Outpatient Embedded Palliative Care for Patients with Advanced Thoracic Malignancy: A Retrospective Cohort Study
Abstract
1. Introduction
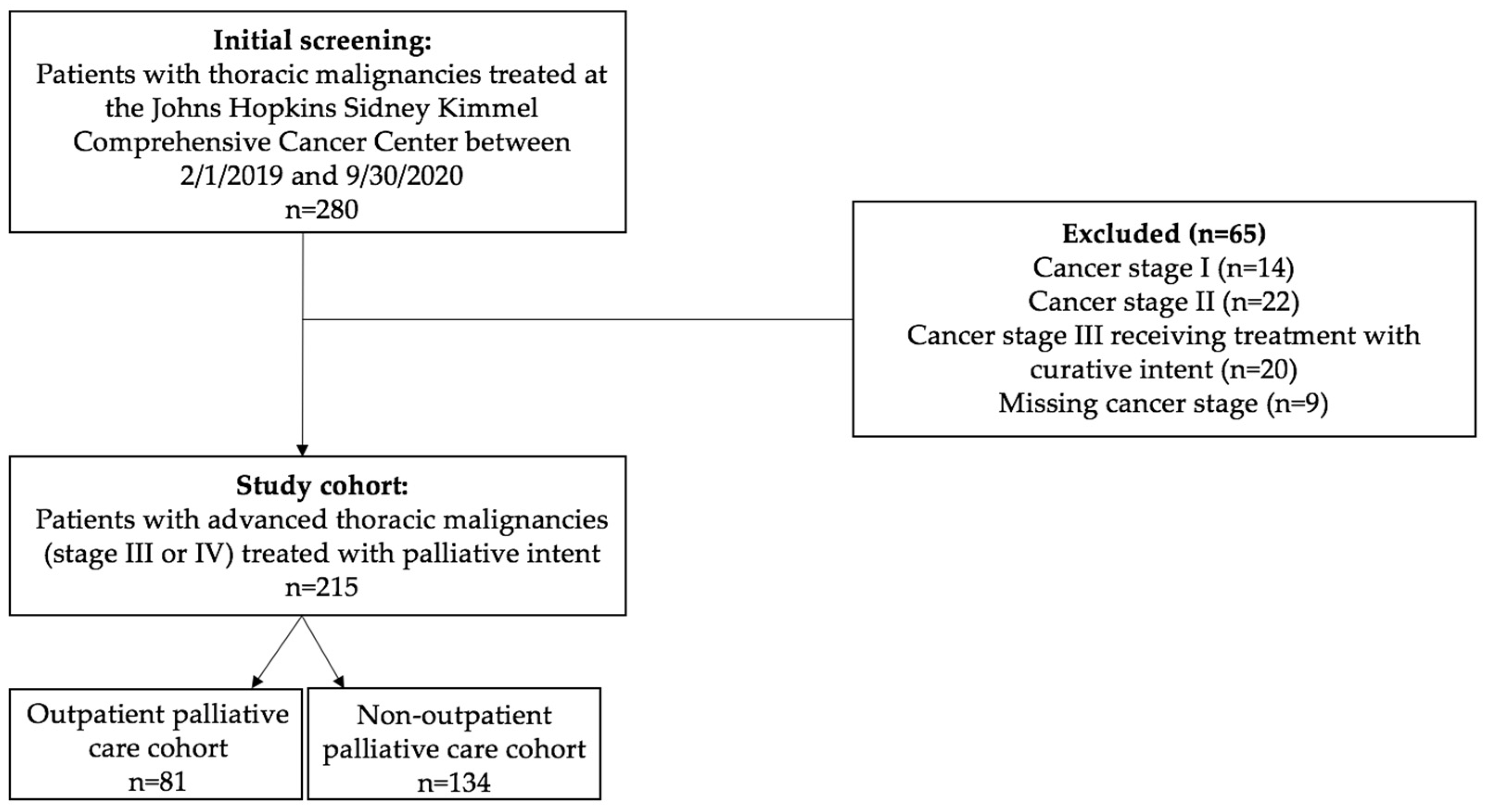
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Survelliance, Epidemiology, and End Results Program: Esophagus. Available online: https://seer.cancer.gov/statistics-network/explorer/application.html?site=17&data_type=4&graph_type=6&compareBy=sex&chk_sex_1=1&race=1&age_range=1&stage=106&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_show_apc=on&advopt_display=2 (accessed on 5 January 2024).
