1. Introduction
Chronic graft-versus-host disease (cGvHD) is a syndrome with diverse clinical features resembling autoimmune and immunological disorders, occurring after allogeneic hematopoietic cell transplantation (HCT) [
1,
2]. It can affect the long-term outcomes of allogeneic HCT patients by increasing morbidity and mortality [
3] and is associated with a reduced quality of life [
4,
5,
6]. It often requires long-term immunosuppressive therapy, which can lead to the development of significant side effects and toxicities [
1,
2,
3]. While there is no national guideline for the treatment of cGvHD in Canada [
7], transplant program specific standardized protocols with similarities across the country do exist in each institution. These protocols include many systemic therapeutic options and are not just limited to systemic corticosteroids, extracorporeal photopheresis [
8,
9], rituximab, sirolimus, or mycophenolate mofetil [
7,
10,
11]. Recently, newer agents have become accessible to some provinces and territories including ibrutinib [
12], ruxolitinib [
13], and belumosudil [
14], while axatilimab is awaiting Health Canada approval [
15]. However, standardized and systemic approaches are still scarce, thus demanding a nationwide standardized guideline.
Although comprehensive international guidelines on cGvHD management have been published [
16,
17,
18], a Canadian-specific guideline is still necessary. There are barriers to prevent the standardization of clinical practice such as Canada’s unique healthcare structure, variable funding mechanisms across the country, differences in provincial medication approval processes, the geographic size of Canada, and the patient populations that exist within the Canadian healthcare system. Accordingly, tailored recommendations are needed to optimize the management of cGvHD in Canada. Particularly, accessibility to newer agents is problematic for countries with a publicly funded healthcare system, where healthcare service models are quite different from the US-based healthcare system. This raises the issue of how to access the best treatment in countries with a restricted healthcare system like Canada, Australia, New Zealand, and other European countries, requiring innovation to manage this challenging patient population.
Through a national consensus guideline, ensuring consistency in the diagnosis and management of cGvHD across the provinces and institutions with different funding and approval systems will eventually lead to facilitating the transplant program’s access to novel therapies and newer agents and will offer our patients standardized care, leading to improved patient outcomes and cost savings in the healthcare system. Accordingly, this document will serve as a consensus-based Canadian guideline for the clinical management of cGvHD patients focusing on (1) the initial assessment and diagnosis of cGvHD, (2) organ severity scoring and global grading, (3) the therapeutic approach, including systemic, topical and supportive treatment, and (4) the management of specific cGvHD patient subpopulations such as pediatric patients [
19]. Finally, this paper will highlight the need for innovative approaches for the management of cGvHD with commentary on the future direction of chronic GvHD treatment development [
20,
21,
22,
23,
24].
2. Consensus Process of the Cell Therapy Transplant Canada (CTTC) Guideline for Chronic GvHD Management
Current clinical practice in cGvHD management is quite heterogeneous in Canada [
7]. Institutions from the various provinces and territories have similar but different practices and policies for the management of cGvHD. Accordingly, experts across Canada reached a consensus and came together to develop this Canadian consensus guideline for cGvHD management.
Initially, a first draft was established within the Princess Margaret Cancer Centre allogeneic hematopoietic stem cell transplant program in early 2023, which was based on internal consensus. Consultation was then expanded to include a broader Canadian perspective under the Cell Therapy Transplant Canada (CTTC) organization, with contributing members from different academic institutions across Canada. All members of the CTTC writing committee have shared their clinical experience and feedback. All the writing committee members have read, reviewed, and approved the final draft.
3. Summary of the Biology and Pathogenesis of Chronic GvHD
cGvHD occurs as a result of an immune response mounted by transplanted donor immune cells against the recipient’s tissues [
1,
2]. In order to discuss the contemporary concepts of cGvHD treatment, it is necessary to review the pathogenesis of cGvHD. The pathophysiology of cGvHD involves a complex interplay between the donor immune cells and the recipient tissues, which leads to the deregulation of immune pathways, chronic inflammation, tissue damage, and fibrosis. The process begins with the activation of donor T cells by recipient antigens, which then proliferate and differentiate into effector T cells and attack recipient tissues, initiating an inflammatory response [
10]. Also, deregulated immune pathways contribute to chronic immune activation by recipient antigens and subsequent chronic inflammation [
10].
The pathogenesis of cGvHD has been characterized as arising in three phases, which occur simultaneously. The inflammatory phase includes the release of cytokines and chemokines, which recruit additional immune cells to the site of injury [
10]. In the immune deregulation phase, these immune cells then amplify the inflammatory response and cause tissue damage, leading to the release of additional danger signals that further stimulate the immune response. Finally, in the fibrotic phase, chronic inflammation can lead to irreversible tissue fibrosis [
10], which can impair organ function and cause long-term damage. The development of fibrosis is thought to be due to the activation of fibroblasts and myofibroblasts by the chronic inflammatory response. A fourth phase or component is suggested by the absence of regulatory populations including regulatory T cells, B cells, NK cells, and macrophages [
25]. Understanding the different involvement of disease phases is fundamental for therapeutic drug selection with different action mechanisms.
4. The Diagnosis of Chronic GvHD
The clinical manifestations of cGvHD are very diverse and heterogeneous. cGvHD generally involves several organs or sites and is rarely restricted to a single organ. It is characterized by features that differ from the typical dermatitis, enteritis, and cholestatic liver manifestations of acute GvHD. In order to reduce the risk of missing the recognition of such symptoms/signs, identifying key features from a systematic review of the patient in the clinic should be the first step [
1].
Supplementary Table S1 summarizes the organs frequently involved and the questions that can be asked in the clinic to capture the early symptoms of cGvHD [
11].
The diagnosis of cGvHD is based on a combination of clinical features and histopathology, functional, and laboratory testing. According to the NIH 2014 criteria [
26], the diagnosis of cGvHD requires the presence of at least one diagnostic clinical sign or feature of cGvHD, such as poikiloderma or esophageal web, lichenoid oral mucosal lesion, or sclerodermatous skin lesion, or the presence of at least one distinctive manifestation confirmed by biopsy or testing, such as keratoconjunctivitis sicca in the same or another organ (
Table 1). In addition, other possible diagnoses for clinical symptoms must be excluded. No time threshold, such as day 100, is set for the diagnosis of cGvHD. cGvHD can be classified into (1) classic cGvHD (i.e., without features or characteristics of acute GvHD) or (2) an overlap syndrome in which diagnostic or distinctive features of chronic GvHD and acute GvHD appear together.
The diagnosis of cGvHD can be supported by histopathologic findings, such as the presence of lymphocytic infiltrates or collagen deposit/fibrosis in the affected tissues. However, histopathologic findings alone are not sufficient for the diagnosis of cGvHD. In cases where the “distinctive” manifestation is the only sign of cGvHD, additional confirmation is required for the diagnosis of cGvHD, such as biopsy, imaging, or a pulmonary function test (PFT).
Whenever cGvHD is suspected, pulmonary function tests (PFTs) are strongly recommended given the frequency of the asymptomatic involvement and the importance of the early recognition of lung GvHD. The bronchodilator response should be assessed to rule out asthma. In addition to objective measures, patient-reported outcome measures, such as the modified Lee Symptom Scale [
27], can be used at the time of the initial diagnosis of cGvHD, particularly in the context of research purposes. Genital manifestations of cGvHD are often overlooked. Accordingly, a directed questionnaire searching for gynecological manifestations is strongly suggested. Gynecology assessment around day 100 will aid in the detection of genital GvHD in female patients, particularly in post-menopausal women where features of atrophic vaginitis may mask early cGvHD symptoms. Immunoglobulin quantitation around day 100 will help detect hypergammaglobulinemia, which is evident when B cell deregulation is the main pathway for cGvHD development, or hypogammaglobulinemia, which can predispose the patient to opportunistic infection during cGvHD treatment.
The diagnosis of cGvHD in children remains challenging. This is especially true in the diagnosis of lung cGvHD due to the fact that children tend to have more potential insults to their lungs with recurrent “childhood” respiratory virus infections, and the inability to perform standard spirometry-based pulmonary function tests before age six [
28]. Alternatives include the multiple breath washout test that can be carried out, as this test is allowed down to the age of three [
29]. Also, young children may not volunteer expressing symptoms such as dry eyes, and it takes an astute clinician to pick up xeropthalmia and usually more frequent exams by an ophthalmologist are required. Since most children and adolescents are not sexually active, vaginal involvement in girls is usually not recognized. Atypical manifestations of cGvHD may be more frequent in children, although this is still to be conclusively determined [
30].
