Quality of Life Longitudinal Evaluation in Prostate Cancer Patients from Radiotherapy Start to 5 Years after IMRT-IGRT
Abstract
1. Introduction
2. Materials and Methods
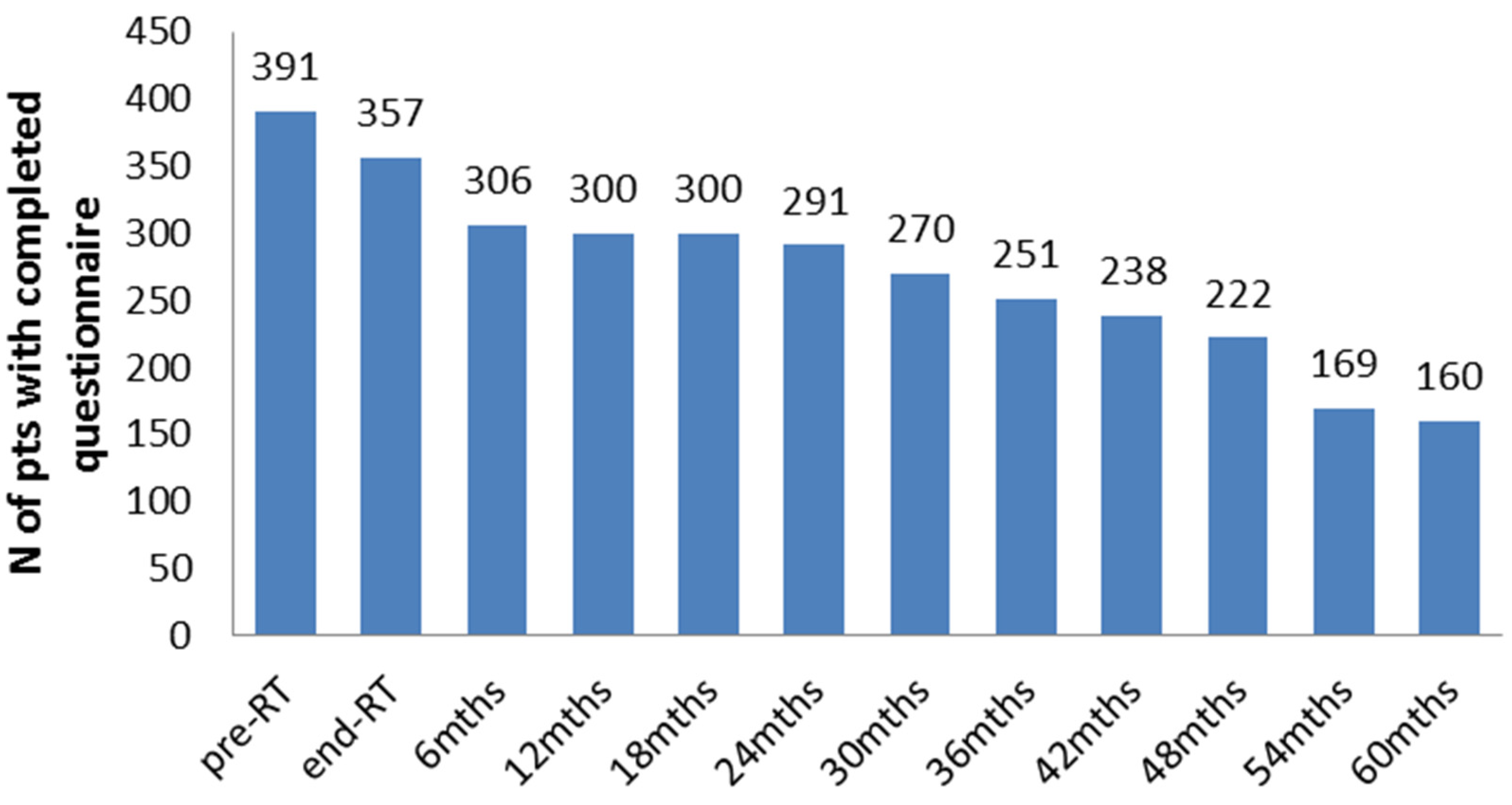
3. Results
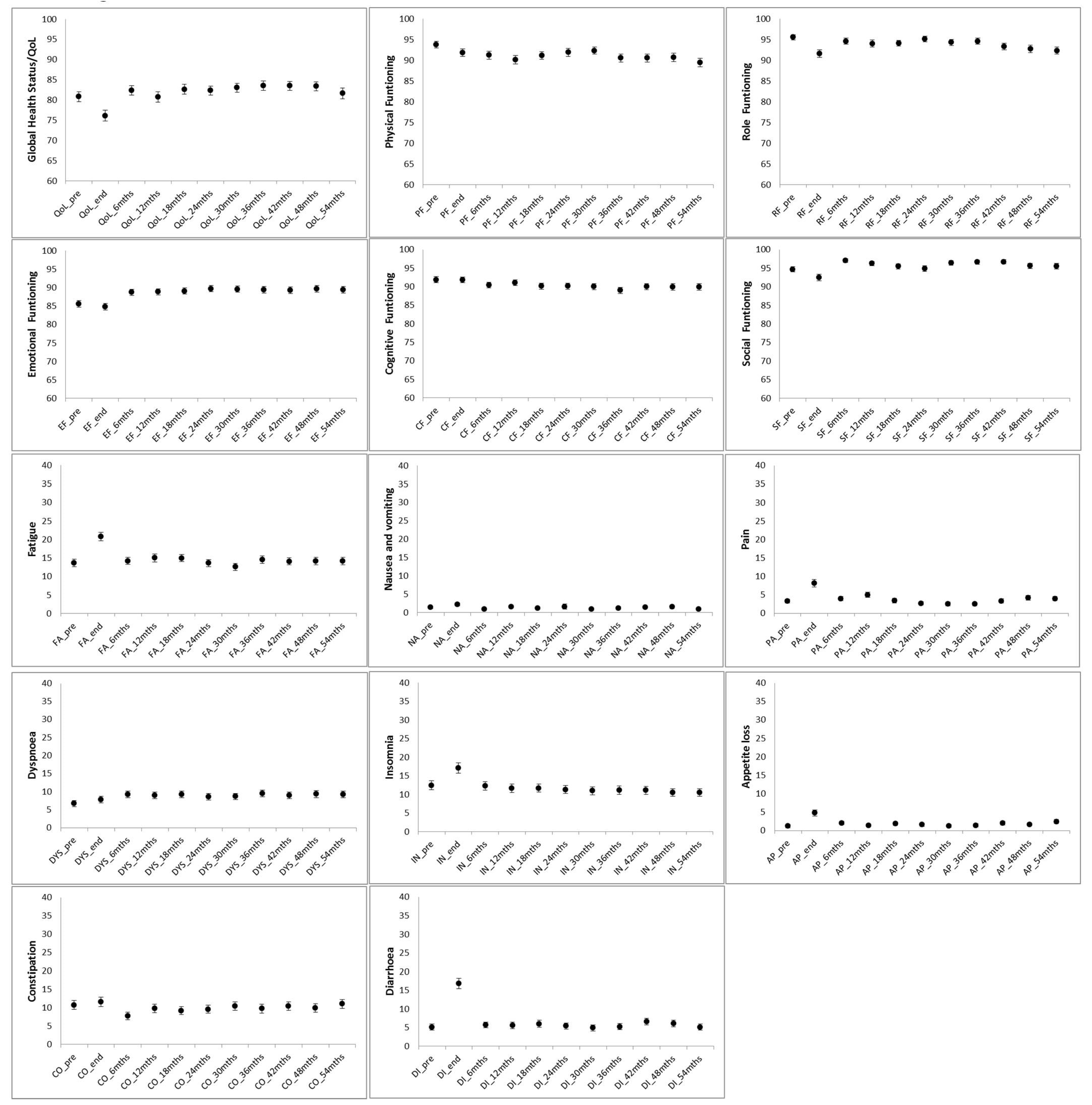
3.1. Longitudinal Changes in QoL Scores
3.2. Predictors of QoL Changes at RT End
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mottet, N.; Bellmunt, J.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Fossati, N.; Gross, T.; Henry, A.M.; Joniau, S.; et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent. Eur. Urol. 2017, 71, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Wilt, T.J.; MacDonald, R.; Rutks, I.; Shamliyan, T.A.; Taylor, B.C.; Kane, R.L. Systematic review: Comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann. Intern. Med. 2008, 148, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Croswell, J.M.; Dana, T.; Bougatsos, C.; Blazina, I.; Fu, R.; Gleitsmann, K.; Koenig, H.C.; Lam, C.; Maltz, A.; et al. Screening for prostate cancer: A review of the evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2011, 155, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.J.; Saskin, R.; Choo, R.; Herschorn, S.; Kodama, R.T.; Satkunasivam, R.; Shah, P.S.; Danjoux, C.; Nam, R.K. Surgery Versus Radiotherapy for Clinically-localized Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2016, 70, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Schröder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Määttänen, L.; Lilja, H.; et al. Screening and prostate cancer mortality: Results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014, 384, 2027–2035. [Google Scholar] [CrossRef]
- Andriole, G.L.; Crawford, E.D.; Grubb, R.L.; Buys, S.S.; Chia, D.; Church, T.R.; Fouad, M.N.; Isaacs, C.; Kvale, P.A.; Reding, D.J.; et al. Prostate cancer screening in the randomized prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: Mortality results after 13 years of follow-up. JNCI J. Natl. Cancer Inst. 2012, 104, 125–132. [Google Scholar] [CrossRef]
- Chen, R.C.; Rumble, R.B.; Loblaw, D.A.; Finelli, A.; Ehdaie, B.; Cooperberg, M.R.; Morgan, S.C.; Tyldesley, S.; Haluschak, J.J.; Tan, W.; et al. Active surveillance for the management of localized prostate cancer (Cancer Care Ontario guideline): American Society of Clinical Oncology clinical practice guideline endorsement. J. Clin. Oncol. 2016, 34, 2182–2190. [Google Scholar] [CrossRef]