- Survelliance, Epidemiology, and End Results Program: Lung and Bronchus. Available online: https://seer.cancer.gov/statistics-network/explorer/application.html?site=47&data_type=4&graph_type=5&compareBy=stage&chk_stage_106=106&series=age_range&chk_age_range_1=1&sex=1&race=1&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_show_apc=on&advopt_display=2 (accessed on 5 January 2024).
- Survelliance, Epidemiology, and End Results Program: Cancer Stat Facts: Lung and Bronchus Cancer. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 5 January 2024).
- Hechtner, M.; Eichler, M.; Wehler, B.; Buhl, R.; Sebastian, M.; Stratmann, J.; Schmidberger, H.; Gohrbandt, B.; Peuser, J.; Kortsik, C.; et al. Quality of life in NSCLC survivors—A multicenter cross-sectional study. J. Thorac. Oncol. 2019, 14, 420–435. [Google Scholar] [CrossRef] [PubMed]
- Dalhammar, K.; Kristensson, J.; Falkenback, D.; Rasmussen, B.H.; Malmström, M. Symptoms, problems and quality of life in patients newly diagnosed with oesophageal and gastric cancer—A comparative study of treatment strategy. BMC Cancer 2022, 22, 434. [Google Scholar] [CrossRef] [PubMed]
- Schnipper, L.E.; Davidson, N.E.; Wollins, D.S.; Blayney, D.W.; Dicker, A.P.; Ganz, P.A.; Hoverman, J.R.; Langdon, R.; Lyman, G.H.; Meropol, N.J.; et al. Updating the American Society of Clinical Oncology Value Framework: Revisions and Reflections in Response to Comments Received. J. Clin. Oncol. 2016, 34, 2925–2934. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.T.; McWilliams, J.M.; Hatfield, L.A.; Gerovich, S.; Chernew, M.E.; Gilstrap, L.G.; Mehrotra, A. Changes in Health Care Use Associated With the Introduction of Hospital Global Budgets in Maryland. JAMA Intern. Med. 2018, 178, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Palliative Care in Cancer. Available online: https://www.cancer.gov/about-cancer/advanced-cancer/care-choices/palliative-care-fact-sheet (accessed on 1 June 2022).
- Thomas, T.H.; Jackson, V.A.; Carlson, H.; Rinaldi, S.; Sousa, A.; Hansen, A.; Kamdar, M.; Jacobsen, J.; Park, E.R.; Pirl, W.F.; et al. Communication Differences between Oncologists and Palliative Care Clinicians: A Qualitative Analysis of Early, Integrated Palliative Care in Patients with Advanced Cancer. J. Palliat. Med. 2019, 22, 41–49. [Google Scholar] [CrossRef]
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.A.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.; Pirl, W.F.; et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N. Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef]
- Greer, J.A.; Jackson, V.A.; Meier, D.E.; Temel, J.S. Early integration of palliative care services with standard oncology care for patients with advanced cancer. CA Cancer J. Clin. 2013, 63, 349–363. [Google Scholar] [CrossRef]
