S.; Xu, W.; Wu, J.W.; Xi, W.Q.; Shi, M.; Wang, Z.Q.; Ni, Z.T.; He, C.Y.; et al. Efficacy and Safety of Conversion Therapy by Intraperitoneal and Intravenous Paclitaxel Plus Oral S-1 in Gastric Cancer Patients With Peritoneal Metastasis: A Prospective Phase II Study. Front. Oncol. 2022, 12, 905922. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Wang, P.; Zhu, Z.; Zhang, J.; Huang, J.; Wang, T.; Chen, J.; Xu, H. Benefits of Surgery After Neoadjuvant Intraperitoneal and Systemic Chemotherapy for Gastric Cancer Patients With Peritoneal Metastasis: A Meta-Analysis. J. Surg. Res. 2020, 245, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Beeharry, M.K.; Ni, Z.T.; Yang, Z.Y.; Zheng, Y.N.; Feng, R.H.; Liu, W.T.; Yan, C.; Yao, X.X.; Li, C.; Yan, M.; et al. Study protocol of a multicenter phase III randomized controlled trial investigating the efficiency of the combination of neoadjuvant chemotherapy (NAC) and neoadjuvant laparoscopic intraperitoneal hyperthermic chemotherapy (NLHIPEC) followed by R0 gastrectomy with intraoperative HIPEC for advanced gastric cancer (AGC): Dragon II trial. BMC Cancer 2020, 20, 224. [Google Scholar] [CrossRef]
- Lu, S.; Yang, Z.Y.; Yan, C.; Liu, W.T.; Ni, Z.T.; Yao, X.X.; Hua, Z.C.; Feng, R.H.; Zheng, Y.N.; Wang, Z.Q.; et al. A phase III trial of neoadjuvant intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis. Future Oncol. 2022, 18, 1175–1183. [Google Scholar] [CrossRef]
- Solass, W.; Kerb, R.; Murdter, T.; Giger-Pabst, U.; Strumberg, D.; Tempfer, C.; Zieren, J.; Schwab, M.; Reymond, M.A. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: First evidence for efficacy. Ann. Surg. Oncol. 2014, 21, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Giger-Pabst, U.; Tempfer, C.B. How to Perform Safe and Technically Optimized Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): Experience After a Consecutive Series of 1200 Procedures. J. Gastrointest. Surg. 2018, 22, 2187–2193. [Google Scholar] [CrossRef]
- Alyami, M.; Hubner, M.; Grass, F.; Bakrin, N.; Villeneuve, L.; Laplace, N.; Passot, G.; Glehen, O.; Kepenekian, V. Pressurised intraperitoneal aerosol chemotherapy: Rationale, evidence, and potential indications. Lancet Oncol. 2019, 20, e368–e377. [Google Scholar] [CrossRef]
- Di Giorgio, A.; Macri, A.; Ferracci, F.; Robella, M.; Visaloco, M.; De Manzoni, G.; Sammartino, P.; Sommariva, A.; Biacchi, D.; Roviello, F.; et al. 10 Years of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC): A Systematic Review and Meta-Analysis. Cancers 2023, 15, 1125. [Google Scholar] [CrossRef]
- Nadiradze, G.; Giger-Pabst, U.; Zieren, J.; Strumberg, D.; Solass, W.; Reymond, M.A. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with Low-Dose Cisplatin and Doxorubicin in Gastric Peritoneal Metastasis. J. Gastrointest. Surg. 2016, 20, 367–373. [Google Scholar] [CrossRef]
- Alyami, M.; Mercier, F.; Siebert, M.; Bonnot, P.E.; Laplace, N.; Villeneuve, L.; Passot, G.; Glehen, O.; Bakrin, N.; Kepenekian, V. Unresectable peritoneal metastasis treated by pressurized intraperitoneal aerosol chemotherapy (PIPAC) leading to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 2021, 47, 128–133. [Google Scholar] [CrossRef]
- Casella, F.; Bencivenga, M.; Brancato, G.; Torroni, L.; Ridolfi, C.; Puccio, C.; Alloggio, M.; Meloni, F.; Fusario, D.; Marrelli, D.; et al. Bidirectional Approach with PIPAC and Systemic Chemotherapy for Patients with Synchronous Gastric Cancer Peritoneal Metastases (GCPM). Ann. Surg. Oncol. 2023, 30, 5733–5742. [Google Scholar] [CrossRef]