- Donovan, J.L.; Hamdy, F.C.; Lane, J.A.; Mason, M.; Metcalfe, C.; Walsh, E.; Blazeby, J.M.; Peters, T.J.; Holding, P.; Bonnington, S.; et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N. Engl. J. Med. 2016, 375, 1425–1437. [Google Scholar] [CrossRef]
- Sureda, A.; Fumadó, L.; Ferrer, M.; Garín, O.; Bonet, X.; Castells, M.; Mir, M.C.; Abascal, J.M.; Vigués, F.; Cecchini, L.; et al. Health-related quality of life in men with prostate cancer undergoing active surveillance versus radical prostatectomy, external-beam radiotherapy, prostate brachytherapy and reference population: A cross-sectional study. Health Qual. Life Outcomes 2019, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Yucel, B.; Akkaş, E.A.; Okur, Y.; Eren, A.A.; Eren, M.F.; Karapınar, H.; Babacan, N.A.; Kılıçkap, S. The impact of radiotherapy on quality of life for cancer patients: A longitudinal study. Support. Care Cancer 2014, 22, 2479–2487. [Google Scholar] [CrossRef] [PubMed]
- Fayers, P.M.; Aaronson, N.K.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A.; on behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 Scoring Manual, 3rd ed.; European Organisation for Research and Treatment of Cancer: Brussels, Belgium, 2001. [Google Scholar]
- Carillo, V.; Cozzarini, C.; Rancati, T.; Avuzzi, B.; Botti, A.; Borca, V.C.; Cattari, G.; Civardi, F.; Degli Esposti, C.; Franco, P.; et al. Relationships between bladder dose–volume/surface histograms and acute urinary toxicity after radiotherapy for prostate cancer. Radiother. Oncol. 2014, 111, 100–105. [Google Scholar] [CrossRef]
- Cozzarini, C.; Rancati, T.; Carillo, V.; Civardi, F.; Garibaldi, E.; Franco, P.; Avuzzi, B.; Degli Esposti, C.; Girelli, G.; Iotti, C.; et al. Multi-variable models predicting specific patient-reported acute urinary symptoms after radiotherapy for prostate cancer: Results of a cohort study. Radiother. Oncol. 2015, 116, 185–191. [Google Scholar] [CrossRef]