- Morrison, R.S.; Penrod, J.D.; Cassel, J.B.; Caust-Ellenbogen, M.; Litke, A.; Spragens, L.; Meier, D.E. Palliative Care Leadership Centers’ Outcomes Group. Cost savings associated with US hospital palliative care consultation programs. Arch. Intern. Med. 2008, 168, 1783–1790. [Google Scholar] [CrossRef]
- Agne, J.L.; Bertino, E.M.; Grogan, M.; Benedict, J.; Janse, S.; Naughton, M.; Eastep, C.; Callahan, M.; Presley, C.J. Too Many Appointments: Assessing Provider and Nursing Perception of Barriers to Referral for Outpatient Palliative Care. Palliat. Med. Rep. 2021, 2, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Bertino, E.M.; Grogan, M.M.; Benedict, J.A.; Agne, J.L.; Janse, S.; Eastep, C.; Sullivan, D.; Gast, K.C.; Naughton, M.J.; Presley, C.J. Feasibility of an embedded palliative care clinic model for patients with an advanced thoracic malignancy. Support Care Cancer 2023, 31, 167. [Google Scholar] [CrossRef] [PubMed]
- Gast, K.C.; Benedict, J.A.; Grogan, M.; Janse, S.; Saphire, M.; Kumar, P.; Bertino, E.M.; Agne, J.L.; Presley, C.J. Impact of an Embedded Palliative Care Clinic on Healthcare Utilization for Patients With a New Thoracic Malignancy. Front. Oncol. 2022, 12, 835881. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, B.R.; Temel, J.S.; Temin, S.; Alesi, E.R.; Balboni, T.A.; Basch, E.M.; Firn, J.I.; Paice, J.A.; Peppercorn, J.M.; Phillips, T.; et al. Integration of Palliative Care Into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 96–112. [Google Scholar] [CrossRef]
- Dans, M.; Smith, T.; Back, A.; Baker, J.N.; Bauman, J.R.; Beck, A.C.; Block, S.; Campbell, T.; Case, A.A.; Dalal, S.; et al. NCCN Guidelines Insights: Palliative Care, Version 2.2017. J. Natl. Compr. Canc. Netw. 2017, 15, 989–997. [Google Scholar] [CrossRef]
- Smith, C.B.; Phillips, T.; Smith, T.J. Using the New ASCO Clinical Practice Guideline for Palliative Care Concurrent with Oncology Care Using the TEAM Approach. Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 714–723. [Google Scholar] [CrossRef]
- Census Bureau, U.S. Explore Census Data. Available online: https://data.census.gov/cedsci/table?g=0500000US48301&tid=ACSST5Y2019.S1901 (accessed on 1 June 2022).
- Greenland, S. Introduction to Regression Models; Modern Epidemiology, K.G., Rothman, S., Lash, T.L., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; pp. 381–417. [Google Scholar]
- Szcklo, M.N.; Nieto, F.J. Epidemiology: Beyond the Basics, 3rd ed.; Jones and Bartlett: Burlington, NJ, USA, 2014. [Google Scholar]
- Brumley, R.; Enguidanos, S.; Jamison, P.; Seitz, R.; Morgenstern, N.; Saito, S.; McIlwane, J.; Hillary, K.; Gonzalez, J. Increased satisfaction with care and lower costs: Results of a randomized trial of in-home palliative care. J. Am. Geriatr. Soc. 2007, 55, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Vranas, K.C.; Lapidus, J.A.; Ganzini, L.; Slatore, C.G.; Sullivan, D.R. Association of Palliative Care Use and Setting With Health-care Utilization and Quality of Care at the End of Life Among Patients With Advanced Lung Cancer. Chest 2020, 158, 2667–2674. [Google Scholar] [CrossRef] [PubMed]
