Primary Central Nervous System Lymphoma (PCNSL) Following Thyroid Cancer Surgery: A Case Report of Misdiagnosed Brain Metastasis and Literature Review
Abstract
1. Introduction
2. Case Description
2.1. Basic Patient Information
2.2. Medical History
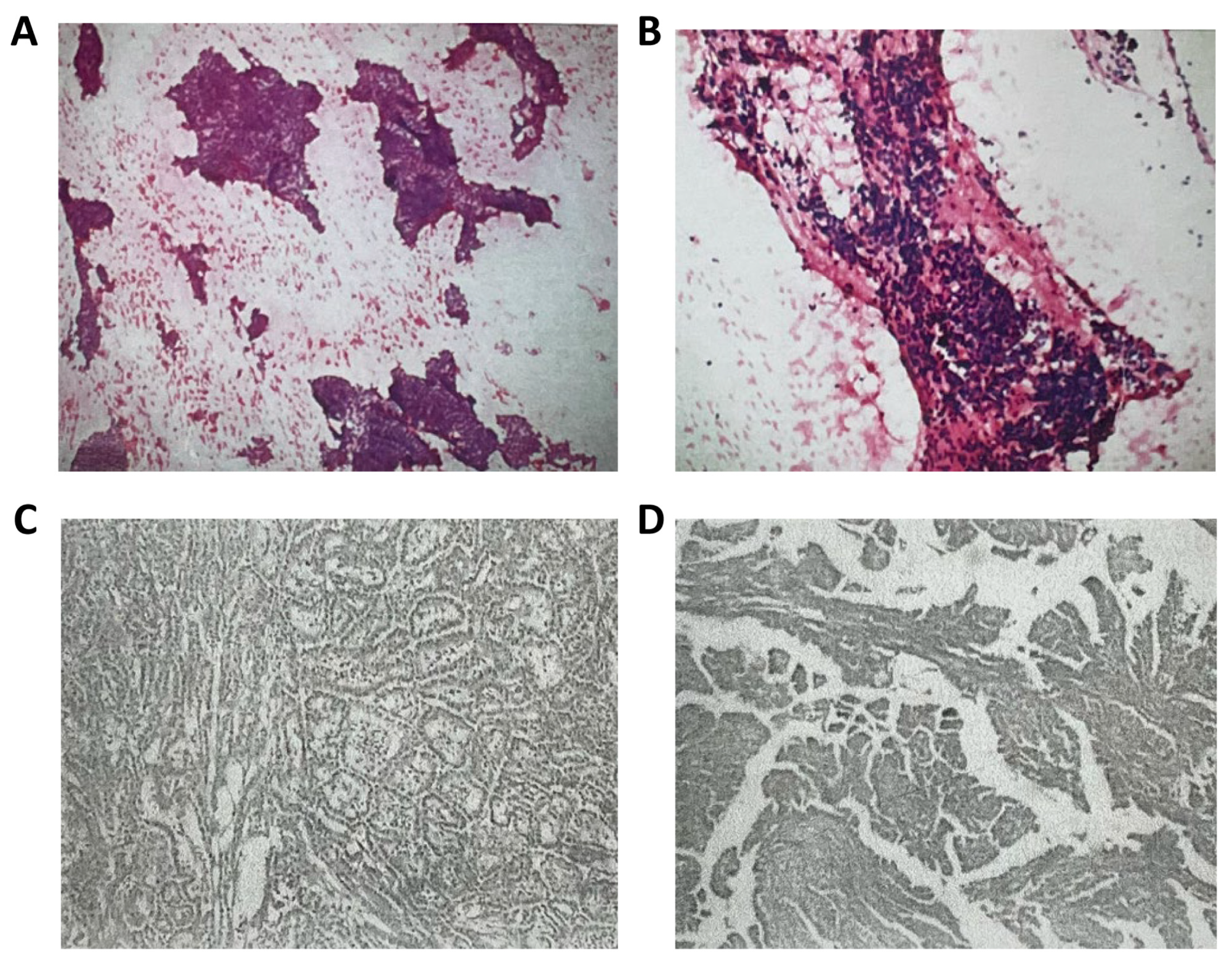
2.3. Diagnostic Process
2.4. Course of Treatment
2.5. Follow-Up and Prognosis
3. Discussion
3.1. Diagnostic Challenges
3.2. Treatment and Prognosis of PCNSL
3.3. Analysis of Causes
3.4. Steps for Clinicians Managing
3.5. Research Innovations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomes-Lima, C.J.; Wu, D.; Rao, S.N.; Punukollu, S.; Hritani, R.; Zeymo, A.; Deeb, H.; Mete, M.; Aulisi, E.F.; Van Nostrand, D.; et al. Brain Metastases from Differentiated Thyroid Carcinoma: Prevalence, Current Therapies, and Outcomes. J. Endocr. Soc. 2018, 3, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, J.W.; Keum, Y.S.; Lee, I.J. The Largest Known Survival Analysis of Patients with Brain Metastasis from Thyroid Cancer Based on Prognostic Groups. PLoS ONE 2016, 11, e0154739. [Google Scholar] [CrossRef]
- Saito, F.; Uruno, T.; Shibuya, H.; Kitagawa, W.; Nagahama, M.; Sugino, K.; Ito, K. Prognosis After Brain Metastasis from Differentiated Thyroid Carcinoma. World J. Surg. 2016, 40, 1. [Google Scholar] [CrossRef] [PubMed]
- Chiu, A.C.; Delpassand, E.S.; Sherman, S.I. Prognosis and Treatment of Brain Metastases in Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 1997, 82, 3637–3642. [Google Scholar] [CrossRef] [PubMed]