In summary, we recommend the use of the NIH 2014 criteria for the diagnosis of cGvHD, with a strong suggestion to include investigations in other organs not showing any symptoms/signs of cGvHD such as the lungs or genitalia.
5. Atypical GvHD
The NIH consensus project of 2020 emphasized atypical GvHD. This is an emerging disease entity that reflects the recently recognized features of cGvHD that differ from the classical manifestations of cGvHD (
Table 2) [
22,
31]. It frequently involves the central nervous system, peripheral nervous system, lungs, serositis, kidney, musculoskeletal system, and immune-mediated cytopenia. Atypical cGvHD affects a substantial number of patients, and it can manifest before or without NIH-defined cGvHD features. Several risk factors for atypical GvHD were proposed such as prior acute GvHD, total body irradiation, and donor lymphocyte infusion [
31].
Clinical suspicion of atypical GvHD is the first step to establish its diagnosis and management. Its pathophysiology is not fully elucidated and strongly warrants further investigation. Provisional diagnostic criteria for suspected atypical manifestations of cGvHD have been published and require further validation. Atypical GvHD manifestations frequently require a different therapeutic approach [
22]. Thus, we recommend that patients with newly diagnosed cGvHD should be screened and monitored for the features of atypical cGvHD.
6. Organ Severity Scoring and Global Grading of Chronic GvHD
The NIH 2014 criteria provide guidelines for the organ severity scoring and global grading of cGvHD, which are based on the severity and extent of organ involvement [
26]. Organs that are commonly involved in cGvHD include the skin, liver, gastrointestinal (GI) tract, oral mucosa, eyes, and lungs. Particularly for sclerotic GvHD, assessment of the photographic range of motion (P-ROM) at the time of initial diagnosis is mandatory for subsequent response assessment. For each organ, specific clinical and laboratory criteria are used to assign a score ranging from 0 to 3, where 0 indicates no involvement and 3 indicates severe involvement (
Table 3).
The global grading of cGvHD is based on the overall severity of the disease, as determined by the organ scoring and clinical assessment [
26]. The global grading system includes three categories: mild, moderate, and severe grade. The classification is based on the maximal organ severity score, the number of organs involved, and the impact of organ involvement on the patient’s quality of life and daily activity level (
Table 4). This allows for a more comprehensive assessment of the disease burden and facilitates the monitoring of disease progression and treatment response.
In summary, we recommend applying the NIH 2024 criteria for organ severity scoring and global grading at the time of the initial diagnosis of cGvHD.
7. Current Strategies for Chronic GvHD Treatment
The treatment paradigm of cGvHD is in evolution, owing to a better understanding of its pathophysiology and the recent development of effective novel therapies. Systemic cGvHD therapy has three ideal goals, including (1) the induction of immunologic tolerance, (2) reversal and limiting organ damage and the preservation of affected organ function, and (3) the successful discontinuation of all systemic immunosuppression without the recurrence of GvHD and without relapse of hematological malignancy. When treating cGvHD, healthcare providers must be cognizant of the balance between the systemic immunosuppression required to control GvHD and the risk of infection/hematologic malignancy relapse and other complications associated with long-term immunosuppression.
Practical measures evaluating the efficacy of cGvHD treatment include: (1) the overall response rate based on the NIH proposed response criteria and a durable response after the achievement of an initial response; (2) the clinical benefit (defined as complete/partial response, as well as a stable disease but a significant reduction or discontinuation of corticosteroids) [
32]; (3) failure-free survival (FFS; defined as treatment switch due to an inadequate response/progression/intolerance to treatment, non-relapse mortality, or the recurrence of a hematologic malignancy) [
33,
34,
35] and overall survival; (4) the duration of systemic immunosuppressive treatment with the rapid reduction and/or discontinuation of corticosteroids to avoid toxicities from prolonged corticosteroid exposure [
36]; and (5) the regain of organ function, limit of adverse effects, and improvement in the quality of life (QoL), using the modified Lee Symptom Scale [
6,
36]. These measures have been frequently used in multiple clinical trials evaluating the efficacy of newer drugs for cGvHD treatment [
13,
14,
15].
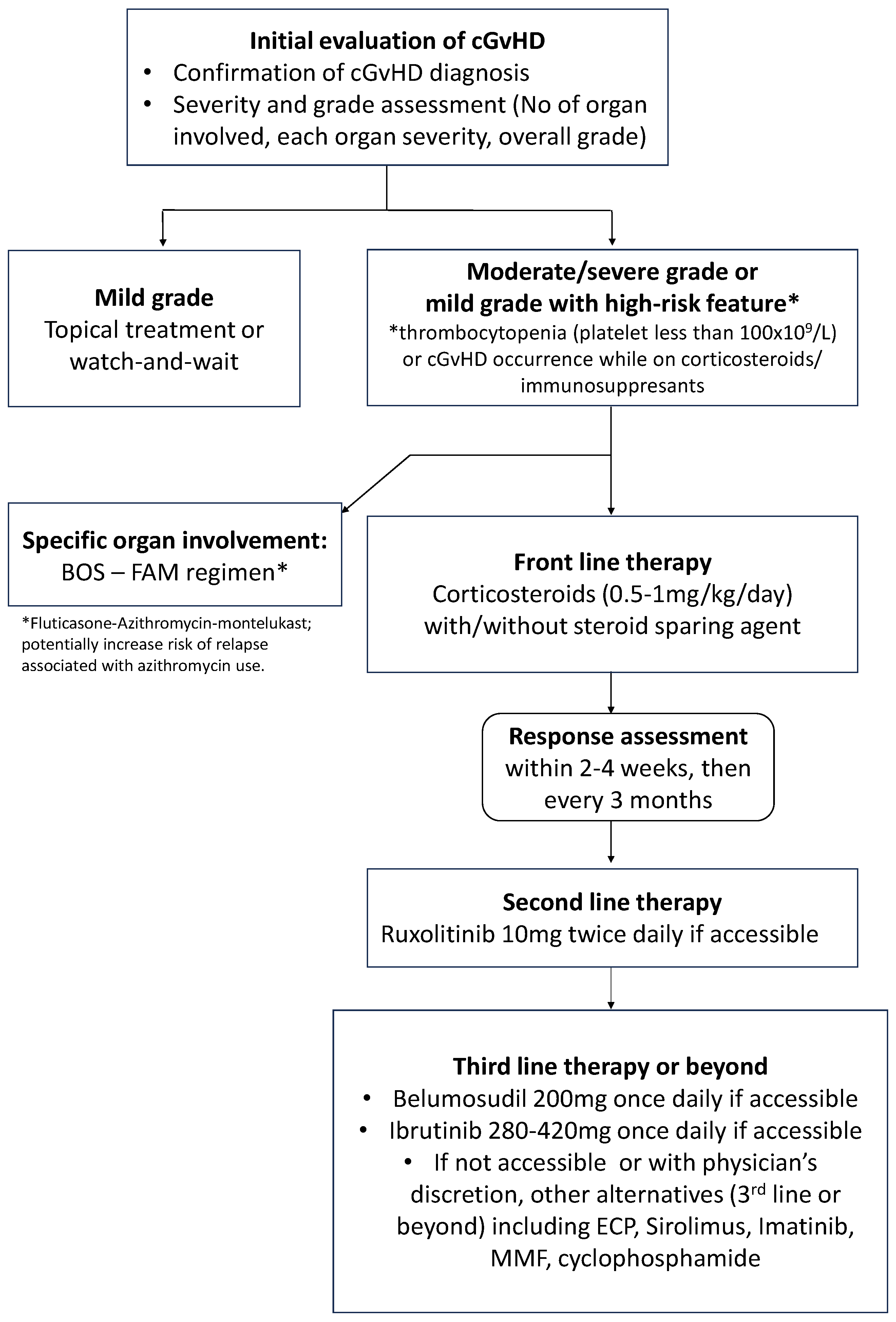
As shown in
Figure 1, the choice of therapy is based on the affected organs or sites, the severity and extent of cGvHD, other comorbidities or medical issues, potential drug–drug interactions, logistics, and reimbursement/individual patients’ drug coverage. Wide variability in practice is observed based on these factors and due to differences in institutional approaches and resources. The choice of steroid-sparing agent largely depends on the physician’s experience and its biological mechanism of action on the immune system [
10]. Children are unusually susceptible to the long-term side effects of steroid usage, including a much higher rate of osteonecrosis in puberty and the inhibition of bone growth leading to short stature as well as osteoporosis.