- Gade, G.; Venohr, I.; Conner, D.; McGrady, K.; Beane, J.; Richardson, R.H.; Williams, M.P.; Liberson, M.; Blum, M.; Della Penna, R. Impact of an inpatient palliative care team: A randomized control trial. J. Palliat. Med. 2008, 11, 180–190. [Google Scholar] [CrossRef]
- Sedhom, R.; Gupta, A.; MacNabb, L.; Smith, T.J. The Impact of Palliative Care Dose Intensity on Outcomes for Patients with Cancer. Oncologist 2020, 25, 913–915. [Google Scholar] [CrossRef]
- Bakitas, M.A.; El-Jawahri, A.; Farquhar, M.; Ferrell, B.; Grudzen, C.; Higginson, I.; Temel, J.S.; Zimmermann, C.; Smith, T.J. The TEAM Approach to Improving Oncology Outcomes by Incorporating Palliative Care in Practice. J. Oncol. Pract. 2017, 19, 557–566. [Google Scholar] [CrossRef]
- Parajuli, J.; Hupcey, J.E. A Systematic Review on Barriers to Palliative Care in Oncology. Am. J. Hosp. Palliat. Care 2021, 38, 1361–1377. [Google Scholar] [CrossRef]
- Schnipper, L.E.; Smith, T.J.; Raghavan, D.; Blayney, D.W.; Ganz, P.A.; Mulvey, T.M.; Wollins, D.S. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. J. Clin. Oncol. 2012, 30, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, M.D.; Hasselaar, J.; Garralda, E.; van der Eerden, M.; Stevenson, D.; McKendrick, K.; Centeno, C.; Meier, D.E. Education, implementation, and policy barriers to greater integration of palliative care: A literature review. Palliat. Med. 2016, 30, 224–239. [Google Scholar] [CrossRef] [PubMed]
- Sedhom, R.; Kamal, A.H. Is Improving the Penetration Rate of Palliative Care the Right Measure? JCO Oncol. Pract. 2022, 18, e1388–e1391. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 215) | OPC (n = 81) | Non-OPC (n = 134) | p-Value | |
|---|---|---|---|---|
| Age (median, IQR) | 66.17 (59.82, 75.12) | 65.78 (59.20, 74.08) | 66.40 (60.15, 75.69) | 0.285 |
| Sex (n (%)) | ||||
| Female | 112 (52.1%) | 33 (40.7%) | 79 (59.0%) | 0.011 * |
| Male | 103 (47.9%) | 48 (59.3%) | 55 (41.0%) | |
| Race (n (%)) | ||||
| White | 148 (68.8%) | 62 (76.5%) | 86 (64.2%) | 0.047 * |
| Black or African American | 46 (21.4%) | 9 (11.1%) | 37 (27.6%) | 0.006 * |
| Asian | 8 (3.7%) | 3 (3.7%) | 5 (3.7%) | 1.000 |
| Other | 12 (5.6%) | 6 (7.4%) | 6 (4.5%) | 0.371 |
| Missing | 1 (0.5%) | 1 (1.2%) | 0 (0%) | |
| Insurance (n (%)) | ||||
| Medicare | 34 (15.3%) | 11 (13.6%) | 23 (17.2%) | 0.565 |
| Medicare with supplemental insurance | 107 (49.8%) | 40 (49.4%) | 67 (50.0%) | 1.000 |
| Medicaid/No insurance | 22 (10.2%) | 6 (7.4%) | 16 (11.9%) | 0.188 |
| Private/Self pay | 52 (24.2%) | 24 (29.6%) | 28 (20.9%) | 0.238 |
| Marital status (n (%)) | ||||
| Single | 36 (16.7%) | 9 (11.1%) | 27 (20.1%) | 0.130 |