- Wolff, L.; Steindl, A.; Popov, P.; Dieckmann, K.; Gatterbauer, B.; Widhalm, G.; Berghoff, A.S.; Preusser, M.; Raderer, M.; Kiesewetter, B. Clinical characteristics, treatment, and long-term outcome of patients with brain metastases from thyroid cancer. Clin. Exp. Metastasis 2023, 40, 217–226. [Google Scholar] [CrossRef]
- Prinzi, A.; van Velsen, E.F.S.; Belfiore, A.; Frasca, F.; Malandrino, P. Brain Metastasis in Differentiated Thyroid Cancer: Clinical Presentation, Diagnosis and Management. Thyroid 2024, 34, 1194–1204. [Google Scholar] [CrossRef]
- Meng, J.; Yan, Z.; Cheng, W.; Wang, Z.; Chen, Z.; You, W.; Wang, Z. Long-term survival of patients with intracranial metastases from thyroid cancer presenting with seizures: A case report and literature review. Transl. Cancer Res. 2023, 12, 439–446. [Google Scholar] [CrossRef]
- Kim, S.S.; Kim, S.-M.; Park, M.; Suh, S.H.; Ahn, S.J. Clinico-radiological features of brain metastases from thyroid cancer. Medicine 2021, 100, e28069. [Google Scholar] [CrossRef]
- Henriques de Figueiredo, B.; Godbert, Y.; Soubeyran, I.; Carrat, X.; Lagarde, P.; Cazeau, A.-L.; Italiano, A.; Sargos, P.; Kantor, G.; Loiseau, H.; et al. Brain Metastases from Thyroid Carcinoma: A Retrospective Study of 21 Patients. Thyroid 2014, 24, 270–276. [Google Scholar] [CrossRef]
- Villano, J.L.; Koshy, M.; Shaikh, H.; Dolecek, T.A.; McCarthy, B.J. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br. J. Cancer 2011, 105, 1414–1418. [Google Scholar] [CrossRef]
