The REthinking Clinical Trials Program Retreat 2023: Creating Partnerships to Optimize Quality Cancer Care
Abstract
1. Introduction
2. Review of Recently Completed and Currently Accruing REaCT Studies
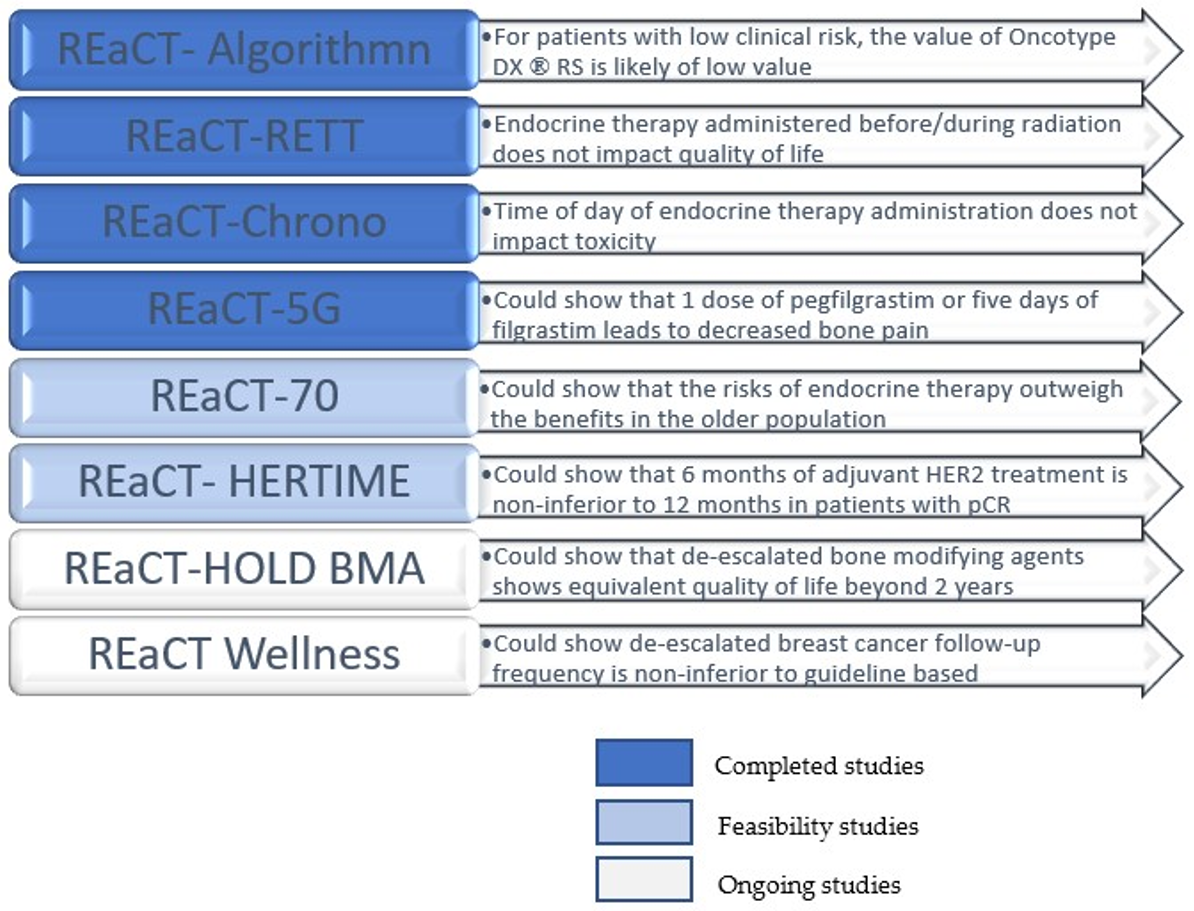
2.1. REaCT-Algorithm (PI: Arif Awan)
2.2. REaCT-RETT (PI: Sharon McGee)
2.3. REaCT-CHRONO (PI: Marie-France Savard)
2.4. REaCT-HER TIME (PI: Sharon McGee)
2.5. REaCT-5G (PI: Terry Ng)
2.6. REaCT-70 (PI: Marie-France Savard)
2.7. REaCT-HOLD BMA (PI: Terry Ng)
2.8. REaCT-Wellness (PI: Ana-Alicia Beltran-Bless)
3. Pragmatic Trial Designs: Understanding Key Statistical Concepts Presented by Gregory Pond
3.1. Per Protocol versus Intent-to-Treat Analyses
3.2. Superiority versus Non-Inferiority Design
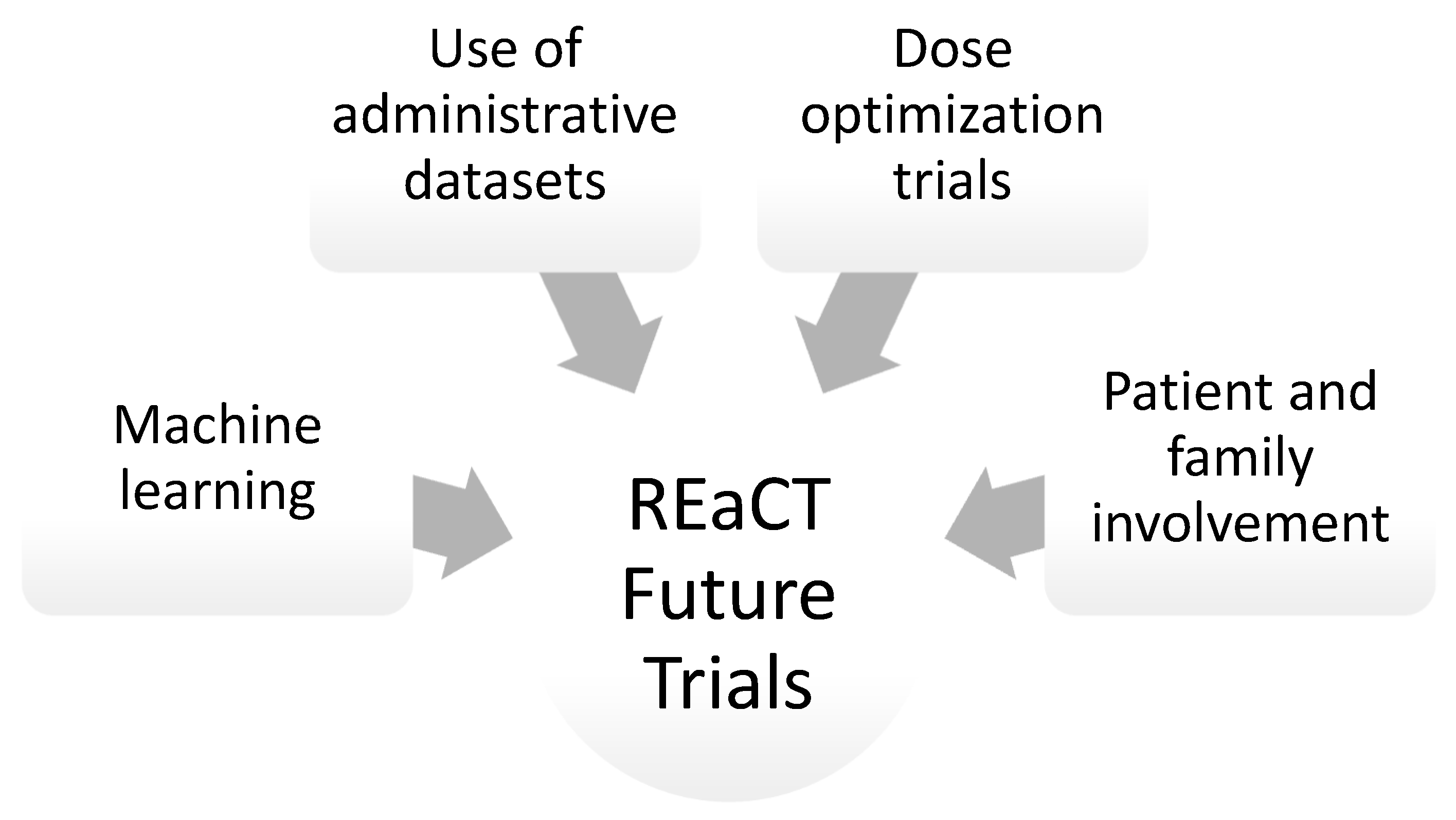
4. Artificial Intelligence and Machine Learning in Clinical Trials Presented by Khaled El-Emam
5. Patient and Family Advisory Council Presented by Julie Renaud and Gwen Barton
6. The Use of Administrative Datasets in Clinical Trials Presented by Brian Hutton
7. The Dose Optimization Trials and Future Perspectives for the REaCT Program Presented by Ian Tannock
8. Discussion
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wouters, O.J.; McKee, M.; Luyten, J. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009–2018. JAMA 2020, 323, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Forum on Drug Discovery. “Current Model for Financing Drug Development: From Concept Through Approval”. 2009. Available online: https://www.ncbi.nlm.nih.gov/books/NBK50972/ (accessed on 17 December 2023).
