Virtual Care for Patients with Advanced Well Differentiated Gastroenteropancreatic Neuroendocrine Tumor (GEP-NET)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Patient Characteristics
2.3. Outcomes of Interest
2.4. Statistical Analysis
3. Results
3.1. Sample Characteristics
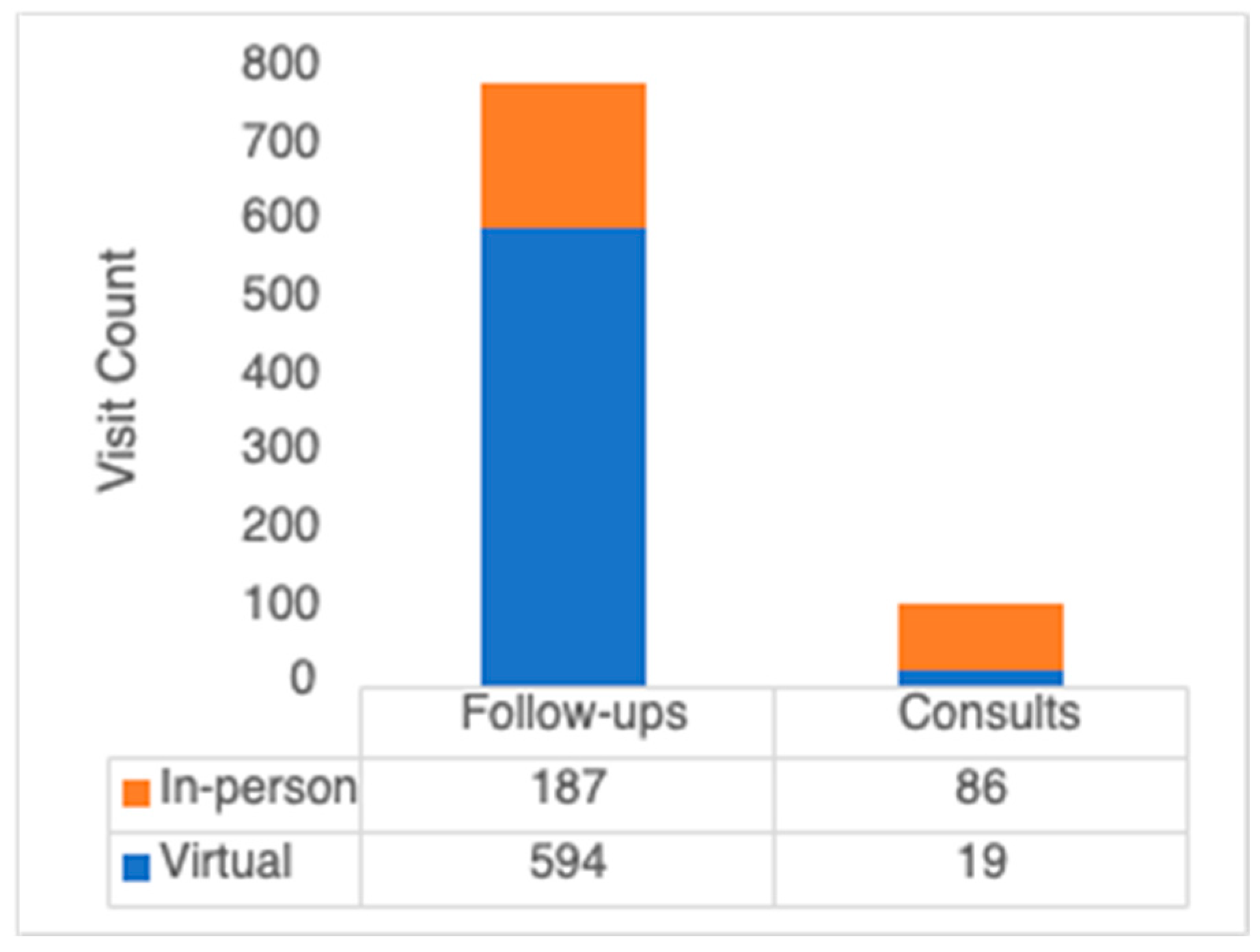
3.2. Virtual Care Use by Year
3.3. The Relationship between Patient Characteristics and Virtual Care Use
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, Z.-Y.; Gong, Y.-F.; Zhuang, H.-K.; Zhou, Z.-X.; Huang, S.-Z.; Zou, Y.-P.; Huang, B.-W.; Sun, Z.-H.; Zhang, C.-Z.; Tang, Y.-Q.; et al. Pancreatic neuroendocrine tumors: A review of serum biomarkers, staging, and management. World J. Gastroenterol. 2020, 26, 2305–2322. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M. Gastrointestinal neuroendocrine tumors in 2020. World J. Gastrointest. Oncol. 2020, 12, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. Available online: https://link.springer.com/article/10.1007/s11912-021-01029-7 (accessed on 5 December 2023). [CrossRef]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. Available online: http://www.ncbi.nlm.nih.gov/pubmed/18565894 (accessed on 5 December 2023). [CrossRef] [PubMed]
- Lee, M.R.; Harris, C.; Baeg, K.J.; Aronson, A.; Wisnivesky, J.P.; Kim, M.K. Incidence Trends of Gastroenteropancreatic Neuroendocrine Tumors in the United States. Clin. Gastroenterol. Hepatol. 2019, 17, 2212–2217.e1. [Google Scholar] [CrossRef] [PubMed]
- Hallet, J.; Law, C.H.L.; Cukier, M.; Saskin, R.; Liu, N.; Singh, S. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015, 121, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Berlin, A.; Lovas, M.; Truong, T.; Melwani, S.; Liu, J.; Liu, Z.A.; Badzynski, A.; Carpenter, M.B.; Virtanen, C.; Morley, L.; et al. Implementation and Outcomes of Virtual Care Across a Tertiary Cancer Center during COVID-19. JAMA Oncol. 2021, 7, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Palkhivala, A. Canada develops models of teleoncology. J. Natl. Cancer Inst. 2011, 103, 1566–1568. [Google Scholar] [CrossRef]
- Petrone, D.; Mateo-Urdiales, A.; Sacco, C.; Riccardo, F.; Bella, A.; Ambrosio, L.; Presti, A.L.; Di Martino, A.; Ceccarelli, E.; Del Manso, M.; et al. Reduction of the risk of severe COVID-19 due to Omicron compared to Delta variant in Italy (November 2021–February 2022). Int. J. Infect. Dis. 2023, 129, 135–141. Available online: http://www.ncbi.nlm.nih.gov/pubmed/36708869 (accessed on 5 December 2023). [CrossRef]
- Harris, E. WHO Declares End of COVID-19 Global Health Emergency. JAMA 2023, 329, 1817. Available online: https://jamanetwork.com/journals/jama/fullarticle/2805298 (accessed on 5 December 2023). [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335. Available online: http://oncology.jamanetwork.com/article.aspx?doi=10.1001/jamaoncol.2017.0589 (accessed on 5 December 2023). [CrossRef]