- Shiels, M.S.; Pfeiffer, R.M.; Besson, C.; Clarke, C.A.; Morton, L.M.; Nogueira, L.; Pawlish, K.; Yanik, E.L.; Suneja, G.; Engels, E.A. Trends in primary central nervous system lymphoma incidence and survival in the U.S. Br. J. Haematol. 2016, 174, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Schaff, L.R.; Grommes, C. Primary central nervous system lymphoma. Blood 2022, 140, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Rozenblum, L.; Houillier, C.; Soussain, C.; Bertaux, M.; Choquet, S.; Galanaud, D.; Hoang-Xuan, K.; Kas, A. Role of Positron Emission Tomography in Primary Central Nervous System Lymphoma. Cancers 2022, 14, 4071. [Google Scholar] [CrossRef]
- Gupta, T.; Manjali, J.J.; Kannan, S.; Purandare, N.; Rangarajan, V. Diagnostic Performance of Pretreatment 18F-Fluorodeoxyglucose Positron Emission Tomography with or Without Computed Tomography in Patients with Primary Central Nervous System Lymphoma: Updated Systematic Review and Diagnostic Test Accuracy Meta-analyses. Clin. Lymphoma Myeloma Leuk. 2021, 21, 497–507. [Google Scholar] [CrossRef]
- Jain, T.K.; Karunanithi, S.; Sharma, P.; Vijay, M.K.; Ballal, S.; Bal, C. Asymptomatic Solitary Cerebral Metastasis from Papillary Carcinoma Thyroid: 131I SPECT/CT for Accurate Staging. Clin. Nucl. Med. 2014, 39, 977–979. [Google Scholar] [CrossRef]
- Tagle, P.; Villanueva, P.; Torrealba, G.; Huete, I. Intracranial metastasis or meningioma?: An uncommon clinical diagnostic dilemma. Surg. Neurol. 2002, 58, 241–245. [Google Scholar] [CrossRef]