| Married | 129 (60.0%) | 53 (65.4%) | 76 (56.7%) | 0.195 |
| Separated, Divorced, Widowed, or Unknown | 49 (22.8%) | 18 (22.2%) | 31 (23.1%) | 1.000 |
| Missing | 1 (0.5%) | 1 (1.2%) | 0 (0%) | |
| High risk zip code (n (%)) | ||||
| No | 188 (87.4%) | 74 (91.4%) | 114 (85.1%) | 0.207 |
| Yes | 27 (12.6%) | 7 (8.6%) | 20 (14.9%) | |
| Median income by zip code (n (%)) | ||||
| <USD 40,000 | 10 (4.7%) | 4 (4.9%) | 6 (4.5%) | 1.000 |
| USD 40,000–100,000 | 139 (64.7%) | 48 (59.3%) | 91 (67.9%) | 0.139 |
| >USD 100,000 | 63 (29.3%) | 29 (35.8%) | 34 (25.4%) | 0.164 |
| Missing | 3 (1.4%) | 0 (0%) | 3 (2.2%) | |
| State of residence (n (%)) | ||||
| Maryland | 179 (83.3%) | 73 (90.1%) | 106 (79.1%) | 0.039 * |
| Other | 36 (16.7%) | 8 (9.9%) | 28 (20.9%) | |
| Cancer type (n (%)) | ||||
| NSCLC | 159 (74.0%) | 63 (77.8%) | 96 (71.6%) | 0.253 |
| SCLC | 18 (8.4%) | 4 (4.9%) | 14 (10.4%) | 0.208 |
| Esophageal | 21 (9.8%) | 8 (9.9%) | 13 (9.7%) | 1.000 |
| Other | 14 (6.5%) | 4 (4.9%) | 10 (7.5%) | 0.577 |
| Missing | 3 (1.4%) | 2 (2.5%) | 1 (0.7%) | |
| Cancer stage at diagnosis (n (%)) | ||||
| III | 38 (17.7%) | 12 (14.8%) | 26 (19.4%) | 0.463 |
| IV | 177 (82.3%) | 69 (85.2%) | 108 (80.6%) | |
| Time from first outpatient oncology visit to date of death (n (%)) | ||||
| <90 days | 43 (20.0%) | 7 (8.6%) | 36 (26.9%) | 0.001 * |
| 90–180 days | 32 (14.9%) | 9 (11.1%) | 23 (17.2%) | 0.242 |
| >180 days | 139 (64.7%) | 65 (80.2%) | 74 (55.2%) | <0.001 * |
| Missing | 1 (0.5%) | 0 (0%) | 1 (0.7%) |
| OPC (n = 81) | Non-OPC (n = 134) | Effect Size (95%CI) | p-Value | |
|---|---|---|---|---|
| Number of ER visits (n/person-years of follow-up) | 1.93 (154/79.8) | 1.47 (191/130.3) | 1.32 (0.89, 1.96) 1 | 0.173 |
| Number of hospitalizations (n/person-years of follow-up) | 1.92 (153/79.8) | 1.74 (227/130.3) | 1.10 (0.91, 1.34) 1 | 0.329 |
| Number of ICU admissions (n/person-years of follow-up) | 0.31 (25/79.8) | 0.32 (42/130.3) | 0.97 (0.66, 1.43) 1 | 0.886 |
| Average length of stay (mean (sd)) | 6.63 (5.61) | 6.84 (6.76) | −0.20 (−2.69, 2.28) 2 | 0.872 |
| Missing (n) | 1 | 2 | ||
| Total length of stay (mean (sd)) | 11.58 (14.63) | 10.75 (14.00) | 0.83 (−2.64, 4.30) 2 | 0.638 |
| Average hospital charge per admission at Johns Hopkins Hospital or Johns Hopkins Bayview (mean (sd)) | 24,887 (22,325) | 29,191 (34,565) | −4304 (−15,419, 6811) 2 | 0.448 |
| Missing (n) | 1 | 2 | ||
| Average hospital charge per day at Johns Hopkins Hospital or Johns Hopkins Bayview (mean (sd)) | 3807 (1465) | 4695 (3693) | −887 (−1657, −118) 2 | 0.024 * |
| Missing (n) | 2 | 3 | ||
| Total hospital charges (mean (sd)) | 42,579 (56,354) | 43,578 (65,811) | −999 (−20,465, 18,467) 2 | 0.920 |
| Missing (n) | 1 | 2 | ||
| Readmission within 30 days from prior discharge (n/person-years of follow-up) | 0.59 (47/79.8) | 0.59 (77/130.3) | 1.00 (0.74, 1.35) 1 | 0.984 |