- Fowler, J.A. The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncol. 2005, 44, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, C.; Cozzarini, C.; Rancati, T.; Briganti, A.; Cattaneo, G.M.; Mangili, P.; Di Muzio, N.G.; Calandrino, R. Modelling the impact of fractionation on late urinary toxicity after postprostatectomy radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Zelefsky, M.J.; Poon, B.Y.; Eastham, J.; Vickers, A.; Pei, X.; Scardino, P.T. Longitudinal assessment of quality of life after surgery, conformal brachytherapy, and intensity-modulated radiation therapy for prostate cancer. Radiother Oncol. 2016, 118, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Lilleby, W.; Stensvold, A.; Dahl, A.A. Fatigue and other adverse effects in men treated by pelvic radiation and long-term androgen deprivation for locally advanced prostate cancer. Acta Oncol. 2016, 55, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Krahn, M.D.; E Bremner, K.; Tomlinson, G.; Naglie, G. Utility and health-related quality of life in prostate cancer patients 12 months after radical prostatectomy or radiation therapy. Prostate Cancer Prostatic Dis. 2009, 12, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Namiki, S.; Ishidoya, S.; Tochigi, T.; Kawamura, S.; Kuwahara, M.; Terai, A.; Yoshimura, K.; Numata, I.; Satoh, M.; Saito, S.; et al. Health-related quality of life after intensity modulated radiation therapy for localized prostate cancer: Comparison with conventional and conformal radiotherapy. Ultrasound Med. Biol. 2006, 36, 224–230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Monga, U.; Kerrigan, A.J.; Thornby, J.; Monga, T.N.; Zimmermann, K.P. Longitudinal study of quality of life in patients with localized prostate cancer undergoing radiotherapy. J. Rehabil. Res. Dev. 2005, 42, 391–400. [Google Scholar] [CrossRef]
- Caffo, O.; Amichetti, M.; Mussari, S.; Romano, M.; Maluta, S.; Tomio, L.; Galligioni, E. Physical side-effects and quality of life during postoperative radiotherapy for uterine cancer. Prospective evaluation by a diary card. Gynecol. Oncol. 2003, 88, 270–276. [Google Scholar] [CrossRef]
- Langston, B.; Armes, J.; Levy, A.; Tidey, E.; Ream, E. The prevalence and severity of fatigue in men with prostate cancer: A systematic review of the literature. Support. Care Cancer 2013, 21, 1761–1771. [Google Scholar] [CrossRef]
- Sveistrup, J.; Mortensen, O.S.; Bjørner, J.B.; Engelholm, S.A.; Rosenschöld, P.M.A.; Petersen, P.M. Prospective assessment of the quality of life before, during and after image guided intensity modulated radiotherapy for prostate cancer. Radiat. Oncol. 2016, 11, 117. [Google Scholar] [CrossRef]