- Zhang, J.Y.; Kunz, P.L. Making Sense of a Complex Disease: A Practical Approach to Managing Neuroendocrine Tumors. JCO Oncol. Pract. 2022, 18, 258–264. Available online: https://ascopubs.org/doi/10.1200/OP.21.00240 (accessed on 5 December 2023). [CrossRef] [PubMed]
- Phan, A.T. Emerging treatment options for patients with gastroenteropancreatic neuroendocrine tumors. Clin. Adv. Hematol. Oncol. 2017, 15, 10–14. [Google Scholar] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177 Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Yeung, J.C.; Bolger, J.C. The safety and acceptability of using telehealth for follow-up of patients following cancer surgery: A systematic review. Eur. J. Surg. Oncol. 2023, 49, 9–15. Available online: http://www.ncbi.nlm.nih.gov/pubmed/36114050 (accessed on 5 December 2023). [CrossRef]
- Phillips, W.J.; Leung, M.; Thavorn, K.; Asmis, T.R. The Impact of Virtual Cancer Care on Chemotherapy Delivery and Clinical Outcomes in Colorectal Cancer Patients Receiving Systemic Therapy: A Pre- and Intra-Pandemic Analysis. Curr. Oncol. 2022, 29, 6226–6235. Available online: http://www.ncbi.nlm.nih.gov/pubmed/36135058 (accessed on 5 December 2023). [CrossRef] [PubMed]
- Chan, B.A.; Larkins, S.L.; Evans, R.; Watt, K.; Sabesan, S. Do teleoncology models of care enable safe delivery of chemotherapy in rural towns? Med. J. Aust. 2015, 203, e1–e406. [Google Scholar] [CrossRef]
- Wessels, H.; Graeff, A.; Wynia, K.; Heus, M.; Kruitwagen, C.L.; Woltjer, G.T.; Teunissen, S.C.; Voest, E.E. Gender-related needs and preferences in cancer care indicate the need for an individualized approach to cancer patients. Oncologist 2010, 15, 648–655. Available online: http://www.ncbi.nlm.nih.gov/pubmed/20507890 (accessed on 5 December 2023). [CrossRef]
- Pritchett, J.C.; Patt, D.; Thanarajasingam, G.; Schuster, A.; Snyder, C. Patient-Reported Outcomes, Digital Health, and the Quest to Improve Health Equity. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e390678. Available online: http://www.ncbi.nlm.nih.gov/pubmed/37290027 (accessed on 5 December 2023). [CrossRef]
- Chu, C.; Nayyar, D.; Bhattacharyya, O.; Martin, D.; Agarwal, P.; Mukerji, G. Patient and Provider Experiences With Virtual Care in a Large, Ambulatory Care Hospital in Ontario, Canada During the COVID-19 Pandemic: Observational Study. J. Med. Internet Res. 2022, 24, e38604. Available online: http://www.ncbi.nlm.nih.gov/pubmed/36194862 (accessed on 5 December 2023). [CrossRef]