- Brendel, A.J.; Lambert, B.; Guyot, M.; Jeandot, R.; Dubourg, H.; Roger, P.; Wynchauk, S.; Manciet, G.; Lefort, G. Low levels of serum thyroglobulin after withdrawal of thyroid suppression therapy in the follow up of differentiated thyroid carcinoma. Eur. J. Nucl. Med. 1990, 16, 35–38. [Google Scholar] [CrossRef]
- Camargo, R.; Limbert, E.; Gillam, M.; Henriques, M.M.; Fernandes, C.; Catarino, A.L.; Soares, J.; Alves, V.A.F.; Kopp, P.; Medeiros-Neto, G. Aggressive Metastatic Follicular Thyroid Carcinoma with Anaplastic Transformation Arising from a Long-Standing Goiter in a Patient with Pendred’s Syndrome. Thyroid 2001, 11, 981–988. [Google Scholar] [CrossRef]
- Bataille, B.; Delwail, V.; Menet, E.; Vandermarcq, P.; Ingrand, P.; Wager, M.; Guy, G.; Lapierre, F. Primary intracerebral malignant lymphoma: Report of 248 cases. J. Neurosurg. 2000, 92, 261–266. [Google Scholar] [CrossRef]
- Houillier, C.; Soussain, C.; Ghesquières, H.; Soubeyran, P.; Chinot, O.; Taillandier, L.; Lamy, T.; Choquet, S.; Ahle, G.; Damaj, G.; et al. Management and outcome of primary CNS lymphoma in the modern era: An LOC network study. Neurology 2020, 94, e1027–e1039. [Google Scholar] [CrossRef] [PubMed]
- Podda, M.G.; Terenziani, M.; Gandola, L.; Collini, P.; Pizzi, N.; Marchianò, A.; Morosi, C.; Luksch, R.; Ferrari, A.; Casanova, M.; et al. Thyroid carcinoma after treatment for malignancies in childhood and adolescence: From diagnosis through follow-up. Med. Oncol. 2014, 31, 121. [Google Scholar] [CrossRef] [PubMed]
- Keegan, T.H.M.; Bleyer, A.; Rosenberg, A.S.; Li, Q.; Goldfarb, M. Second Primary Malignant Neoplasms and Survival in Adolescent and Young Adult Cancer Survivors. JAMA Oncol. 2017, 3, 1554–1557. [Google Scholar] [CrossRef] [PubMed]