- Sindayigaya, R.; Dogan, C.; Demtroder, C.R.; Fischer, B.; Karam, E.; Buggisch, J.R.; Tempfer, C.B.; Lecomte, T.; Ouaissi, M.; Giger-Pabst, U. Clinical Outcome for Patients Managed with Low-Dose Cisplatin and Doxorubicin Delivered as Pressurized Intraperitoneal Aerosol Chemotherapy for Unresectable Peritoneal Metastases of Gastric Cancer. Ann. Surg. Oncol. 2022, 29, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Khomyakov, V.; Ryabov, A.; Ivanov, A.; Bolotina, L.; Utkina, A.; Volchenko, N.; Kaprin, A. Bidirectional chemotherapy in gastric cancer with peritoneal metastasis combining intravenous XELOX with intraperitoneal chemotherapy with low-dose cisplatin and Doxorubicin administered as a pressurized aerosol: An open-label, Phase-2 study (PIPAC-GA2). Pleura Peritoneum 2016, 1, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Struller, F.; Horvath, P.; Solass, W.; Weinreich, F.J.; Strumberg, D.; Kokkalis, M.K.; Fischer, I.; Meisner, C.; Konigsrainer, A.; Reymond, M.A. Pressurized intraperitoneal aerosol chemotherapy with low-dose cisplatin and doxorubicin (PIPAC C/D) in patients with gastric cancer and peritoneal metastasis: A phase II study. Ther. Adv. Med. Oncol. 2019, 11, 1758835919846402. [Google Scholar] [CrossRef] [PubMed]
- Graversen, M.; Detlefsen, S.; Ainsworth, A.P.; Fristrup, C.W.; Knudsen, A.O.; Pfeiffer, P.; Tarpgaard, L.S.; Mortensen, M.B. Treatment of Peritoneal Metastasis with Pressurized Intraperitoneal Aerosol Chemotherapy: Results from the Prospective PIPAC-OPC2 Study. Ann. Surg. Oncol. 2023, 30, 2634–2644. [Google Scholar] [CrossRef] [PubMed]
- Kryh-Jensen, C.G.; Fristrup, C.W.; Ainsworth, A.P.; Detlefsen, S.; Mortensen, M.B.; Pfeiffer, P.; Tarpgaard, L.S.; Graversen, M. What is long-term survival in patients with peritoneal metastasis from gastric, pancreatic, or colorectal cancer? A study of patients treated with systemic chemotherapy and pressurized intraperitoneal aerosol chemotherapy (PIPAC). Pleura Peritoneum 2023, 8, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Ellebaek, S.B.; Graversen, M.; Detlefsen, S.; Lundell, L.; Fristrup, C.W.; Pfeiffer, P.; Mortensen, M.B. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) of peritoneal metastasis from gastric cancer: A descriptive cohort study. Clin. Exp. Metastasis 2020, 37, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Sgarbura, O.; Eveno, C.; Alyami, M.; Bakrin, N.; Guiral, D.C.; Ceelen, W.; Delgadillo, X.; Dellinger, T.; Di Giorgio, A.; Kefleyesus, A.; et al. Consensus statement for treatment protocols in pressurized intraperitoneal aerosol chemotherapy (PIPAC). Pleura Peritoneum 2022, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Casella, F.; Bencivenga, M.; Rosati, R.; Fumagalli, U.R.; Marrelli, D.; Pacelli, F.; Macri, A.; Donini, A.; Torroni, L.; Pavarana, M.; et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) in multimodal therapy for patients with oligometastatic peritoneal gastric cancer: A randomized multicenter phase III trial PIPAC VEROne. Pleura Peritoneum 2022, 7, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Eveno, C.; Jouvin, I.; Pocard, M. PIPAC EstoK 01: Pressurized IntraPeritoneal Aerosol Chemotherapy with cisplatin and doxorubicin (PIPAC C/D) in gastric peritoneal metastasis: A randomized and multicenter phase II study. Pleura Peritoneum 2018, 3, 20180116. [Google Scholar] [CrossRef] [PubMed]