In keeping with published guidelines, we recommend the following as our current standard practice of cGvHD treatment:
- (1)
Front-line therapy of moderate-to-severe grade chronic GvHD is corticosteroids (prednisone 0.5–1 mg/kg or equivalent) with or without the addition of another systemic agent for steroid-sparing purpose such as calcineurin inhibitors, as these agents were reported to alter the natural course of the disease [
37,
38].
- (2)
To avoid any unnecessary drug-related side effects or toxicities from systemic immunosuppression and to avoid drug–drug interaction; the number of systemic agents is advised to be minimized as much as possible. However, with advances in newer therapeutic agents, combination strategies may now be reconsidered.
- (3)
Local or topical therapy such as corticosteroid-containing cream, a steroid bronchodilator, or an enteric form of corticosteroids should be maximized to avoid systemic steroid-induced, long-term toxicities.
- (4)
Supportive management, antimicrobial prophylaxis, and access to physiotherapy or rehabilitation programs are strongly recommended to avoid long-term side-effects and toxicities from chronic exposure to corticosteroids.
- (5)
Regular assessment of the response to the treatment per target organ is advised to expedite corticosteroid tapering.
8. Current Front-Line Therapy for Chronic GvHD Treatment
Systemic therapy is indicated for patients with moderate-to-severe grade cGvHD, which is defined according to the NIH 2014 criteria as the involvement of three or more organs, moderate or severe grade organ involvement in any organ, or any highly morbid form of GvHD, such as lung or sclerotic GvHD [
11]. Systemic treatment is also indicated for patients with mild grade cGvHD but presenting in conjunction with high-risk features such as thrombocytopenia (less than 100 × 10
9/L) or the development of cGvHD while on corticosteroid treatment. Symptomatic but mild grade cGvHD is often treated with topical therapies alone. Topical agents may also be used as adjuncts to systemic therapy to improve and accelerate the local response.
Front-line therapy for cGvHD treatment typically involves corticosteroids, which are potent anti-inflammatory agents that can suppress the underlying immune response causing cGvHD [
11]. The initial dose of corticosteroids is based on the severity of the disease, with higher doses indicated for more severe cases. It is recommended that prednisone is to be started at the dose of 0.5 to 1 mg/kg/day, with the goal of achieving at least a partial response within 2–4 weeks. When the patient is responding to corticosteroids, the dose can be gradually tapered over several months, with careful monitoring for GvHD recurrence or steroid-related toxicity.
Additional drugs can be added for steroid-sparing purposes. Given that prolonged systemic corticosteroid treatment causes significant toxicity, including weight gain, bone loss, myopathy, diabetes, hypertension, anxiety/depression, cataracts, and increased risk of infection, the combined therapy of corticosteroids with a steroid-sparing agent is generally recommended. While a calcineurin inhibitor (CNI) is the most commonly used steroid-sparing agent, its inhibitory effect on Treg lymphocytes can be detrimental. Past history of intolerance to calcineurin inhibitors, renal function impairment, and previous microangiopathy needs to be carefully reviewed before its selection as the steroid-sparing agent [
38].
Sirolimus (rapamycin) is a lipophilic macrocytic lactone with immunosuppressive properties [
39]. Its unique properties give it potential advantages over other immunosuppressive agents, including (1) immunosuppressive action through T cell inhibition, while promoting CD4
+CD25
+FoxP3
+ regulatory T cells (Tregs), a T cell population involved in graft-versus-leukemia reaction, (2) the inhibition of antigen presentation and dendritic cell maturation, (3) antifibrotic properties, (4) antineoplastic activity, (5) antiviral activity, and (6) the steroid-sparing effect. Although more frequently used for aGvHD prevention and treatment, its use as a steroid-sparing agent in cGvHD could be of interest [
39,
40].
Mycophenolate mofetil has failed to demonstrate its efficacy as a front-line therapy for cGvHD treatment, with an increased risk of hematologic malignancies and relapse [
41], while azathioprine is also known to increase the risk of secondary malignancies [
42]. Thus, several treatment guidelines, including the National Comprehensive Cancer Network (NCCN) guideline [
18], do not recommend the use of mycophenolate mofetil for front-line therapy and have advised against the use of azathioprine for cGvHD treatment [
43].
Steroid tapering is undertaken following the improvement or resolution of cGvHD-related symptoms and signs. Tapering schedules vary across different transplant centers [
11]. While some taper on an alternate-day tapering schedule [
11], others use a daily dose tapering policy. The efficacy of alternate-day vs. daily administration of corticosteroids has been reported in pediatric renal transplantation, but has not been tested in HCT [
11].
In summary, we recommend systemic treatment in patients with moderate-to-severe grade cGvHD as well as mild grade cGvHD with high-risk features. We also recommend the use of systemic corticosteroids with consideration of steroid-sparing agents as the current standard treatment. Diverse options for steroid-sparing agents exist including CNI and sirolimus, which are widely available in Canada.
9. Response Assessment following Chronic GvHD Treatment
The NIH 2014 criteria provide provisional response criteria for evaluating the efficacy of therapeutic interventions in patients with cGvHD [
44]. These criteria are based on the assessment of organ severity score, liver enzyme values, FEV1 value, photographic range of motion (P-ROM) score, or symptom score. Responses are classified as complete response (CR), partial response (PR), or lack of response (unchanged, mixed response, or progression), based on the improvement or worsening of the organ severity score, lab or PFT value, P-ROM score, or symptoms in each organ system. A detailed definition of each response, i.e., CR, PR, and lack of response, is summarized in
Table 5.
Therefore, we strongly recommend evaluating the response to cGvHD treatment within at least 2–4 weeks of therapy starting, with regular assessment every three months.
10. The Current Definition of Corticosteroid Failure following Chronic GvHD Treatment
In cases where there is no response to initial therapy, or if the response is incomplete or transient, systemic treatment should be switched to another therapy. The definition of steroid failure following frontline treatment is not fully established for cGvHD, in contrast to steroid-refractory acute GvHD, which has been well defined and standardized. Per the NIH 2014 criteria, the following criteria were proposed for the diagnosis of steroid-refractory cGvHD [
10,
36]: (1) a lack of response or disease progression after the administration of minimum prednisone 1 mg/kg/day for at least 1 week (i.e., steroid refractoriness), (2) disease persistence without improvement despite continued treatment with prednisone at >0.5 mg/kg/day or 1 mg/kg/every other day for at least 4 weeks (i.e., steroid refractory cGvHD), and (3) an increase in the prednisone dose to >0.25 mg/kg/day after two unsuccessful attempts to taper prednisone (i.e., steroid-dependent cGvHD).
We recommend paying special attention to corticosteroid failure to determine steroid refractory cGvHD, based on the NIH 2014 criteria.
11. Current Consensus for Second-Line Therapy or beyond for Chronic GvHD after Steroid Failure
The choice of second-line therapy is typically ruxolitinib, based on a randomized controlled trial [
13]. However, beyond second-line therapy, there is no standard and the treatment should be individualized based on the specific organ involvement, the severity and extent of cGvHD, the potential side effects of the medications, other comorbidities or medical issues, drug–drug interactions, logistics, and reimbursement. Before switching treatment due to progression or non-response in one organ, it is recommended that screening for other organ involvement by active cGvHD is undertaken by repeating PFTs and/or P-ROM score assessments.
Ruxolitinib, a Janus kinase (JAK) inhibitor, is the only drug that demonstrated a clinical benefit and superior efficacy over the best available therapy in a phase 3 trial as a second-line treatment [
13]. The REACH3 study and Canadian real-world experience study have shown that ruxolitinib can lead to improvements in symptoms, overall response, and failure-free survival (FFS) and can reduce the need for corticosteroids [
13,
32,
45]. Accordingly, it should be strongly considered as a second-line option for steroid-refractory cGvHD [
33,
45].
The choice of third-line or later therapies is not standardized and there are no well-controlled randomized trials to guide treatment selection. The following are variably available and effective cGvHD-active treatment options for third-line treatment or beyond: belumosudil [
14], ibrutinib [
46], ECP [
8,
9], sirolimus [
8,
9,
10,
11,
44], mycophenolate mofetil, and rituximab.
Table 6 summarizes the currently available and frequently used third-line and beyond treatment options in Canada [
7].
Table 7 compares the three newest options, i.e., ruxolitinib, belumosudil, and ibrutinib, which had been approved by Health Canada.