- Saunders, D.; Liu, M.; Vandermeer, L.; Alzahrani, M.J.; Hutton, B.; Clemons, M. The Rethinking Clinical Trials (REaCT) Program. A Canadian-Led Pragmatic Trials Program: Strategies for Integrating Knowledge Users into Trial Design. Curr. Oncol. 2021, 28, 3959–3977. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.; Mazzarello, S.; Caudrelier, J.; Lima, M.; Hutton, B.; Sienkiewicz, M.; Stober, C.; Fernandes, R.; Ibrahim, M.; Vandermeer, L.; et al. Optimal sequence of adjuvant endocrine and radiation therapy in early-stage breast cancer—A systematic review. Cancer Treat. Rev. 2018, 69, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.L.; Tu, M.M.; Ibrahim, M.F.K.; Basulaiman, B.; McGee, S.F.; Srikanthan, A.; Fernandes, R.; Vandermeer, L.; Stober, C.; Sienkiewicz, M.; et al. Long-term impact of bone-modifying agents for the treatment of bone metastases: A systematic review. Support. Care Cancer 2020, 29, 925–943. [Google Scholar] [CrossRef] [PubMed]
- Savard, M.-F.; Clemons, M.; Hutton, B.; Alzahrani, M.J.; Caudrelier, J.-M.; Vandermeer, L.; Liu, M.; Saunders, D.; Sienkiewicz, M.; Stober, C.; et al. De-escalating adjuvant therapies in older patients with lower risk estrogen receptor-positive breast cancer treated with breast-conserving surgery: A systematic review and meta-analysis. Cancer Treat. Rev. 2021, 99, 102254. [Google Scholar] [CrossRef] [PubMed]
- Awan, A.A.; Hutton, B.; Hilton, J.; Mazzarello, S.; Van Poznak, C.; Vandermeer, L.; Bota, B.; Stober, C.; Sienkiewicz, M.; Fergusson, D.; et al. De-escalation of bone-modifying agents in patients with bone metastases from breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2019, 176, 507–517. [Google Scholar] [CrossRef]
- Basulaiman, B.; Awan, A.A.; Fergusson, D.; Vandermeer, L.; Arnaout, A.; Hilton, J.; Hutton, B.; Joy, A.A.; Robinson, A.; Califaretti, N.; et al. Creating a pragmatic trials program for breast cancer patients: Rethinking Clinical Trials (REaCT). Breast Cancer Res. Treat. 2019, 177, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Awan, A. Evaluating Whether Integration of Prognostic and Predictive Algorithms into Routine Clinical Practice Effect Whether Oncologists Order Multigene Assays in Patients with Early Stage Breast Cancer (REaCT-Algorith). Available online: https://clinicaltrials.gov/study/NCT04131933 (accessed on 28 November 2023).
- Awan, A.A.; Saunders, D.; Pond, G.; Hamm, C.; Califaretti, N.; Mates, M.; Kumar, V.; Ibrahim, M.F.K.; Beltran-Bless, A.-A.; Vandermeer, L.; et al. Does pre-emptive availability of PREDICT 2.1 results change ordering practices for Oncotype DX? A multi-center prospective cohort study. Curr. Oncol. 2024, 31, 1278–1290. [Google Scholar] [CrossRef]
- Bourque, J.-M.; McGee, S. “Evaluating Optimal Timing of Endocrine Therapy and Radiation Therapy in Early-stage Breast Cancer (REaCT-RETT),” ClinicalTrials.gov: NCT03948568. Available online: https://clinicaltrials.gov/study/NCT03948568 (accessed on 28 November 2023).