- Bansal, M.; Mohanti, B.; Shah, N.; Chaudhry, R.; Bahadur, S.; Shukla, N. Radiation related morbidities and their impact on quality of life in head and neck cancer patients receiving radical radiotherapy. Qual. Life Res. 2004, 13, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Lips, I.M.; van Gils, C.H.; van der Heide, U.A.; Kruger, A.E.B.; van Vulpen, M. Health-related quality of life 3 years after high-dose intensity-modulated radiotherapy with gold fiducial marker-based position verification. BJU Int. 2009, 103, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Gogou, P.; Tsilika, E.; Parpa, E.; Kouvaris, I.; Damigos, D.; Balafouta, M.; Mystakidou, V.M.A.K. The Impact of Radiotherapy on Symptoms, Anxiety and QoL in Patients with Cancer. Anticancer. Res. 2015, 35, 1771–1776. [Google Scholar] [PubMed]
- Clark, J.A.; Rieker, P.; Propert, K.J.; A Talcott, J. Changes in quality of life following treatment for early prostate cancer. Urology 1999, 53, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Jereczek-Fossa, B.A.; Santoro, L.; Zerini, D.; Fodor, C.; Vischioni, B.; Dispinzieri, M.; Bossi-Zanetti, I.; Gherardi, F.; Bonora, M.; Caputo, M.; et al. Image guided hypofractionated radiotherapy and quality of life for localized prostate cancer: Prospective longitudinal study in 337 patients. J. Urol. 2013, 189, 2099–2103. [Google Scholar] [CrossRef]
- Sanda, M.G.; Dunn, R.L.; Michalski, J.; Sandler, H.M.; Northouse, L.; Hembroff, L.; Lin, X.; Greenfield, T.K.; Litwin, M.S.; Saigal, C.S.; et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N. Engl. J. Med. 2008, 358, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Marchand, V.; Bourdin, S.; Charbonnel, C.; Rio, E.; Munos, C.; Campion, L.; Bonnaud-Antignac, A.; Lisbona, A.; Mahé, M.-A.; Supiot, S. No impairment of quality of life 18 months after high-dose intensity-modulated radiotherapy for localized prostate cancer: A prospective study. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Alemozaffar, M.; Regan, M.M.; Cooperberg, M.R.; Wei, J.T.; Michalski, J.M.; Sandler, H.M.; Hembroff, L.; Sadetsky, N.; Saigal, C.S.; Litwin, M.S.; et al. Prediction of erectile function following treatment for prostate cancer. JAMA 2011, 306, 1205–1214. [Google Scholar] [CrossRef]