- (1)
Belumosudil: Belumosudil is a selective inhibitor of rho-associated coiled-coil kinase 2 (ROCK2), which is involved in the signaling pathways that lead to inflammation and tissue damage in cGvHD. Belumosudil has been shown to be effective in the treatment of cGvHD, particularly in the cases of sclerotic or mild-to-moderate lung GvHD [
14]. Clinical trials have shown that belumosudil can lead to improvements in symptoms, overall response, and FFS and can reduce the need for systemic immunosuppression [
14].
- (2)
Ibrutinib: Ibrutinib is a Bruton’s tyrosine kinase inhibitor that has been shown to be effective in the treatment of cGvHD. It works by blocking the activation of B cells and T cells, which play a key role in the development of cGvHD. Phase 2 clinical trials have shown that ibrutinib can lead to improvements in symptoms and organ function and can reduce the need for systemic immunosuppression [
12]. However, a real-world, experience-based study reported only a 9% failure-free survival (FFS) rate at two years with a median of 4.5 months of FFS [
46].
- (3)
Extracorporeal photopheresis (ECP): ECP is a therapeutic apheresis procedure that entails the separation of activated T lymphocytes from the
patient’s blood, which are subsequently subjected to ultraviolet light and a photosensitizing agent before being reinfused into the patient’s circulation. ECP has demonstrated efficacy in the management of steroid-resistant cGvHD, particularly in the setting of sclerotic GvHD and in Canadian practice [
8,
9]. Several studies have demonstrated its corticosteroid-sparing effect and potential role in combination with other drugs. Accessibility, tolerability, and appropriate blood counts are pre-requisites to the successful use of this modality. Several months of ECP may be needed before tangible improvement can be seen.
- (4)
Sirolimus: This agent operates by suppressing the activity of mTOR, a protein involved in the regulation of cellular growth and proliferation.
Sirolimus may be employed either as monotherapy or in conjunction with calcineurin inhibitors, and the dosage is tailored to the individual patient’s response and tolerance [
47]. Emerging data suggest sirolimus may be superior to cyclosporine or tacrolimus, based on its beneficial sparing effect on regulatory T cells [
47,
48], but this is still not confirmed in large trials with sufficient follow-up.
- (5)
Other systemic agents: Other immunosuppressive agents, such as mycophenolate mofetil [
49], rituximab, imatinib [
50], methotrexate, or
cyclophosphamide, may be used as salvage therapy in cases of steroid-refractory cGvHD. These medications are often reserved for cases where other therapies have failed, as they can be associated with significant toxicity. Axatilimab is also very promising and is awaiting approval by the US-FDA, as of December 2023 [
15].
Multiple aspects need to be considered for third-line therapy or beyond such as the mode of action of the drug, organ-specific treatment outcome, infection history, comorbidity, relapse risk, compliance/logistics, and funding and treatment access. The organ-specific action of the treatment should also be taken into consideration. For instance, cyclophosphamide has been adopted for renal GvHD manifesting as nephrotic syndrome [
51], while the FAM regimen (i.e., fluticasone-azithromycin-montelukast) has been used for pulmonary GvHD [
52,
53], although there is a concern for an increased risk of relapse associated with the use of azithromycin [
54]. It is recognized that ruxolitinib has little evidence supporting its use in some of the atypical cGvHD manifestations (e.g., immune cytopenias and renal and neurologic manifestations) and that other immunosuppressive agents (e.g., rituximab) may be preferable in these settings.
In conclusion, we recommend ruxolitinib as a second-line therapy in the patients experiencing corticosteroid failure following front-line cGvHD treatment, while other factors are to be considered for other therapeutic options. As a third-line option or beyond, newer agents, other systemic agents, and ECP can be used, while considering their funding accessibility.
Table 6.
Summary of commonly used treatment options in Canada for steroid-refractory chronic GvHD as second-line therapy or beyond, reprinted/adapted from Ref. [
10].
Table 6.
Summary of commonly used treatment options in Canada for steroid-refractory chronic GvHD as second-line therapy or beyond, reprinted/adapted from Ref. [
10].
| Therapy | Type | Recommendation | Overall Response | Overall Survival | Toxicities | Study Type |
|---|
| Ruxolitinib | Janus kinase 1/2 inhibitor | ≥second-line | BOR 76% (CR 12%, PR 64%) in 165 patients with SR-cGvHD [13]; 85% (CR 7%, PR 78%) in 41 patients with SR-cGvHD [33,46,55] | 97% at 6 months [55] | Viral reactivation/infection, peripheral neuropathy, anemia, thrombocytopenia, and neutropenia [13,56]; viral reactivation, cytopenia, and malignancy relapse [55] | Phase 3 randomized trial |
| Ibrutinib | Bruton’s tyrosine kinase inhibitor | ≥third-line | BOR 67% (CR 21%, PR 45%) in 42 patients with cGvHD, with median follow-up of 13.9 months [12] | 71% at 2 years in cGvHD [57] | Pneumonia and impaired platelet function [58] | Phase 2a trial |
| Extracorporeal photopheresis | UVA treatment of mononucleated blood cells via leukapheresis | ≥second-line | Rates dependent on site and severity—highest responses in skin, liver, mouth, and BOS [59,60,61,62]: 67% (CR 23%, PR 44%) in 48 patients with SR-cGvHD [61] | 53–78% at 1 year [8,9,11,59]. | Vascular access complications [58] | Phase 2 randomized trial |
| Mycophenolate mofetil | Antimetabolite immunosuppressant | ≥third-line | 26–64% [11,49] | 67–96% at 1 year [11] | Viral reactivation, hypertension, pneumonia, and post-transplantation lymphoproliferative disease [58] | Retrospective cohorts |
| Rituximab | CD20 (B cell surface antigen) monoclonal antibody | ≥third-line | 65% in 38 patients with SR-cGvHD [59]; 70% (CR 10%) in 20 patients with SR-cGvHD [63]; 27% in 37 patients with sclerotic cGvHD [64]; 17% (CR 17%) in 6 patients with SR-cGvHD [64] | 72% at 1 year; 76% at 2 years [11,55] | Infections, infusion-related symptoms, and late neutropenia [62,63] | Phase 2b randomized trial |
| Sirolimus | mTOR inhibitor | ≥third-line | 81% (CR 38%, PR 43%) in 47 patients with SR-cGvHD [65]; 94% of 16 patients with cGvHD [47,66] | – | Thrombotic microangiopathy, renal insufficiency, and proteinuria [65,66,67] | Phase 2a trials |
| Imatinib | Multi-kinase inhibitor | ≥third-line | 79% (CR 37%, PR 42%) in 19 patients with SR-cGvHD [68]; 26% in 35 patients with sclerotic cGvHD [64] | 84% at 1.5 years [68] | Fluid retention, myelosuppression, and anemia [68] | Phase 2b trial |
| Cyclophosphamide (either pulse or low dose) | Alkylating agent | ≥third-line | 100% of 3 patients with cGvHD showed response in treatment of skin and oral cavity [69]; 60% of 15 patients showed improvement after 8–12 monthly cycles [70] | – | Short-term myelosuppression, neutropenia, fatigue, and nausea [51,69,70] | Retrospective cohorts |
| Belumosudil | ROCK2 inhibitor | ≥third-line | 74% (CR 3%, PR 71%) of 132 patients with cGvHD [14] | FFS 77% at 6 months [14] | Pneumonia, hypertension, hyperglycemia, and increased gamma-glutamyltransferase [14] | Phase 2 open-label, randomized clinical trial |
| Axatilimab | IgG4 antibody targeting the CSF-1 receptor | Available in clinical trial only | 58% of 12 patients with cGvHD across doses [15] | – | Increased gamma-glutamyltransferase, asparatate aminotransferase, and creating phosphokinase, periorbital edema [15] | Phase 1/2 dose-escalation and dose-expansion study |
Table 7.
Summary of three newer treatment options for steroid-refractory chronic GvHD approved by Health Canada.
Table 7.