- Mc Gee, S.F.; Clemons, M.; Liu, M.; Alzahrani, M.J.; Ng, T.; Awan, A.; Sehdev, S.; Hilton, J.; Caudrelier, J.M.; Savard, M.F.; et al. Abstract OT1-01-01: A randomized, pragmatic trial investigating the timing of radiotherapy and endocrine in patients with early stage breast cancer (REaCT-RETT trial). Cancer Res 2022, 82, OT1-01. [Google Scholar] [CrossRef]
- McGee, S.F.; Clemons, M.; Liu, M.; Alzahrani, M.J.; Ng, T.; Awan, A.; Sehdev, S.; Hilton, J.; Caudrelier, J.M.; Savard, M.F.; et al. REaCT-RETT: A pragmatic clinical trial successfully conducted during the COVID-19 pandemic. In Proceedings of the 2021 Canadian Cancer Research Alliance Conference, Virtual, 8–11 November 2021. [Google Scholar]
- Aiello Bowles, E.J.; Boudreau, D.M.; Chubak, J.; Yu, O.; Fujii, M.; Chestnut, J.; Buist, D.S. Patient-reported discontinuation of endocrine therapy and related adverse effects among women with early-stage breast cancer. J. Oncol. Pract. 2012, 8, e149–e157. [Google Scholar] [CrossRef]
- Savard, M.-F. Evaluating the Dose Timing (Morning vs Evening) of Endocrine Therapy and Its Effects on Tolerability and Compliance,” ClinicalTrials.gov: NCT04928261. Available online: https://clinicaltrials.gov/study/NCT04864405 (accessed on 28 November 2023).
- Savard, M.-F.; Ibrahim, M.; Pond, G.; Saunders, D.; Vandermeer, L. A pragmatic, randomised, multicentre trial evaluating the dose timing (morning vs. evening) of endocrine therapy and its effects on tolerability and compliance (REaCT-CHRONO Study). In Proceedings of the San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 5–9 December 2023. [Google Scholar]
- Wang, B.-C.; Xiao, B.-Y.; Fan, J.-Q.; Lin, G.-H.; Wang, C.; Liu, Q.; Zhao, Y.-X. 6 versus 12 months of adjuvant trastuzumab in HER2+ early breast cancer: A systematic review and meta-analysis. Medicine 2021, 100, e24995. [Google Scholar] [CrossRef]
- Deng, H.; Du, X.; Wang, L.; Chen, M. Six Months vs. 12 Months of Adjuvant Trastuzumab Among Women with HER2-Positive Early-Stage Breast Cancer: A Meta-Analysis of Randomized Controlled Trials. Front. Oncol. 2020, 10, 288. [Google Scholar] [CrossRef]
- McGee, S. Evaluating 6-months of HER2-targeted Therapy in Patients with HER2 Positive Early-stage Breast Cancer That Achieve a Pathological Complete Response to Neoadjuvant Systemic Therapy,” ClinicalTrials.gov: NCT04928261. Available online: https://clinicaltrials.gov/study/NCT04928261 (accessed on 28 November 2023).
- Ng, T. A Randomized, Multicenter Pragmatic Trial Comparing Bone Pain from a Single Dose of Pegfilgrastim to 5 Doses of Daily Filgrastim in Breast Cancer Patients Receiving Neoadjuvant/Adjuvant Chemotherapy,” ClinicalTrials.gov: NCT04781959. Available online: https://clinicaltrials.gov/study/NCT04781959 (accessed on 28 November 2023).
- Ng, T.L.; Taljaard, M.; Savard, M.F.; Stober, C.; Nicholls, S.; Vandermeer, L.; Thavorn, K.; Hampel, C.; Shamess, J.; Mills, N.; et al. REaCT 5G: A randomized study comparing bone pain after 5 days of filgrastim or one day of pegfilgrastim for primary febrile neutropenia prophylaxis during neo-/adjuvant chemotherapy for early breast cancer. In Proceedings of the San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 5–9 December 2023. [Google Scholar]
- Savard, M.-F. Evaluating Harms and Benefits of Endocrine Therapy in Patients ≥70 Years of Age with Lower Risk Breast Cancer,” ClinicalTrials.gov: NCT04921137. Available online: https://clinicaltrials.gov/study/NCT04921137 (accessed on 28 November 2023).
- Ng, T. Comparing Continuation or De-Escalation of Bone Modifying Agents (BMA) in Patients Treated for Over 2 Years for Bone Metastases from Either Breast or Castration-Resistant Prostate Cancer,” ClinicalTrials.gov: NCT04549207. Available online: https://clinicaltrials.gov/study/NCT04549207 (accessed on 28 November 2023).