| Hospital admission within 30 days of death (n/N) | 0.45 (25/56) | 0.59 (56/95) | 0.56 (0.36, 0.88) 3 | 0.011 * |
| Missing (n) | 1 | 6 | ||
| Inpatient mortality rate (n/person-years of follow-up) | 0.19 (14/74.8) | 0.30 (33/109.0) | 0.62 (0.44, 0.87) 1 | 0.006 * |
| Missing (n) | 3 | 13 | ||
| Inpatient mortality risk (n/N) | 0.18 (14/78) | 0.27 (33/121) | 0.58 (0.39, 0.87) 3 | 0.009 * |
| Missing (n) | 3 | 13 | ||
| Average number of days in hospice for those enrolled in hospice (mean (sd)) | 14.7 (20.7) | 13.9 (31.0) | 0.87 (−6.49, 8.22) 2 | 0.818 |
| Missing (n) | 1 | 1 | ||
| Received inpatient palliative care consult during any inpatient admission (n/N) | 0.40 (32/81) | 0.31 (41/134) | 1.48 (0.68, 3.22) 3 | 0.321 |
| Emergency Room Visits | Hospitalizations | ICU Admissions | Readmission within 30 Days | Hospital Admission within 30 Days of Death | Inpatient Mortality (Rate) | Inpatient Mortality (Odds) | Time in Hospice < 7 Days | Time in Hospice 8–14 Days | Time in Hospice > 14 Days | |
|---|---|---|---|---|---|---|---|---|---|---|
| IRR(95%CI); p-Value | IRR(95%CI); p-Value | IRR(95%CI); p-Value | IRR(95%CI); p-Value | OR(95%CI); p-Value | IRR(95%CI; p-Value | OR(95%CI); p-Value | OR(95%CI); p-Value | OR(95%CI); p-Value | OR(95%CI); p-Value | |
| Outpatient PC vs. no outpatient PC | 1.45 (1.00, 2.10); 0.050 | 1.16 (0.86, 1.58); 0.336 | 1.03 (0.69, 1.54); 0.889 | 0.92 (0.54, 1.59); 0.775 | 0.45 (0.29, 0.68); <0.001 * | 0.67 (0.48, 0.95); 0.024 * | 0.65 (0.36, 1.18); 0.158 | 0.64 (0.36, 1.12); 0.120 | 1.20 (0.60, 2.39); 0.609 | 1.52 (0.70, 3.28); 0.291 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boulanger, M.C.; Krasne, M.D.; Gough, E.K.; Myers, S.; Browner, I.S.; Feliciano, J.L. Outpatient Embedded Palliative Care for Patients with Advanced Thoracic Malignancy: A Retrospective Cohort Study. Curr. Oncol. 2024, 31, 1389-1399. https://doi.org/10.3390/curroncol31030105
Boulanger MC, Krasne MD, Gough EK, Myers S, Browner IS, Feliciano JL. Outpatient Embedded Palliative Care for Patients with Advanced Thoracic Malignancy: A Retrospective Cohort Study. Current Oncology. 2024; 31(3):1389-1399. https://doi.org/10.3390/curroncol31030105
Chicago/Turabian StyleBoulanger, Mary C., Margaret D. Krasne, Ethan K. Gough, Samantha Myers, Ilene S. Browner, and Josephine L. Feliciano. 2024. "Outpatient Embedded Palliative Care for Patients with Advanced Thoracic Malignancy: A Retrospective Cohort Study" Current Oncology 31, no. 3: 1389-1399. https://doi.org/10.3390/curroncol31030105
APA StyleBoulanger, M. C., Krasne, M. D., Gough, E. K., Myers, S., Browner, I. S., & Feliciano, J. L. (2024). Outpatient Embedded Palliative Care for Patients with Advanced Thoracic Malignancy: A Retrospective Cohort Study. Current Oncology, 31(3), 1389-1399. https://doi.org/10.3390/curroncol31030105