- Deangelis, L.M. Primary central nervous system lymphoma as a secondary malignancy. Cancer 1991, 67, 1431–1435. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.; Choi, J.-W.; Kim, Y.-S. The association between papillary thyroid carcinoma and histologically proven Hashimoto’s thyroiditis: A meta-analysis. Eur. J. Endocrinol. 2013, 168, 343–349. [Google Scholar] [CrossRef]
- Singh, B.; Shaha, A.R.; Trivedi, H.; Carew, J.F.; Poluri, A.; Shah, J.P. Coexistent Hashimoto’s thyroiditis with papillary thyroid carcinoma: Impact on presentation, management, and outcome. Surgery 1999, 126, 1070–1077. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Mahale, P.; Herr, M.M.; Engels, E.A.; Pfeiffer, R.M.; Shiels, M.S. Autoimmune conditions and primary central nervous system lymphoma risk among older adults. Br. J. Haematol. 2020, 188, 516–521. [Google Scholar] [CrossRef]
- Iskra, I.; Tomaš, M.I.; Crnčić, T.B.; Kukić, E.; Hadžisejdić, I.; Avirović, M.; Girotto, N. Two lymphoma histotypes and papillary thyroid carcinoma coexisting on Hashimoto ground: A case report and review of the literature. Diagn. Pathol. 2024, 19, 52. [Google Scholar] [CrossRef]
- Speiser, D.E.; Ho, P.-C.; Verdeil, G. Regulatory circuits of T cell function in cancer. Nat. Rev. Immunol. 2016, 16, 599–611. [Google Scholar] [CrossRef]
| Time | Diagnosis and Treatment |
|---|---|
| December 2019 | Thyroid isthmus and by lobe resection |