- Beltran-Bless, A.-A.; Clemons, M. How Frequently Should Patients with Breast Cancer Have Routine Follow-Up Visits? NEJM Evid. 2023, 2, EVIDtt2300062. [Google Scholar] [CrossRef]
- Clemons, M.; Hilton, J. A Randomized Trial Evaluating Personalized vs Guideline-based Well Follow-up Strategies for Patients with Early-stage Breast Cancer,” ClinicalTrials.gov: NCT05365230. Available online: https://clinicaltrials.gov/study/NCT05365230 (accessed on 28 November 2023).
- Ranganathan, P.; Pramesh, C.; Aggarwal, R. Common pitfalls in statistical analysis: Intention-to-treat versus per-protocol analysis. Perspect. Clin. Res. 2016, 7, 144–146. [Google Scholar] [CrossRef]
- Tripepi, G.; Chesnaye, N.C.; Dekker, F.W.; Zoccali, C.; Jager, K.J. Intention to treat and per protocol analysis in clinical trials. Nephrology 2020, 25, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Pramesh, C.; Aggarwal, R. Non-inferiority trials. Perspect. Clin. Res. 2022, 13, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Bertsimas, D.; Wiberg, H. Machine Learning in Oncology: Methods, Applications, and Challenges. JCO Clin. Cancer Inform. 2020, 4, 885–894. [Google Scholar] [CrossRef]
- Maceachern, S.J.; Forkert, N.D. Machine learning for precision medicine. Genome 2021, 64, 416–425. [Google Scholar] [CrossRef]
- Kocak, B. Key concepts, common pitfalls, and best practices in artificial intelligence and machine learning: Focus on radiomics. Diagn. Interv. Radiol. 2022, 28, 450–462. [Google Scholar] [CrossRef] [PubMed]
- El Kababji, S.; El Kababji, S.; Mitsakakis, N.; Mitsakakis, N.; Fang, X.; Fang, X.; Beltran-Bless, A.-A.; Beltran-Bless, A.-A.; Pond, G.; Pond, G.; et al. Evaluating the Utility and Privacy of Synthetic Breast Cancer Clinical Trial Data Sets. JCO Clin. Cancer Inform. 2023, 7, e2300116. [Google Scholar] [CrossRef]
- Hay, A.E.; Leung, Y.W.; Pater, J.L.; Brown, M.C.; Bell, E.; Howell, D.; Kassam, Z.; Willing, S.; Tian, C.; Liu, G. Linkage of clinical trial and administrative data: A survey of cancer patient preferences. Curr. Oncol. 2017, 24, 161–167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shah, H.; Wolfe, D.; Clemons, M.; Liu, M.; Thavorn, K.; Veroniki, A.-A.; Lunny, C.; Pond, G.; McGee, S.; Skidmore, B.; et al. Can routinely collected administrative data effectively be used to evaluate and validate endpoints used in breast cancer clinical trials? Protocol for a scoping review of the literature. Syst. Rev. 2023, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Clemons, M. Comparing a Single-Dose vs. Twice Yearly Zoledronate in Patients with Early Stage Breast Cancer (REaCT-ZOL) (REaCT-ZOL),” ClinicalTrials.gov: NCT03664687. Available online: https://clinicaltrials.gov/study/NCT03664687 (accessed on 29 November 2023).