Summary of three newer treatment options for steroid-refractory chronic GvHD approved by Health Canada.
| | Ibrutinib | Belumosudil | Ruxolitinib |
|---|
| Publication | Miklos (2017) [12], Phase 1b/2 | Cutler (2021) [14], Phase 2 | Zeiser (2021) [13], Phase 3 |
| No. of patients | n = 42 | n = 66/66 | n = 165 |
| Dose | 420 mg once daily | 200 once daily/200 mg twice daily | 10 mg twice daily |
| Indication | Second-line or beyond | Third-line or beyond | Second-line or beyond |
| Follow-up/exposure | 13.9 months | 12 months | 9.5 months |
| ORR at 6 months | NR | NR | 50% (CR 6.7%) |
| ORR, max | 67% (CR 21%, PR 45%) | 74–77% (CR, 5.3%) | 76% (CR 12.1%) |
| Steroid stop | 5/42 at 12 months (12%) | 21% | - |
| FFS duration | NR | 14–15 months | Not reached |
| FFS at 12 months | NR | 56% | 62% |
| Adverse events | Fatigue, diarrhea, muscle spasms, nausea, and bruising | Fatigue, diarrhea, nausea, and upper respiratory tract infection | Anemia and thrombocytopenia |
| Public reimbursement | Not available | Under review (third-line or beyond) | Available (second-line or beyond) |
12. Future Directions for Chronic GvHD Treatment Development
The NIH 2020 consensus report highlighted the importance of the early clinical recognition of cGvHD, even before meeting NIH diagnostic criteria [
20], early biomarker development for cGvHD diagnosis, and early diagnosis when presented in the form of atypical GvHD [
22]. It also underscores the fact that when patients meet the NIH 2014 criteria, many of them already have a high burden of disease and fibrotic insults to their organs [
21]. Accordingly, pre-emptive therapy for future development was emphasized [
23].
With recent advances in cGvHD therapeutics and the approvals of ibrutinib [
12], ruxolitinib [
13], belumosudil [
14], and the soon-to-be approved axatilimab [
15], the therapeutic paradigm is rapidly evolving. Here is a summary of its developmental direction.
Mode-of-action-based cGvHD drug selection: An emerging concept of cGvHD treatment involves selecting therapies based on their modes of action. As discussed, the pathogenesis of cGvHD can be stratified into inflammatory, immune deregulation, and fibrotic phases [
10]. Each drug has its own mode of action that targets one or more of these phases. For example, ruxolitinib has robust anti-inflammatory activity, while belumosudil and axatilimab have strong antifibrotic properties. Depending on the clinical manifestation and suspected pathogenesis of cGvHD in a particular patient, the most appropriate could be selected.
Combination strategy: While minimizing the number of immunosuppressive drugs was one of the main principles of cGvHD management in the past, with the introduction of newer targeted drugs, a combination strategy is now rational and revisited. One such combination strategy could include a novel agent, e.g., ruxolitinib or belumosudil, along with another therapeutic agent that has been extensively evaluated [
71]. A combined approach of ECP with belumosudil or with ruxolitinib seems promising and is currently under investigation [
72]. Alternatively, a combination of two novel agents is also promising and might be therapeutically valuable, if accessible.
Steroid-free regimen: Development of a corticosteroid-free, front-line regimen is highly warranted. Corticosteroids have been the mainstay of cGvHD treatment for over five decades. When feasible, clinical trials of initial systemic therapy should investigate steroid-free therapeutic strategies in front-line therapy [
71].
Pre-emptive approach: In order to improve the quality of life and long-term outcomes in patients suffering from highly morbid forms of cGvHD, a pre-emptive treatment approach could be an optimal approach. This strategy could potentially prevent the progression of cGvHD in high-risk patients destined to develop severe and highly morbid forms of cGvHD [
23]. Such an approach will likely require further research on cGvHD biomarker development.
13. Multidisciplinary Approach and Supportive Care in the Management of Chronic GvHD
The management of cGvHD requires a multidisciplinary approach, as it involves the coordination of multiple medical specialists to address the various symptoms and complications associated with cGvHD.
The multidisciplinary team typically includes specialists in infectious diseases, ophthalmology, dermatology, gynecology, gastroenterology, respirology, hepatology, nephrology, dentistry, psychiatry, and rehabilitation medicine. In addition to specialist physicians, the multidisciplinary team must include pharmacists, nurse practitioners, physician assistants, dietitians, physiotherapists, and social workers. A dedicated team approach with relevant expertise is necessary to provide optimal and holistic care for patients dealing with a chronic illness that can significantly affect their QoL. Also, the development of a dedicated “cGvHD program” in each center that operates in parallel with a dedicated multidisciplinary team has the potential to improve the quality of care, patient-reported outcomes, and QoL in cGvHD patients.
Chronic GvHD is associated with a severe impairment in QoL [
73]. Patients with cGvHD may present with a diverse range of manifestations affecting multiple organ systems. These symptoms may be intrusive and can include chronic pain, fatigue, xerostomia and xerophthalmia, skin rashes and ulcers, joint stiffness, dyspnea, and intractable and persistent gastrointestinal disturbances such as chronic diarrhea and nausea. Supportive measures to control and improve these symptoms are needed and may include physiotherapy, exercise, pain clinic consultation, and the care of many specialists.
Patients with cGvHD experience excess comorbidities versus survivors of allogeneic HCT without cGvHD, including loss of bone density, hyperlipidemia, hyperglycemia, and subsequent malignancies amongst others. Consideration should be given to regular bone mineral densitometry, measurement of glucose and lipids, and age-appropriate malignancy screening [
74]. In addition to the physical manifestations, cGvHD can exert a considerable financial, emotional, and psychological toll on patients. The disease can be prolonged, necessitating ongoing medical intervention and monitoring, which may trigger feelings of anxiety, depression, and social isolation. The burden of disease management may also impact patients’ ability to carry out daily activities, work, and interact with others, further impinging upon their quality of life.
A multidisciplinary approach to the management of cGvHD strives to address these diverse aspects of the disease and to enhance patients’ QoL. This may involve treating the physical symptoms with pharmaceutical and supportive modalities, providing psychological and social support, and facilitating patients’ navigation of the practical aspects of their treatment, such as medication adherence and appointment scheduling. By attending to these varied needs, the multidisciplinary team can assist patients with cGvHD in sustaining their independence and promoting their overall well-being.
Author Contributions
Conceptualization, D.D.H.K. and K.R.S.; writing—original draft preparation, D.D.H.K.; writing—review and editing, D.D.H.K., G.P., K.L., K.P., D.A., R.V.N., S.L., U.D., J.W., M.E., K.J., C.F., C.L., I.N.-B., A.D.L., R.K., I.W. and K.R.S.; visualization, D.D.H.K.; supervision, D.D.H.K., I.W., and K.R.S.; project administration, D.D.H.K. and K.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The current work was conducted without financial support from pharmaceutical companies.
Conflicts of Interest
D.D.H.K.—honoraria from Novartis, Sanofi, mallinckrodt and Jazz, and research grant from Novartis and Sanofi. Advisory board member and constancy for Novartis and Sanofi; G.P.—honoraria and/or consulting: sanofi, servier, mallinckrodt, abbvie, jazz, medexus, pfizer, seattle genetics, takeda, amgen, merck, gilead, novartis, kyowa kirin, paladin, sobi / research support: Syndax, Abbvie, Equilium—for being local PI; K.L.—honorarium from Sanofi and advisory boards for Novartis and Sanofi; K.P.—advisory board for Sanofi; J.W.—honoraria from Sanofi and Novartis; M.E.—honoraria from Novartis, Sanofi and Advisory board member and constancy for Novartis and Sanofi; K.J.—Ad board: Sanofi, Paladin, Jazz, Avir, Pfizer, Vertex/Research funding: Jazz; I.W.—Research funds and Advisory Board-Sanofi; K.R.S.—Ad Board: Novartis, Sanofi, Incyte and DSMB: BMS, Seres. Other authors declare no conflicts of interest.