- Singh, S.; Law, C. Multidisciplinary reference centers: The care of neuroendocrine tumors. J. Oncol. Pract. 2010, 6, e11–e16. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21358944 (accessed on 5 December 2023). [CrossRef] [PubMed]
- Dcruz, J.; McIntyre, N.; Devlin, M.; Nicholson, M.; Mrkobrada, M.; Lau, S.Y.; Spicer, E. Is it Virtually Worth It? Cost Analysis of Telehealth Monitoring for Community-based COVID-19-positive Patients. Can. J. Gen. Intern. Med. 2022, 17, 22–33. Available online: https://www.cjgim.ca/index.php/csim/article/view/634 (accessed on 5 December 2023). [CrossRef]
- Singh, S.; Fletcher, G.G.; Yao, X.; Sussman, J. Virtual care in patients with cancer: A systematic review. Curr. Oncol. 2021, 28, 3488–3506. [Google Scholar] [CrossRef] [PubMed]
- Levine, O.H.; McGillion, M.; Levine, M. Virtual cancer care during the COVID-19 pandemic and beyond: A call for evaluation. JMIR Cancer 2020, 6, e24222. [Google Scholar] [CrossRef]

| Characteristic | Description | Frequency | Percentage |
|---|---|---|---|
| Age | <50 | 19 | 18.5% |
| 50–59 | 29 | 28.2% | |
| 60–69 | 34 | 33.0% | |
| 70–79 | 17 | 16.5% | |
| 80+ | 4 | 3.9% | |
| Sex | Female | 49 | 47.1% |
| Charlston score (modified) | 0–1 | 36 | 34.6% |
| 2–4 | 56 | 53.8% | |
| 5+ | 12 | 11.5% | |
| Employment | Employed | 55 | 56.1% |
| Retired | 41 | 41.8% | |
| Disability | 2 | 2.0% | |
| Distance to cancer center | <20 | 58 | 55.8% |
| 20–49 | 22 | 21.2% | |
| 50+ | 24 | 23.1% | |
| Provider | Provider A | 35 | 33.7% |
| Provider B | 46 | 44.2% | |
| Provider C | 23 | 22.1% | |
| Primary location | Small Bowel | 57 | 55.3% |
| Colon | 14 | 13.6% | |
| Pancreas | 22 | 21.4% | |
| Other/Unknown | 10 | 9.7% | |
| De novo metastatic disease | - | 50 | 48.1% |
| Ki67 | <3 | 63 | 67.7% |
| 3–20 | 30 | 32.3% | |
| Year of oncology consultation | 2019.0 | 19 | 18.3% |
| 2020.00 | 27 | 26.0% | |
| 2021.00 | 38 | 36.5% | |
| 2022.00 | 20 | 19.2% | |
| Radical surgery | - | 68 | 66.0% |
| Debulking surgery | - | 5 | 4.9% |
| Radiation to metastatic site | - | 3 | 2.9% |
| Any systemic therapy | - | 50 | 48.1% |
| Somatostatin analogue | - | 48 | 46.2% |
| Chemotherapy | - | 6 | 5.8% |
| Targeted therapy | - | 1 | 1.0% |
| Peptide receptor radionucleotide therapy | - | 2 | 1.9% |
| Characteristic | Description | OR (95% CI) | p-Value |
|---|---|---|---|
| Year of follow-up appointment | 2020 (comparison) | - | - |
| 2019 | - | - | |
| 2021 | 4.32 (1.98–9.44) | <0.001 | |
| 2022 | 0.39 (0.24–0.66) | <0.001 | |
| 2023 | 0.14 (0.058–0.33) | <0.001 | |
| De novo metastases | - | 0.54 (0.63–1.27) | 0.89 |
| Sex | Female | 0.80 (0.57–1.11) | 0.18 |
| Age | - | 1.06 (0.91–1.25) | 0.45 |
| Employment | Working (comparison) | - | - |
| Retired | 0.57 (0.41–0.80) | 0.001 | |