- Hirsch, I.; Goldstein, D.A.; Tannock, I.F.; Butler, M.O.; Gilbert, D.C. Optimizing the dose and schedule of immune checkpoint inhibitors in cancer to allow global access. Nat. Med. 2022, 28, 2236–2237. [Google Scholar] [CrossRef]
- Jatoi, I.; Gail, M.H. The Need for Combined Assessment of Multiple Outcomes in Noninferiority Trials in Oncology. JAMA Oncol. 2020, 6, 420–424. [Google Scholar] [CrossRef]
- Renner, A.; Burotto, M.; Rojas, C. Immune checkpoint inhibitor dosing: Can we go lower without compromising clinical efficacy? J. Glob. Oncol. 2019, 5, 1–5. [Google Scholar] [CrossRef]
- Rocque, G.B.; Williams, C.P.; Andrews, C.; Childers, T.C.; Wiseman, K.D.; Gallagher, K.; Tung, N.; Balch, A.; Lawhon, V.M.; Ingram, S.A.; et al. Patient perspectives on chemotherapy de-escalation in breast cancer. Cancer Med. 2021, 10, 3288–3298. [Google Scholar] [CrossRef]
- Rocque, G.B.; Andrews, C.; Lawhon, V.M.; Frazier, R.; Ingram, S.A.; Smith, M.L.; Wagner, L.I.; Zubkoff, L.; Tung, N.; Wallner, L.P.; et al. Oncologist-Reported Barriers and Facilitators to Enrolling Patients in Optimization Trials That Test Less Intense Cancer Treatment. JCO Oncol. Pract. 2023, 19, e263–e273. [Google Scholar] [CrossRef]
- Kolesar, J.M.; Liu, G.X. Low-Fat Abiraterone Food Effect Is of Little Consequence. J. Clin. Oncol. 2018, 36, 1385–1386. [Google Scholar] [CrossRef] [PubMed]
- Velho, P.I.; Eisenberger, M.A. There is now compelling evidence to further evaluate lower doses of abiraterone acetate in men with metastatic prostate cancer: It should be safer, may be as effective and less expensive. J. Clin. Oncol. 2018, 36, 3059–3060. [Google Scholar] [CrossRef] [PubMed]
| Study | REaCT- Algorithm | REaCT-RETT | REaCT-CHRONO | REaCT-HER TIME | REaCT-5G | REaCT-70 | REaCT-HOLD BMA | REaCT- Wellness |
|---|---|---|---|---|---|---|---|---|
| Objective | Assess how clinicians use PREDICT 2.1 to guide their decision making when ordering Oncotype Dx | Assess whether concomitant administration of ET throughout RT increases risk of toxicities | Assess whether the time of day of ET administration affects side effects and compliance | Assess feasibility of conducting larger trial examining 6 months of HER2-targeted therapy | Assess difference in bone pain between PEG and FIL in patients with early breast cancer receiving neo/adjuvant chemotherapy | Assess whether the omission of adjuvant ET for patients ≥ 70 with a lower-risk HR+ breast cancer (treated with standard loco-regional therapy) affects outcomes | Evaluate the optimal frequency of BMA use after 2 years of prior BMA treatment for bone metastases in patients with metastatic breast cancer and CRPC | Assess quality of life with de-escalated follow-up for patients with a history of breast cancer |
| Arms | N/A (before and after an educational intervention with PREDICT 2.1 results) | Sequential RT and ET vs. concurrent RT and ET | Morning ET dosing vs. evening ET dosing | N/A (single-arm study) | Five days of FIL vs. 1 dose of PEG | Omission of ET vs. administration of ET | Continuation of BMA every 4 or 12 weeks vs. de-escalating BMA to every 26 weeks | Guideline-based survivorship vs. annual phone call post-mammogram with on-demand access |
| Enrollment | Mar 2020– Nov 2021 | Sep 17 2019–Jan 15 2021 | Jun 2021– Mar 2022 | Dec 2021–Present | Jun 2021–Mar 2023 | Sep 2021–Present | Oct 2020–Present | Sep 2022– Mar 2023 |
| Number of patients enrolled | 620 | 262 | 245 | 20 | 233 | 72 | 171 | 244 |
| Endpoints | Primary: proportion of patients for whom Oncotype Dx was ordered, defined as the number of patients with Oncotype DX orders divided by the number of patients eligible for Oncotype DX testing | Primary: ET toxicity per FACT-ES Secondary: FACT-TOI, EQ-5D-5L, RT toxicity and compliance | Primary: FACT-ES score from baseline to 12 weeks following the beginning of ET. Secondary: total score and individual items of FACT-ES and FACT-B from baseline to 4, 8, 12 and 52 weeks. Rates of discontinuation, interruption and compliance. | Primary: feasibility Secondary: cardiac events, Rx discontinuation EQ-5D-5L | Primary: bone pain during cycle 1 of chemotherapy Secondary: febrile neutro-penia rate, treat-ment-related hospitalizations, compliance, healthcare utilization, HR-QoL, cost utility, patient preference pre- vs. post-chemo | Primary: feasibility Secondary: significant AEs, PROs, treatment discontinuation and reasons | Primary: quality of life and physical function (C30+ BM22) at 48 weeks Secondary: Quality of life other time points, BMA toxicity, cost utility and EQ-5D-5L until year 1 | Primary: quality of life by FACT-G 1 year post-randomization. Secondary outcome: fear of recurrence, anxiety levels, treatment-related toxicity, recurrences, frequency, type and reasoning behind any breast-cancer-related healthcare contacts, incremental cost-effectiveness ratio |