References
- Lee, S.J.; Vogelsang, G.; Flowers, M.E. Chronic graft-versus-host disease. Biol. Blood Marrow Transplant. 2003, 9, 215–233. [Google Scholar] [CrossRef]
- Cooke, K.R.; Luznik, L.; Sarantopoulos, S.; Hakim, F.T.; Jagasia, M.; Fowler, D.H.; van den Brink, M.R.M.; Hansen, J.A.; Parkman, R.; Miklos, D.B.; et al. The Biology of Chronic Graft-versus-Host Disease: A Task Force Report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2017, 23, 211–234. [Google Scholar] [CrossRef]
- Nieder, M.L.; McDonald, G.B.; Kida, A.; Hingorani, S.; Armenian, S.H.; Cooke, K.R.; Pulsipher, M.A.; Baker, K.S. National Cancer Institute-National Heart, Lung and Blood Institute/pediatric Blood and Marrow Transplant Consortium First International Consensus Conference on late effects after pediatric hematopoietic cell transplantation: Long-term organ damage and dysfunction. Biol. Blood Marrow Transplant. 2011, 17, 1573–1584. [Google Scholar] [CrossRef]
- Wong, F.L.; Francisco, L.; Togawa, K.; Bosworth, A.; Gonzales, M.; Hanby, C.; Sabado, M.; Grant, M.; Forman, S.J.; Bhatia, S. Long-term recovery after hematopoietic cell transplantation: Predictors of quality-of-life concerns. Blood 2010, 115, 2508–2519. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.R.; Syrjala, K.L.; Martin, P.J.; Flowers, M.E.; Carpenter, P.A.; Salit, R.B.; Baker, K.S.; Lee, S.J. Resilience, health, and quality of life among long-term survivors of hematopoietic cell transplantation. Cancer 2015, 121, 4250–4257. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Logan, B.; Westervelt, P.; Cutler, C.; Woolfrey, A.; Khan, S.P.; Waller, E.K.; Maziarz, R.T.; Wu, J.; Shaw, B.E.; et al. Comparison of Patient-Reported Outcomes in 5-Year Survivors Who Received Bone Marrow vs Peripheral Blood Unrelated Donor Transplantation: Long-term Follow-up of a Randomized Clinical Trial. JAMA Oncol. 2016, 2, 1583–1589. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Taparia, M.; Robinson, E.; McGee, M.; Merali, T. Navigating the complexity of chronic graft-versus-host disease: Canadian insights into real-world treatment sequencing. In Transplantation Proceedings; Elsevier: New York, NY, USA, 2024. [Google Scholar]
- Linn, S.M.; Novitzky-Basso, I.; Patriquin, C.; Pasic, I.; Lam, W.; Law, A.; Michelis, F.V.; Gerbitz, A.; Viswabandya, A.; Lipton, J.; et al. Single centre retrospective analysis of extracorporeal photopheresis (ECP) therapy in patients heavily pre-treated for chronic graft-versus-host disease (cGvHD) with steroid failure. Leuk. Res. 2023, 134, 107387. [Google Scholar] [CrossRef] [PubMed]
- Novitzky-Basso, I.; Patriquin, C.; Linn, S.M.; Chiarello, C.; Pasic, I.; Lam, W.; Law, A.; Michelis, F.V.; Gerbitz, A.; Viswabandya, A.; et al. Propensity Score Matching Analysis Comparing the Efficacy and Steroid Tapering Benefit of Extracorporeal Photopheresis to Best Available Therapy in Third-Line or Beyond Treatment for Chronic GvHD. Transplant. Cell. Ther. 2023, 29, 773.e1–773.e10. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.; Fatobene, G.; Rocha, V.; Kroger, N.; Flowers, M.E. Steroid-refractory chronic graft-versus-host disease: Treatment options and patient management. Bone Marrow Transplant. 2021, 56, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Flowers, M.E.; Martin, P.J. How we treat chronic graft-versus-host disease. Blood 2015, 125, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Miklos, D.; Cutler, C.S.; Arora, M.; Waller, E.K.; Jagasia, M.; Pusic, I.; Flowers, M.E.; Logan, A.C.; Nakamura, R.; Blazar, B.R.; et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood 2017, 130, 2243–2250. [Google Scholar] [CrossRef]
- Zeiser, R.; Polverelli, N.; Ram, R.; Hashmi, S.K.; Chakraverty, R.; Middeke, J.M.; Musso, M.; Giebel, S.; Uzay, A.; Langmuir, P.; et al. Ruxolitinib for Glucocorticoid-Refractory Chronic Graft-versus-Host Disease. N. Engl. J. Med. 2021, 385, 228–238. [Google Scholar] [CrossRef]
- Cutler, C.; Lee, S.J.; Arai, S.; Rotta, M.; Zoghi, B.; Lazaryan, A.; Ramakrishnan, A.; DeFilipp, Z.; Salhotra, A.; Chai-Ho, W.; et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: The ROCKstar Study. Blood 2021, 138, 2278–2289. [Google Scholar] [CrossRef]
- Kitko, C.L.; Arora, M.; DeFilipp, Z.; Zaid, M.A.; Di Stasi, A.; Radojcic, V.; Betts, C.B.; Coussens, L.M.; Meyers, M.L.; Qamoos, H.; et al. Axatilimab for Chronic Graft-Versus-Host Disease After Failure of at Least Two Prior Systemic Therapies: Results of a Phase I/II Study. J. Clin. Oncol. 2023, 41, 1864–1875. [Google Scholar] [CrossRef]
- Schoemans, H.M.; Lee, S.J.; Ferrara, J.L.; Wolff, D.; Levine, J.E.; Schultz, K.R.; Shaw, B.E.; Flowers, M.E.; Ruutu, T.; Greinix, H.; et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018, 53, 1401–1415. [Google Scholar] [CrossRef]
- Penack, O.; Marchetti, M.; Ruutu, T.; Aljurf, M.; Bacigalupo, A.; Bonifazi, F.; Ciceri, F.; Cornelissen, J.; Malladi, R.; Duarte, R.F.; et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: Updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020, 7, e157–e167. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network: Hematopoietic Cell Transplantation Version 3.2023. 2023. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1501 (accessed on 9 October 2023).
- Cuvelier, G.D.E.; Li, A.; Drissler, S.; Kariminia, A.; Abdossamadi, S.; Rozmus, J.; Chanoine, J.P.; Ng, B.; Mostafavi, S.; Brinkman, R.R.; et al. Age Related Differences in the Biology of Chronic Graft-Versus-Host Disease After Hematopoietic Stem Cell Transplantation. Front. Immunol. 2020, 11, 571884. [Google Scholar] [CrossRef]
- Williams, K.M.; Inamoto, Y.; Im, A.; Hamilton, B.; Koreth, J.; Arora, M.; Pusic, I.; Mays, J.W.; Carpenter, P.A.; Luznik, L.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2020 Etiology and Prevention Working Group Report. Transplant. Cell Ther. 2021, 27, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Kitko, C.L.; Pidala, J.; Schoemans, H.M.; Lawitschka, A.; Flowers, M.E.; Cowen, E.W.; Tkaczyk, E.; Farhadfar, N.; Jain, S.; Steven, P.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IIa. The 2020 Clinical Implementation and Early Diagnosis Working Group Report. Transplant. Cell Ther. 2021, 27, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Cuvelier, G.D.E.; Schoettler, M.; Buxbaum, N.P.; Pinal-Fernandez, I.; Schmalzing, M.; Distler, J.H.W.; Penack, O.; Santomasso, B.D.; Zeiser, R.; Angstwurm, K.; et al. Toward a Better Understanding of the Atypical Features of Chronic Graft-Versus-Host Disease: A Report from the 2020 National Institutes of Health Consensus Project Task Force. Transplant. Cell Ther. 2022, 28, 426–445. [Google Scholar] [CrossRef] [PubMed]
- Pidala, J.; Kitko, C.; Lee, S.J.; Carpenter, P.; Cuvelier, G.D.E.; Holtan, S.; Flowers, M.E.; Cutler, C.; Jagasia, M.; Gooley, T.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IIb. The 2020 Preemptive Therapy Working Group Report. Transplant. Cell Ther. 2021, 27, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.; Radojcic, V.; Lafyatis, R.; Cinar, R.; Rosenstein, R.K.; Cowen, E.W.; Cheng, G.S.; Sheshadri, A.; Bergeron, A.; Williams, K.M.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. The 2020 Highly morbid forms report. Transplant. Cell Ther. 2021, 27, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Schultz, K.R.; Kariminia, A.; Ng, B.; Abdossamadi, S.; Lauener, M.; Nemecek, E.R.; Wahlstrom, J.T.; Kitko, C.L.; Lewis, V.A.; Schechter, T.; et al. Immune profile differences between chronic GVHD and late acute GVHD: Results of the ABLE/PBMTC 1202 studies. Blood 2020, 135, 1287–1298. [Google Scholar] [CrossRef]