| Disability | 2.96 (0.68–12.81) | 0.15 | |
| Charlston score (modified) | - | 1.28 (0.96–1.70) | 0.091 |
| Distance to cancer center | - | 1.05 (0.86–1.28) | 0.65 |
| Location | Small bowel (comparison) | - | - |
| Pancreas | 0.80 (0.43–1.49) | 0.49 | |
| Colon | 1.45 (0.95–2.21) | 0.082 | |
| Unknown/other | 0.66 (0.31–1.40) | 0.28 | |
| Ki67 | >3% | 1.21 (0.94–1.56) | 0.14 |
| Active systemic therapy | - | 0.76 (0.53–1.09) | 0.13 |
| Provider | A (comparison) | - | - |
| B | 1.10 (0.76–1.61) | 0.61 | |
| C | 0.98 (0.63–1.54) | 0.94 |
| Characteristic | Description | OR (95% CI) | p-Value |
|---|---|---|---|
| Year of follow-up appointment | 2019 | - | - |
| 2020 (comparison) | - | - | |
| 2021 | 4.67 (2.03–10.73) | <0.001 | |
| 2022 | 0.40 (0.23–0.69) | <0.001 | |
| 2023 | 0.14 (0.055–0.37) | <0.001 | |
| De novo metastases | - | 0.76 (0.36–1.60) | 0.47 |
| Sex | Female | 0.63 (0.40–0.98) | 0.04 |
| Age | - | 1.29 (0.90–1.84) | 0.16 |
| Employment | Working (comparison) | - | - |
| Retired | 0.25 (0.13–0.45) | 0.040 | |
| Disability | 1.58 (0.26–9.43) | 0.62 | |
| Charlston score | - | 1.88 (1.03–3.43) | 0.039 |
| Distance to cancer center (km) | - | 1.14 (0.87–1.49) | 0.34 |
| Location | Small bowel (comparison) | - | - |
| Pancreas | 0.68 (0.30–1.54) | 0.36 | |
| Colon | 1.29 (0.75–2.21) | 0.36 | |
| Unknown/other | 0.71(0.25–2.00) | 0.52 | |
| Ki67 | >3% | 0.98 (0.68–1.41) | 0.91 |
| Systemic therapy | 0.73 (0.36–1.48) | 0.38 | |
| Provider | A (comparison) | - | - |
| B | 1.09 (0.63–1.90) | 0.76 | |
| C | 1.45 (0.79–2.65) | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phillips, W.J.; Pradier, M.; Goodwin, R.; Vickers, M.; Asmis, T. Virtual Care for Patients with Advanced Well Differentiated Gastroenteropancreatic Neuroendocrine Tumor (GEP-NET). Curr. Oncol. 2024, 31, 952-961. https://doi.org/10.3390/curroncol31020071
Phillips WJ, Pradier M, Goodwin R, Vickers M, Asmis T. Virtual Care for Patients with Advanced Well Differentiated Gastroenteropancreatic Neuroendocrine Tumor (GEP-NET). Current Oncology. 2024; 31(2):952-961. https://doi.org/10.3390/curroncol31020071
Chicago/Turabian StylePhillips, William J., Michelle Pradier, Rachel Goodwin, Michael Vickers, and Tim Asmis. 2024. "Virtual Care for Patients with Advanced Well Differentiated Gastroenteropancreatic Neuroendocrine Tumor (GEP-NET)" Current Oncology 31, no. 2: 952-961. https://doi.org/10.3390/curroncol31020071
APA StylePhillips, W. J., Pradier, M., Goodwin, R., Vickers, M., & Asmis, T. (2024). Virtual Care for Patients with Advanced Well Differentiated Gastroenteropancreatic Neuroendocrine Tumor (GEP-NET). Current Oncology, 31(2), 952-961. https://doi.org/10.3390/curroncol31020071