- Kopecky, K.; Monton, O.; Rosman, L.; Johnston, F. Palliative interventions for patients with advanced gastric cancer: A systematic review. Chin. Clin. Oncol. 2022, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Shrestha, R.D.; Kokubun, M.; Ohta, M.; Takahashi, M.; Kobayashi, K.; Kiuchi, S.; Okui, K.; Miyoshi, T.; Arimizu, N.; et al. Intraperitoneal hyperthermic perfusion combined with surgery effective for gastric cancer patients with peritoneal seeding. Ann. Surg. 1988, 208, 36–41. [Google Scholar] [CrossRef]
- Valle, S.J.; Alzahrani, N.A.; Alzahrani, S.E.; Liauw, W.; Morris, D.L. Laparoscopic hyperthermic intraperitoneal chemotherapy (HIPEC) for refractory malignant ascites in patients unsuitable for cytoreductive surgery. Int. J. Surg. 2015, 23, 176–180. [Google Scholar] [CrossRef]
- Facchiano, E.; Scaringi, S.; Kianmanesh, R.; Sabate, J.M.; Castel, B.; Flamant, Y.; Coffin, B.; Msika, S. Laparoscopic hyperthermic intraperitoneal chemotherapy (HIPEC) for the treatment of malignant ascites secondary to unresectable peritoneal carcinomatosis from advanced gastric cancer. Eur. J. Surg. Oncol. 2008, 34, 154–158. [Google Scholar] [CrossRef]
- Jiao, J.; Li, C.; Yu, G.; Zhang, L.; Shi, X.; Yan, J.; Zhang, H.; Guo, P. Efficacy of hyperthermic intraperitoneal chemotherapy (HIPEC) in the management of malignant ascites. World J. Surg. Oncol. 2020, 18, 180. [Google Scholar] [CrossRef]
- Zhao, C.; Dai, C.; Chen, X. Whole-body hyperthermia combined with hyperthermic intraperitoneal chemotherapy for the treatment of stage IV advanced gastric cancer. Int. J. Hyperthermia 2012, 28, 735–741. [Google Scholar] [CrossRef]
- Yarema, R.R.; Ohorchak, M.A.; Zubarev, G.P.; Mylyan, Y.P.; Oliynyk, Y.Y.; Zubarev, M.G.; Gyrya, P.I.; Kovalchuk, Y.J.; Safiyan, V.I.; Fetsych, T.G. Hyperthermic intraperitoneal chemoperfusion in combined treatment of locally advanced and disseminated gastric cancer: Results of a single-centre retrospective study. Int. J. Hyperthermia 2014, 30, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ning, M.Y.; Han, D.; Li, L.; Li, Z.Z. Hyperthermic Intraperitoneal Chemotherapy plus Intravenous Chemotherapy of Paclitaxel with or without Sintilimab in Gastric Cancer: A Comparative Study. J. Oncol. 2022, 2022, 3054485. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Hofheinz, R.D.; Pauligk, C.; Kopp, H.G.; Haag, G.M.; Luley, K.B.; Meiler, J.; Homann, N.; Lorenzen, S.; Schmalenberg, H.; et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): Results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016, 17, 1697–1708. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Illerhaus, G.; Martens, U.M.; Stoehlmacher, J.; Schmalenberg, H.; Luley, K.B.; Prasnikar, N.; Egger, M.; et al. Effect of Neoadjuvant Chemotherapy Followed by Surgical Resection on Survival in Patients With Limited Metastatic Gastric or Gastroesophageal Junction Cancer: The AIO-FLOT3 Trial. JAMA Oncol. 2017, 3, 1237–1244. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef]