- Jagasia, M.H.; Greinix, H.T.; Arora, M.; Williams, K.M.; Wolff, D.; Cowen, E.W.; Palmer, J.; Weisdorf, D.; Treister, N.S.; Cheng, G.S.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol. Blood Marrow Transplant. 2015, 21, 389–401.e1. [Google Scholar] [CrossRef]
- Teh, C.; Onstad, L.; Lee, S.J. Reliability and Validity of the Modified 7-Day Lee Chronic Graft-versus-Host Disease Symptom Scale. Biol. Blood Marrow Transplant. 2020, 26, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, G.C.; Fazekas, T.; Lawitschka, A.; Bertz, H.; Greinix, H.; Halter, J.; Pavletic, S.Z.; Holler, E.; Wolff, D. Diagnosis and treatment of pulmonary chronic GVHD: Report from the consensus conference on clinical practice in chronic GVHD. Bone Marrow Transplant. 2011, 46, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, S.; Bowerman, C.; Robinson, P. Multiple breath washout: Measuring early manifestations of lung pathology. Breathe 2021, 17, 210016. [Google Scholar] [CrossRef]
- Sobkowiak-Sobierajska, A.; Lindemans, C.; Sykora, T.; Wachowiak, J.; Dalle, J.H.; Bonig, H.; Gennery, A.; Lawitschka, A. Management of Chronic Graft-vs.-Host Disease in Children and Adolescents With ALL: Present Status and Model for a Personalised Management Plan. Front. Pediatr. 2022, 10, 808103. [Google Scholar] [CrossRef]
- Doering, J.; Perl, M.; Weber, D.; Banas, B.; Schulz, C.; Hamer, O.W.; Angstwurm, K.; Holler, E.; Herr, W.; Edinger, M.; et al. Incidence and Outcome of Atypical Manifestations of Chronic Graft-versus-Host Disease: Results From a Retrospective Single-Center Analysis. Transplant. Cell Ther. 2023, 29, 772.e1–772.e10. [Google Scholar] [CrossRef]
- White, J.; Elemary, M.; Linn, S.M.; Novitzky-Basso, I.; Culos, S.; Tan, S.K.; Kelly, K.; Deotare, U.; Xenocostas, A.; Hamad, N.; et al. A Multicenter, Retrospective Study Evaluating Clinical Outcomes of Ruxolitinib Therapy In Heavily Pretreated Chronic GVHD Patients With Steroid Failure. Transplant. Cell Ther. 2023, 29, 120.e1–120.e9. [Google Scholar] [CrossRef]
- Inamoto, Y.; Storer, B.E.; Lee, S.J.; Carpenter, P.A.; Sandmaier, B.M.; Flowers, M.E.; Martin, P.J. Failure-free survival after second-line systemic treatment of chronic graft-versus-host disease. Blood 2013, 121, 2340–2346. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, Y.; Flowers, M.E.; Sandmaier, B.M.; Aki, S.Z.; Carpenter, P.A.; Lee, S.J.; Storer, B.E.; Martin, P.J. Failure-free survival after initial systemic treatment of chronic graft-versus-host disease. Blood 2014, 124, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.; Chai, X.; Martin, P.J.; Weisdorf, D.; Inamoto, Y.; Pidala, J.; Jagasia, M.; Pavletic, S.; Cutler, C.; Vogelsang, G.; et al. Failure-free survival in a prospective cohort of patients with chronic graft-versus-host disease. Haematologica 2015, 100, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.J.; Lee, S.J.; Przepiorka, D.; Horowitz, M.M.; Koreth, J.; Vogelsang, G.B.; Walker, I.; Carpenter, P.A.; Griffith, L.M.; Akpek, G.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: VI. The 2014 Clinical Trial Design Working Group Report. Biol. Blood Marrow Transplant. 2015, 21, 1343–1359. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.M.; Shulman, H.M.; Storb, R.; Weiden, P.L.; Witherspoon, R.P.; McDonald, G.B.; Schubert, M.M.; Atkinson, K.; Thomas, E.D. Chronic graft-versus-host disease in 52 patients: Adverse natural course and successful treatment with combination immunosuppression. Blood 1981, 57, 267–276. [Google Scholar] [CrossRef]
- Koc, S.; Leisenring, W.; Flowers, M.E.; Anasetti, C.; Deeg, H.J.; Nash, R.A.; Sanders, J.E.; Witherspoon, R.P.; Storb, R.; Appelbaum, F.R.; et al. Therapy for chronic graft-versus-host disease: A randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood 2002, 100, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Abouelnasr, A.; Roy, J.; Cohen, S.; Kiss, T.; Lachance, S. Defining the role of sirolimus in the management of graft-versus-host disease: From prophylaxis to treatment. Biol. Blood Marrow Transplant. 2013, 19, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Pidala, J.; Hamadani, M.; Dawson, P.; Martens, M.; Alousi, A.M.; Jagasia, M.; Efebera, Y.A.; Chhabra, S.; Pusic, I.; Holtan, S.G.; et al. Randomized multicenter trial of sirolimus vs prednisone as initial therapy for standard-risk acute GVHD: The BMT CTN 1501 trial. Blood 2020, 135, 97–107. [Google Scholar] [CrossRef]
- Martin, P.J.; Storer, B.E.; Rowley, S.D.; Flowers, M.E.; Lee, S.J.; Carpenter, P.A.; Wingard, J.R.; Shaughnessy, P.J.; DeVetten, M.P.; Jagasia, M.; et al. Evaluation of mycophenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood 2009, 113, 5074–5082. [Google Scholar] [CrossRef]
- Chien, S.H.; Liu, C.J.; Hong, Y.C.; Teng, C.J.; Hu, Y.W.; Shen, C.C.; Ku, F.C.; Chen, S.C.; Yeh, C.M.; Chiou, T.J.; et al. Use of azathioprine for graft-vs-host disease is the major risk for development of secondary malignancies after haematopoietic stem cell transplantation: A nationwide population-based study. Br. J. Cancer 2015, 112, 177–184. [Google Scholar] [CrossRef]
- Dignan, F.L.; Amrolia, P.; Clark, A.; Cornish, J.; Jackson, G.; Mahendra, P.; Scarisbrick, J.J.; Taylor, P.C.; Shaw, B.E.; Potter, M.N.; et al. Diagnosis and management of chronic graft-versus-host disease. Br. J. Haematol. 2012, 158, 46–61. [Google Scholar] [CrossRef]
- Lee, S.J.; Wolff, D.; Kitko, C.; Koreth, J.; Inamoto, Y.; Jagasia, M.; Pidala, J.; Olivieri, A.; Martin, P.J.; Przepiorka, D.; et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol. Blood Marrow Transplant. 2015, 21, 984–999. [Google Scholar] [CrossRef]
- Novitzky-Basso, I.; Linn, S.M.; White, J.; Elemary, M.; Xenocostas, A.; Deotare, U.; Kelly, K.; Hamad, N.; Tan, S.; Culos, S.; et al. Propensity score matching analysis comparing the efficacy of Ruxolitinib to historical controls in second-line or beyond treatment for chronic GvHD after steroid failure. Bone Marrow Transplant. 2023, 58, 1024–1032. [Google Scholar] [CrossRef]
- Chin, K.K.; Kim, H.T.; Inyang, E.A.; Ho, V.; Koreth, J.; Romee, R.; Gooptu, M.; Shapiro, R.; Antin, J.; Soiffer, R.; et al. Ibrutinib in Steroid-Refractory Chronic Graft-versus-Host Disease, a Single-Center Experience. Transplant. Cell Ther. 2021, 27, 990.e1–990.e7. [Google Scholar] [CrossRef] [PubMed]
- Pidala, J.; Kim, J.; Anasetti, C. Sirolimus as primary treatment of acute graft-versus-host disease following allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2009, 15, 881–885. [Google Scholar] [CrossRef]
- Coenen, J.J.; Koenen, H.J.; van Rijssen, E.; Hilbrands, L.B.; Joosten, I. Rapamycin, and not cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood 2006, 107, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Sohn, S.K.; Kim, D.H.; Lee, N.Y.; Suh, J.S.; Lee, K.S.; Lee, K.B. Different efficacy of mycophenolate mofetil as salvage treatment for acute and chronic GVHD after allogeneic stem cell transplant. Eur. J. Haematol. 2004, 73, 56–61. [Google Scholar] [CrossRef]