- Lips, I.; Dehnad, H.; Kruger, A.B.; van Moorselaar, J.; van der Heide, U.; Battermann, J.; van Vulpen, M. Health-related quality of life in patients with locally advanced prostate cancer after 76 gy intensity-modulated radiotherapy vs. 70 gy conformal radiotherapy in a prospective and longitudinal study. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 656–661. [Google Scholar] [CrossRef] [PubMed]
| Institution | Patients | N | |
|---|---|---|---|
| Conventional Fractionation | Moderate Hypofractionation | ||
| 1 | 111 | 21 | 132 |
| 2 | - | 76 | 76 |
| 3 | - | 12 | 12 |
| 4 | 13 | 12 | 25 |
| 5 | 9 | 21 | 30 |
| 6 | 27 | 28 | 55 |
| 7 | - | 32 | 32 |
| 8 | - | 29 | 29 |
| Age (y) | 71 (67–74) |
| BMI (kg/m2) | 26 (19-42) |
| PSA (ng/mL) | 6.7 (0.3–277) |
| Gleason score: | |
| <7 | 135 |
| =7 | 186 |
| >7 | 40 |
| n.a. | 30 |
| T stage: | |
| T1 | 217 |
| T2 | 117 |
| T3-4 | 46 |
| TX | 11 |
| Lymph node staging | |
| Nx | 349 |
| N0 | 39 |
| N1 | 3 |
| Diabetes | 63 (16%) |
| Cardiovascular disease | 102 (26%) |
| Hypercholesterolemia | 23 (6%) |
| Urological disease | 23 (6%) |
| Anticoagulants | 27 (7%) |
| Antidepressive | 16 (4%) |
| TURP | 39 (10%) |
| Previous abdominal surgery | 180 (46%) |
| Smoke | 63 (16%) |
| Alcohol | 188 (48%) |
| Hormone therapy before/during RT | 227 (58%) |
| Pelvic irradiation (Yes/No) | 167 (Yes 42.7%)/224 (No 57.3%) |
| Hormone therapy after RT 166 (56%) | 226 (58%) |
| Prescribed dose (Gy) | HYPO (n = 231): 70.2 (54.3–74.2) |
| CONV (n = 160): 76 (74–83.2) | |
| Daily dose (Gy/fr) | HYPO: 2.55 (2.2–3.8) |
| CONV: 2.0 (1.8–2.0) | |
| CTV volume (cc) 51 (34–66) | 52 (11–180) |
| PTV volume (cc) 131 (93–170) | 132 (28–350) |
| Qol Dimension | Significant Trend with Time from ANOVA | p-Value | Time and Size of Significant Variation |
|---|---|---|---|
| Global health/QoL | quadratic | 0.05 | 5-point worsening at RT end with respect to baseline, then recovery |
| Physical functioning | cubic | 0.04 | 4-point worsening at RT end with respect to baseline, then recovery, further 4-point worsening at 5 years |
| Role functioning | cubic | 0.04 | 5-point worsening at RT end with respect to baseline, then recovery, further 5-point worsening at 5 years |
| Social functioning | linear | 0.04 | 2-point increase in the 5-year period |