- Mullen, J.T. Top Gastric Cancer Articles from 2022 and 2023 to Inform Your Cancer Practice. Ann. Surg. Oncol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Petrioli, R.; Francini, E.; Cherri, S.; Marrelli, D.; Rovello, F.; Fiaschi, A.I.; Miano, S.T.; Savelli, V.; Calomino, N.; Farsi, M.; et al. Feasibility of modified docetaxel, oxaliplatin, capecitabine followed by capecitabine as maintenance chemotherapy as first-line therapy for patients with metastatic gastric or gastroesophageal cancer. Anticancer Drugs 2020, 31, 292–297. [Google Scholar] [CrossRef]
- Badgwell, B.; Blum, M.; Das, P.; Estrella, J.; Wang, X.; Ho, L.; Fournier, K.; Royal, R.; Mansfield, P.; Ajani, J. Phase II Trial of Laparoscopic Hyperthermic Intraperitoneal Chemoperfusion for Peritoneal Carcinomatosis or Positive Peritoneal Cytology in Patients with Gastric Adenocarcinoma. Ann. Surg. Oncol. 2017, 24, 3338–3344. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Gilly, F.N.; Arvieux, C.; Cotte, E.; Boutitie, F.; Mansvelt, B.; Bereder, J.M.; Lorimier, G.; Quenet, F.; Elias, D.; et al. Peritoneal carcinomatosis from gastric cancer: A multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann. Surg. Oncol. 2010, 17, 2370–2377. [Google Scholar] [CrossRef]
- Piso, P.; Slowik, P.; Popp, F.; Dahlke, M.H.; Glockzin, G.; Schlitt, H.J. Safety of gastric resections during cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Ann. Surg. Oncol. 2009, 16, 2188–2194. [Google Scholar] [CrossRef]
- Shen, P.; Levine, E.A.; Hall, J.; Case, D.; Russell, G.; Fleming, R.; McQuellon, R.; Geisinger, K.R.; Loggie, B.W. Factors predicting survival after intraperitoneal hyperthermic chemotherapy with mitomycin C after cytoreductive surgery for patients with peritoneal carcinomatosis. Arch. Surg. 2003, 138, 26–33. [Google Scholar] [CrossRef]
- van der Kaaij, R.T.; Wassenaar, E.C.E.; Koemans, W.J.; Sikorska, K.; Grootscholten, C.; Los, M.; Huitema, A.; Schellens, J.H.M.; Veenhof, A.; Hartemink, K.J.; et al. Treatment of PERItoneal disease in Stomach Cancer with cytOreductive surgery and hyperthermic intraPEritoneal chemotherapy: PERISCOPE I initial results. Br. J. Surg. 2020, 107, 1520–1528. [Google Scholar] [CrossRef]
- Yarema, R.; Mielko, J.; Fetsych, T.; Ohorchak, M.; Skorzewska, M.; Rawicz-Pruszynski, K.; Mashukov, A.; Maksimovsky, V.; Jastrzebski, T.; Polkowski, W.; et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) in combined treatment of locally advanced and intraperitonealy disseminated gastric cancer: A retrospective cooperative Central-Eastern European study. Cancer Med. 2019, 8, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, Y.; Kawamura, T.; Bandou, E.; Takahashi, S.; Sawa, T.; Matsuki, N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br. J. Surg. 2005, 92, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Newhook, T.E.; Agnes, A.; Blum, M.; Estrella, J.S.; Das, P.; Ho, L.; Ajani, J.A.; Minsky, B.D.; Mansfield, P.; Badgwell, B.D. Laparoscopic Hyperthermic Intraperitoneal Chemotherapy is Safe for Patients with Peritoneal Metastases from Gastric Cancer and May Lead to Gastrectomy. Ann. Surg. Oncol. 2019, 26, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, Y.; Ishibashi, H.; Hirano, M.; Mizumoto, A.; Takeshita, K.; Noguchi, K.; Takao, N.; Ichinose, M.; Liu, Y.; Li, Y. Effects of Neoadjuvant Laparoscopic Hyperthermic Intraperitoneal Chemotherapy and Neoadjuvant Intraperitoneal/Systemic Chemotherapy on Peritoneal Metastases from Gastric Cancer. Ann. Surg. Oncol. 2017, 24, 478–485. [Google Scholar] [CrossRef]