- Linn, S.M.; Novitzky-Basso, I.; Abduljalil, O.; Pasic, I.; Lam, W.; Law, A.; Michelis, F.V.; Gerbitz, A.; Viswabandya, A.; Lipton, J.; et al. A Single-center, Real-world Experience of Chronic GVHD Treatment Using Ibrutinib, Imatinib, and Ruxolitinib and its Treatment Outcomes. Hematol. Oncol. Stem Cell Ther. 2023, 17, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Fante, M.A.; Holler, B.; Weber, D.; Angstwurm, K.; Bergler, T.; Holler, E.; Edinger, M.; Herr, W.; Wertheimer, T.; Wolff, D. Cyclophosphamide for salvage therapy of chronic graft-versus-host disease: A retrospective analysis. Ann. Hematol. 2020, 99, 2181–2190. [Google Scholar] [CrossRef]
- Norman, B.C.; Jacobsohn, D.A.; Williams, K.M.; Au, B.K.; Au, M.A.; Lee, S.J.; Moravec, C.K.; Chien, J.W. Fluticasone, azithromycin and montelukast therapy in reducing corticosteroid exposure in bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: A case series of eight patients. Bone Marrow Transplant. 2011, 46, 1369–1373. [Google Scholar] [CrossRef][Green Version]
- Williams, K.M.; Cheng, G.S.; Pusic, I.; Jagasia, M.; Burns, L.; Ho, V.T.; Pidala, J.; Palmer, J.; Johnston, L.; Mayer, S.; et al. Fluticasone, Azithromycin, and Montelukast Treatment for New-Onset Bronchiolitis Obliterans Syndrome after Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2016, 22, 710–716. [Google Scholar] [CrossRef]
- Vallet, N.; Le Grand, S.; Bondeelle, L.; Hoareau, B.; Corneau, A.; Bouteiller, D.; Tournier, S.; Derivry, L.; Bohineust, A.; Tourret, M.; et al. Azithromycin promotes relapse by disrupting immune and metabolic networks after allogeneic stem cell transplantation. Blood 2022, 140, 2500–2513. [Google Scholar] [CrossRef]
- Zeiser, R.; Burchert, A.; Lengerke, C.; Verbeek, M.; Maas-Bauer, K.; Metzelder, S.K.; Spoerl, S.; Ditschkowski, M.; Ecsedi, M.; Sockel, K.; et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: A multicenter survey. Leukemia 2015, 29, 2062–2068. [Google Scholar] [CrossRef]
- Jagasia, M.; Perales, M.A.; Schroeder, M.A.; Ali, H.; Shah, N.N.; Chen, Y.B.; Fazal, S.; Dawkins, F.W.; Arbushites, M.C.; Tian, C.; et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): A multicenter, open-label phase 2 trial. Blood 2020, 135, 1739–1749. [Google Scholar] [CrossRef]
- Waller, E.K.; Miklos, D.; Cutler, C.; Arora, M.; Jagasia, M.H.; Pusic, I.; Flowers, M.E.D.; Logan, A.C.; Nakamura, R.; Chang, S.; et al. Ibrutinib for Chronic Graft-versus-Host Disease After Failure of Prior Therapy: 1-Year Update of a Phase 1b/2 Study. Biol. Blood Marrow Transplant. 2019, 25, 2002–2007. [Google Scholar] [CrossRef] [PubMed]
- Sarantopoulos, S.; Cardones, A.R.; Sullivan, K.M. How I treat refractory chronic graft-versus-host disease. Blood 2019, 133, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Couriel, D.R.; Hosing, C.; Saliba, R.; Shpall, E.J.; Anderlini, P.; Rhodes, B.; Smith, V.; Khouri, I.; Giralt, S.; de Lima, M.; et al. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood 2006, 107, 3074–3080. [Google Scholar] [CrossRef]
- Santiago, P.; Schwartz, I.; Tamariz, L.; Levy, C. Systematic review with meta-analysis: Mycophenolate mofetil as a second-line therapy for autoimmune hepatitis. Aliment. Pharmacol. Ther. 2019, 49, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Oarbeascoa, G.; Lozano, M.L.; Guerra, L.M.; Amunarriz, C.; Saavedra, C.A.; Garcia-Gala, J.M.; Viejo, A.; Revilla, N.; Acosta Fleitas, C.; Arroyo, J.L.; et al. Retrospective Multicenter Study of Extracorporeal Photopheresis in Steroid-Refractory Acute and Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2020, 26, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Zaja, F.; Bacigalupo, A.; Patriarca, F.; Stanzani, M.; Van Lint, M.T.; Fili, C.; Scime, R.; Milone, G.; Falda, M.; Vener, C.; et al. Treatment of refractory chronic GVHD with rituximab: A GITMO study. Bone Marrow Transplant. 2007, 40, 273–277. [Google Scholar] [CrossRef]
- Cutler, C.; Miklos, D.; Kim, H.T.; Treister, N.; Woo, S.B.; Bienfang, D.; Klickstein, L.B.; Levin, J.; Miller, K.; Reynolds, C.; et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood 2006, 108, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Pidala, J.; Pusic, I.; Chai, X.; Jaglowski, S.; Khera, N.; Palmer, J.; Chen, G.L.; Jagasia, M.H.; Mayer, S.A.; et al. A Randomized Phase II Crossover Study of Imatinib or Rituximab for Cutaneous Sclerosis after Hematopoietic Cell Transplantation. Clin. Cancer Res. 2016, 22, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Jurado, M.; Vallejo, C.; Perez-Simon, J.A.; Brunet, S.; Ferra, C.; Balsalobre, P.; Perez-Oteyza, J.; Espigado, I.; Romero, A.; Caballero, D.; et al. Sirolimus as part of immunosuppressive therapy for refractory chronic graft-versus-host disease. Biol. Blood Marrow Transplant. 2007, 13, 701–706. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johnston, L.J.; Brown, J.; Shizuru, J.A.; Stockerl-Goldstein, K.E.; Stuart, M.J.; Blume, K.G.; Negrin, R.S.; Chao, N.J. Rapamycin (sirolimus) for treatment of chronic graft-versus-host disease. Biol. Blood Marrow Transplant. 2005, 11, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Mielke, S.; Lutz, M.; Schmidhuber, J.; Kapp, M.; Ditz, D.; Ammer, J.; Einsele, H.; Grigoleit, G.U.; Holler, E.; Wolff, D. Salvage therapy with everolimus reduces the severity of treatment-refractory chronic GVHD without impairing disease control: A dual center retrospective analysis. Bone Marrow Transplant. 2014, 49, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, A.; Locatelli, F.; Zecca, M.; Sanna, A.; Cimminiello, M.; Raimondi, R.; Gini, G.; Mordini, N.; Balduzzi, A.; Leoni, P.; et al. Imatinib for refractory chronic graft-versus-host disease with fibrotic features. Blood 2009, 114, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.; Krejci, M.; Doubek, M.; Pospisil, Z.; Brychtova, Y.; Tomiska, M.; Racil, Z. Pulse cyclophosphamide for corticosteroid-refractory graft-versus-host disease. Bone Marrow Transplant. 2005, 35, 699–705. [Google Scholar] [CrossRef]
- Chao, N.J.; Foster, Y.G.; Rowe, K.; Shah, A.; Cardones, A.R. Pulse cyclophosphamide for steroid-refractory chronic graft-versus-host disease. Biol. Blood Marrow Transplant. 2016, 22, S393–S395. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Couriel, D.R.; Lazaryan, A.; Bhatt, V.R.; Buxbaum, N.P.; Alousi, A.M.; Olivieri, A.; Pulanic, D.; Halter, J.P.; Henderson, L.A.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: III. The 2020 Treatment of Chronic GVHD Report. Transplant. Cell Ther. 2021, 27, 729–737. [Google Scholar] [CrossRef]
- Maas-Bauer, K.; Kiote-Schmidt, C.; Bertz, H.; Apostolova, P.; Wasch, R.; Ihorst, G.; Finke, J.; Zeiser, R. Ruxolitinib-ECP combination treatment for refractory severe chronic graft-versus-host disease. Bone Marrow Transplant. 2021, 56, 909–916. [Google Scholar] [CrossRef]
- Pidala, J.; Kurland, B.; Chai, X.; Majhail, N.; Weisdorf, D.J.; Pavletic, S.; Cutler, C.; Jacobsohn, D.; Palmer, J.; Arai, S.; et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: Report on baseline data from the Chronic GVHD Consortium. Blood 2011, 117, 4651–4657. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.; Herzberg, P.Y.; Herrmann, A.; Pavletic, S.Z.; Heussner, P.; Mumm, F.; Hofer, C.; Hilgendorf, I.; Hemmati, P.G.; Holler, E.; et al. Post-transplant multimorbidity index and quality of life in patients with chronic graft-versus-host disease-results from a joint evaluation of a prospective German multicenter validation trial and a cohort from the National Institutes of Health. Bone Marrow Transplant. 2021, 56, 243–256. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).