| Papillary thyroid carcinoma (T1N0M0) with BRAF V600E mutation | |
| April 2024 | Unfavorable movement of left limb, dizziness |
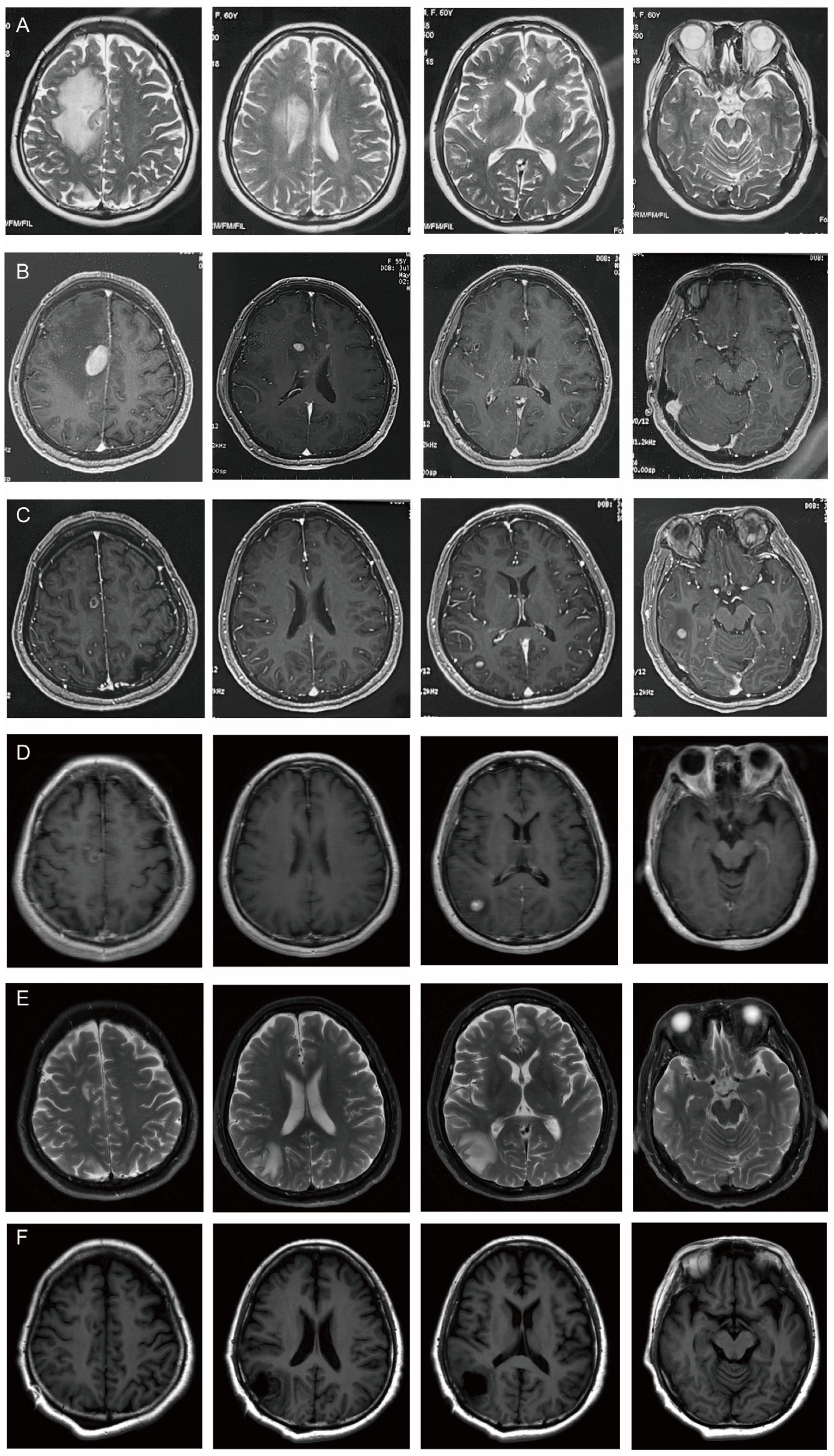
| 18 April–6 May 2024 | CT (4.18): small lymph nodes in both necks, patchy hypodense shadow in the right side of the brain after right lobectomy of the thyroid gland |
| MRI (4.18): nodular abnormal signals in the right frontal lobe of the brain next to the anterior horn of the lateral ventricle. It was considered to be metastatic tumor accompanied by peripheral odema. | |
| Ultrasound of the thyroid gland (4.18): heterogeneous echogenicity of the left lobe of the thyroid gland—Hashimoto’s thyroiditis perhaps | |
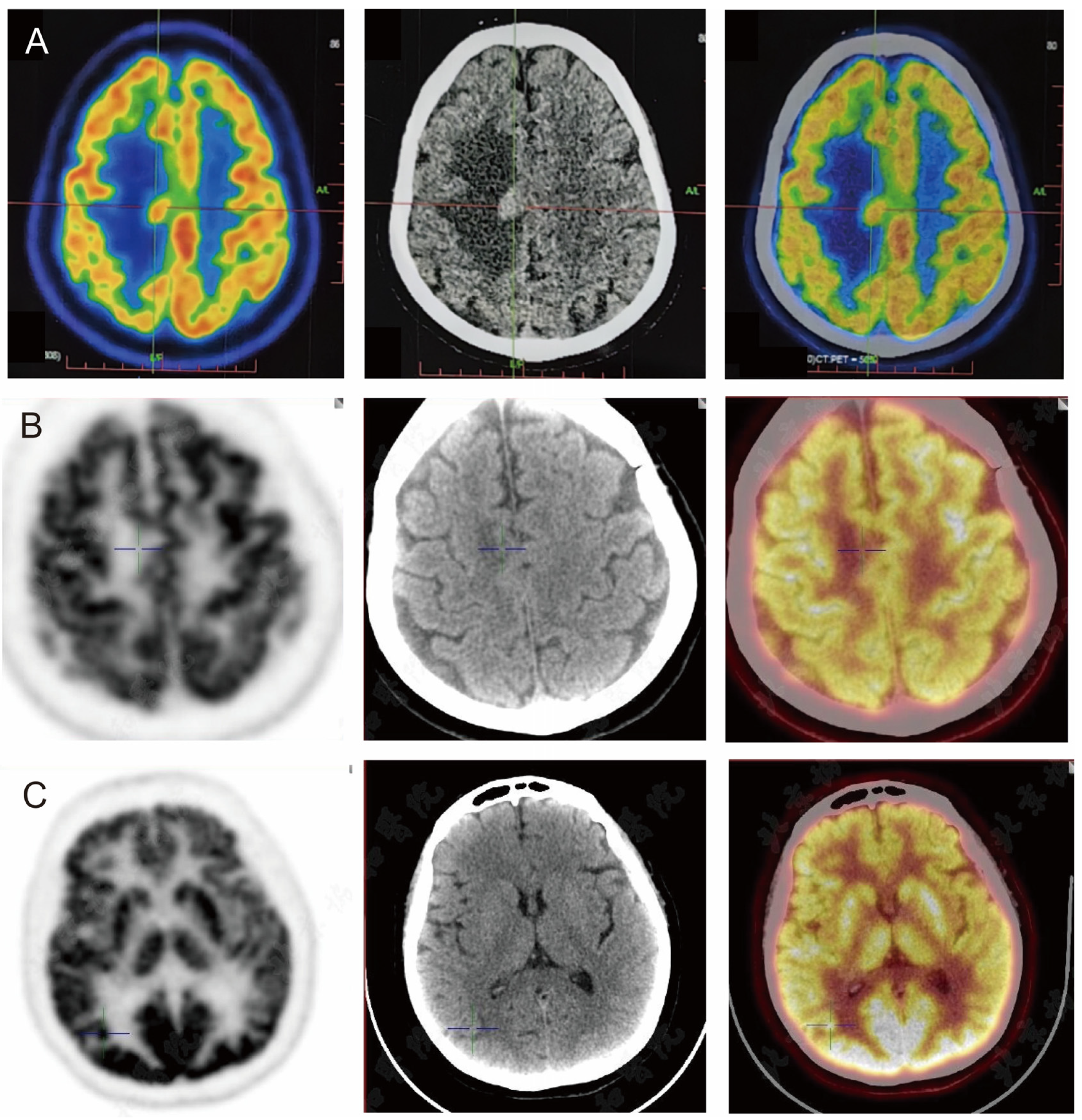
| PET/CT (4.22): hypermetabolic foci with peripheral odema in the right centrum semiovale; uneven metabolism in left lobe glands, multiple slightly hypermetabolic lymph nodes in neck and mediastinum bilaterally | |
| 6 May 2024 | MRI (5.6): multiple intracranial lesions in the right frontal lobe and top of the lateral ventricles. |
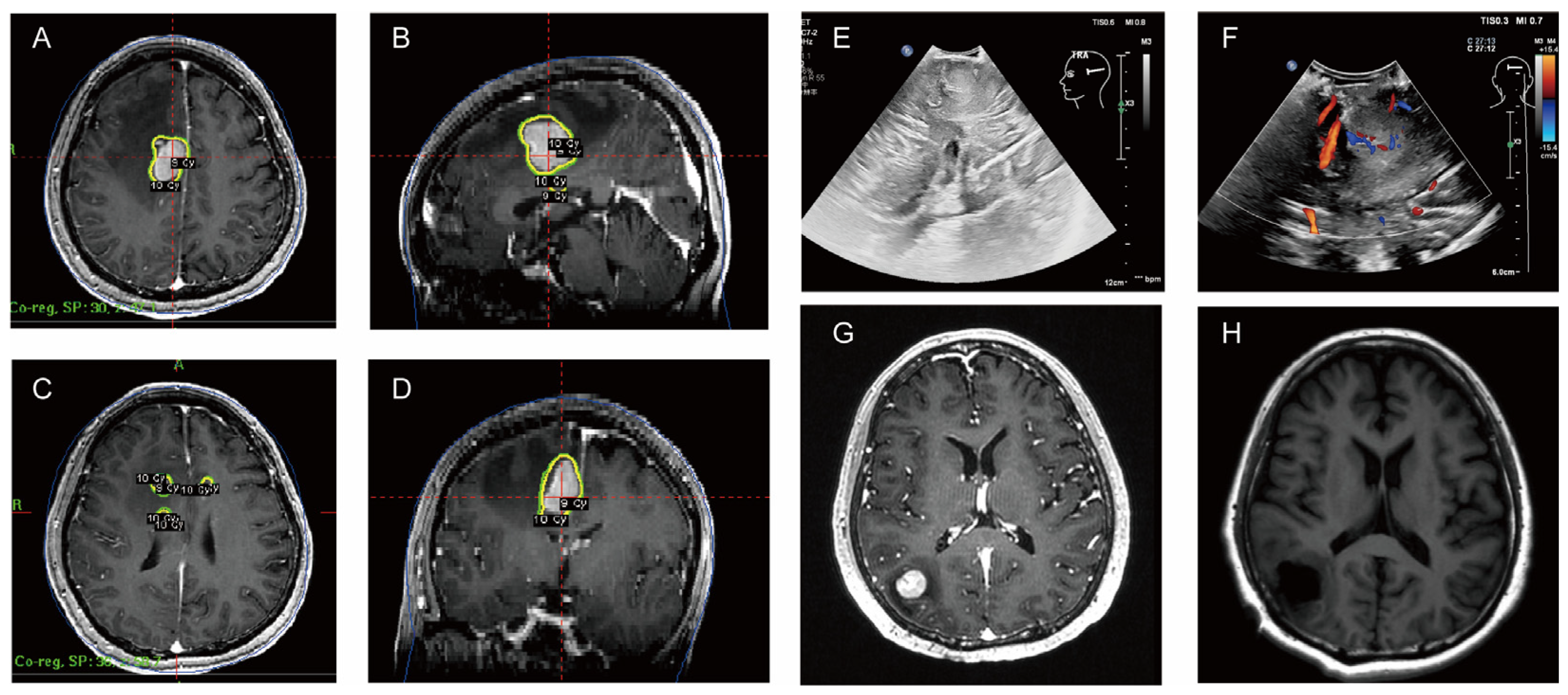
| gamma knife radiation therapy | |
| 24 June–9 July 2024 | MRI (6.24): The lesions in the right frontal lobe and the top of the lateral ventricle reduced, but new lesions appeared in the right temporal lobe and occipital lobe. |
| MRI (7.9): Right temporal lobe lesions were smaller, but right occipital lobe lesions were larger. | |
| PET/CT (7.8): A metabolically increased nodule in the right occipital lobe (SUVmax 17.4); a nodule in the right centrum semiovale and the right subject lobe, with metabolism lower than that of the cortex | |