| Trial ID | Phase | Allocation | N | Start | End (est.) | Land | Status |
|---|---|---|---|---|---|---|---|
| NCT02381847 | phase III | randomized | 60 | 2015 | 2020 | China | unknown |
| NCT03179579 | phase III | randomized | 88 | 2017 | 2022 | China | unknown |
| NCT03023436 | phase III | single-arm | 220 | 2016 | 2022 | China | unknown |
| NCT03348150 | phase III | randomized | 182 | 2017 | 2029 | The Netherlands | recruiting |
| Author | Study Design | Morbidity, SAE [%] | Mortality [%] | Histological Response [%] | Median OS [Months] |
|---|---|---|---|---|---|
| Di Giorgio et al. [53] | meta-analysis | 4 | 1.3 | 69.2 | 12.3 |
| Nadiradze et al. [54] | retrospective | 12.5 | 8.3 | 50 | 15.4 |
| Alyami et al. [55] | retrospective | 6.1 | 4.7 | n.a. | 19.1 |
| Casella et al. [56] | retrospective | 4 | 0 | n.a. | 10.5 |
| Sindayigaya et al. [57] | retrospective | 4.9 | 1.4 | 73 | 11 |
| Khomyakov et al. [58] | phase II | 3.2 | 0 | 60 | 13 |
| Struller et al. [59] | phase II | 12 | 0 | 75 | 6.7 |
| Graversen et al. [60] | phase II | 4.5 | 4 | 61 * | 7.4 ** |
| Author | HIPEC Regimen | Duration of Perfusion | RCT |
|---|---|---|---|
| Badgwell et al. [79] | mitomycin C 30 mg + cisplatin 200 mg intravenous sodium thiosulfate | 60 min | no |
| Glehen et al. [80] | mitomycin C 30–50 mg/m2 ± cisplatin 50–100 mg/m2 | 60–120 min | no |
| Glehen et al. [80] | oxaliplatin 360–460 mg/m2 ± irinotecan 100–200 mg/m2 ± intravenous 5-FU/FA | 30 min | no |
| Koemans et al. [37] | oxaliplatin 460 mg/m2 + docetaxel 50 mg/m2 | 30 + 90 min | yes |
| Piso et al. [81] | cisplatin 75 mg/m2 + doxorubicin 15 mg/m2 | 60 min | no |
| Rau et al. [30] | mitomycin C 15 mg/m2 + cisplatin 75 mg/m2 | 60 min | yes |
| Rudloff et al. [29] | oxaliplatin 460 mg/m2 + intravenous 5-FU/FA | 30 min | yes |
| Shen et al. [82] | mitomycin C 30 mg + additional 10 mg at 60 min | 120 min | no |
| Van der Kaaij [83] | oxaliplatin 460 mg/m2 + docetaxel 0, 50, 75 mg/m2 | 30 + 90 min | no |
| Yang et al. [28] | mitomycin C 30 mg + cisplatin 120 mg | 60–90 min | yes |
| Yarema et al. [72] | mitomycin C 12.5 mg/m2 + cisplatin 75 mg/m2 + intravenous 5-FU | 90 min | no |
| Yarema et al. [84] | mitomycin C 10–15 mg/m2 ± cisplatin 75 mg/m2 ± intravenous 5-FU cisplatin 75 mg/m2 + doxorubicin 15 mg/m2 oxaliplatin 460 mg/m2 + intravenous 5-FU | 30–90 min | no |
| Yonemura et al. [85] | mitomycin C 30 mg + cisplatin 300 mg + etoposide 150 mg | 60 min | no |
| Yonemura et al. [45] | mitomycin C 12.5 mg/m2 + cisplatin 50 mg/m2 | 60 min | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acs, M.; Piso, P.; Glockzin, G. Peritoneal Metastatic Gastric Cancer: Local Treatment Options and Recommendations. Curr. Oncol. 2024, 31, 1445-1459. https://doi.org/10.3390/curroncol31030109
Acs M, Piso P, Glockzin G. Peritoneal Metastatic Gastric Cancer: Local Treatment Options and Recommendations. Current Oncology. 2024; 31(3):1445-1459. https://doi.org/10.3390/curroncol31030109
Chicago/Turabian StyleAcs, Miklos, Pompiliu Piso, and Gabriel Glockzin. 2024. "Peritoneal Metastatic Gastric Cancer: Local Treatment Options and Recommendations" Current Oncology 31, no. 3: 1445-1459. https://doi.org/10.3390/curroncol31030109
APA StyleAcs, M., Piso, P., & Glockzin, G. (2024). Peritoneal Metastatic Gastric Cancer: Local Treatment Options and Recommendations. Current Oncology, 31(3), 1445-1459. https://doi.org/10.3390/curroncol31030109