| Results | Results showed that the educational intervention did not impact molecular assay requests such as Oncotype DX. However, the study suggests that routine ordering of molecular assays for patients with low-clinical-risk disease is of poor value for most patients(9). | No difference in ET toxicity from baseline to 3 months and no difference in quality of life, ET compliance or RT toxicity at twelve months. | The 12-week FACT-ES scores mean changed from baseline and the proportion of patients with a clinically important decrease in their FACT-ES scores was not statistically different between the two groups. Secondary endpoint results, including 12-month adherence rate and quality of life were presented at a later symposium in 2023 (15). | N/A | No significant difference in patient-reported bone pain or quality of life between 5 days FIL and a single dose of PEG and no difference in chemotherapy delay, dose reduction or premature discontinuation. At the end of the study, preference for PEG increased in the PEG-treated group. | N/A | N/A | N/A |
| Successes | Important question potentially resulting in savings for the healthcare system, as well as substantial data generation regarding adjuvant decision making. | Strong multidisciplinary involvement, rapid recruitment of a large cohort and nimble response to pandemic restrictions (virtual recruitment and online questionnaire) | Incredible interest and engagement from patients and medical oncologists, as well as rapid accrual | Important question addressed, funding from CURE foundation, excellent patient identification and MD engagement | Developed in partnership with patient partners, using PROs as primary endpoint and strong engagement | First trial using integrated consent model to open in Saskatchewan. It had a steady rate of accrual, was designed for older patients and had good patient engagement | Trial caught attention of international advocacy group (GRASP) and had slow and steady accrual | Strong patient and physician engagement, as well as quick enrolment |
| Challenges | Impact of education intervention was minimal in changing clinical practice. Lower-than-expected accrual due to changes in practice due to COVID-19 pandemic. | Peripheral site activation and recruitment. Pandemic impacts on data collection and publication | Heavy workload for the CRA due to rapid accrual. Use of patient-reported outcomes as a primary endpoint | Small patient population, short window for enrollment, peripheral site activation and competing de-escalation studies | Peripheral site activation, completion of questionnaire pre-randomization, and heterogeneity across centres for chemotherapy regimens eligible for G-CSF prophylaxis | Time commitment to explain the study to patients, accrual in an older population, peripheral site recruitment and heavy workload due to being a mult-centre study | Peripheral site recruitment, recruitment of patients with CRPC due to short life expectancy and accuracy of data regarding toxicity for peripheral sites | Peripheral site recruitment and difficulty with data collection due to different follow-ups |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltran-Bless, A.-A.; Clemons, M.; Vandermeer, L.; El Emam, K.; Ng, T.L.; McGee, S.; Awan, A.A.; Pond, G.; Renaud, J.; Barton, G.; et al. The REthinking Clinical Trials Program Retreat 2023: Creating Partnerships to Optimize Quality Cancer Care. Curr. Oncol. 2024, 31, 1376-1388. https://doi.org/10.3390/curroncol31030104
Beltran-Bless A-A, Clemons M, Vandermeer L, El Emam K, Ng TL, McGee S, Awan AA, Pond G, Renaud J, Barton G, et al. The REthinking Clinical Trials Program Retreat 2023: Creating Partnerships to Optimize Quality Cancer Care. Current Oncology. 2024; 31(3):1376-1388. https://doi.org/10.3390/curroncol31030104
Chicago/Turabian StyleBeltran-Bless, Ana-Alicia, Mark Clemons, Lisa Vandermeer, Khaled El Emam, Terry L. Ng, Sharon McGee, Arif Ali Awan, Gregory Pond, Julie Renaud, Gwen Barton, and et al. 2024. "The REthinking Clinical Trials Program Retreat 2023: Creating Partnerships to Optimize Quality Cancer Care" Current Oncology 31, no. 3: 1376-1388. https://doi.org/10.3390/curroncol31030104
APA StyleBeltran-Bless, A.-A., Clemons, M., Vandermeer, L., El Emam, K., Ng, T. L., McGee, S., Awan, A. A., Pond, G., Renaud, J., Barton, G., Hutton, B., & Savard, M.-F. (2024). The REthinking Clinical Trials Program Retreat 2023: Creating Partnerships to Optimize Quality Cancer Care. Current Oncology, 31(3), 1376-1388. https://doi.org/10.3390/curroncol31030104