| Emotional functioning | linear | 0.01 | 3-point increase in the 5-year period |
| Cognitive functioning | no significant trend | ||
| Appetite loss | quadratic | 0.004 | 5-point worsening at RT end with respect to baseline, then recovery |
| Diarrhoea | quadratic + linear decrease | 0.05 | 14-point worsening at RT end with respect to baseline, then recovery, and at 5 years, 1.5 points better with respect to baseline |
| Fatigue | quadratic | 0.03 | 7-point worsening at RT end with respect to baseline, then recovery |
| Insomnia | no significant trend | ||
| Dyspnoea | no significant trend | ||
| Pain | quadratic | 0.03 | 5-point worsening at RT end with respect to baseline, then recovery |
| Constipation | no significant trend | ||
| Nausea | no significant trend |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggio, A.; Rancati, T.; Gatti, M.; Cante, D.; Avuzzi, B.; Bianconi, C.; Badenchini, F.; Farina, B.; Ferrari, P.; Giandini, T.; et al. Quality of Life Longitudinal Evaluation in Prostate Cancer Patients from Radiotherapy Start to 5 Years after IMRT-IGRT. Curr. Oncol. 2024, 31, 839-848. https://doi.org/10.3390/curroncol31020062
Maggio A, Rancati T, Gatti M, Cante D, Avuzzi B, Bianconi C, Badenchini F, Farina B, Ferrari P, Giandini T, et al. Quality of Life Longitudinal Evaluation in Prostate Cancer Patients from Radiotherapy Start to 5 Years after IMRT-IGRT. Current Oncology. 2024; 31(2):839-848. https://doi.org/10.3390/curroncol31020062
Chicago/Turabian StyleMaggio, Angelo, Tiziana Rancati, Marco Gatti, Domenico Cante, Barbara Avuzzi, Cinzia Bianconi, Fabio Badenchini, Bruno Farina, Paolo Ferrari, Tommaso Giandini, and et al. 2024. "Quality of Life Longitudinal Evaluation in Prostate Cancer Patients from Radiotherapy Start to 5 Years after IMRT-IGRT" Current Oncology 31, no. 2: 839-848. https://doi.org/10.3390/curroncol31020062
APA StyleMaggio, A., Rancati, T., Gatti, M., Cante, D., Avuzzi, B., Bianconi, C., Badenchini, F., Farina, B., Ferrari, P., Giandini, T., Girelli, G., Landoni, V., Magli, A., Moretti, E., Petrucci, E., Salmoiraghi, P., Sanguineti, G., Villa, E., Waskiewicz, J. M., ... Cozzarini, C. (2024). Quality of Life Longitudinal Evaluation in Prostate Cancer Patients from Radiotherapy Start to 5 Years after IMRT-IGRT. Current Oncology, 31(2), 839-848. https://doi.org/10.3390/curroncol31020062