| July 2024 | Surgical resection |
| Pathology: considered aggressive B-cell non-Hodgkin’s lymphoma | |
| August 2024 | Referred to the haematology department, and after further refinement of investigations, diagnosis of primary central diffuse large B-cell lymphoma (GCB subtype, DE+, DH to be investigated, IELSG score 1, low risk) |
| A C1-POR regimen |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liang, T.; Xing, H.; Wang, Y.; Wang, K.; Ma, W. Primary Central Nervous System Lymphoma (PCNSL) Following Thyroid Cancer Surgery: A Case Report of Misdiagnosed Brain Metastasis and Literature Review. Curr. Oncol. 2024, 31, 7555-7565. https://doi.org/10.3390/curroncol31120556
Li Y, Liang T, Xing H, Wang Y, Wang K, Ma W. Primary Central Nervous System Lymphoma (PCNSL) Following Thyroid Cancer Surgery: A Case Report of Misdiagnosed Brain Metastasis and Literature Review. Current Oncology. 2024; 31(12):7555-7565. https://doi.org/10.3390/curroncol31120556
Chicago/Turabian StyleLi, Yilin, Tingyu Liang, Hao Xing, Yu Wang, Kuanyu Wang, and Wenbin Ma. 2024. "Primary Central Nervous System Lymphoma (PCNSL) Following Thyroid Cancer Surgery: A Case Report of Misdiagnosed Brain Metastasis and Literature Review" Current Oncology 31, no. 12: 7555-7565. https://doi.org/10.3390/curroncol31120556
APA StyleLi, Y., Liang, T., Xing, H., Wang, Y., Wang, K., & Ma, W. (2024). Primary Central Nervous System Lymphoma (PCNSL) Following Thyroid Cancer Surgery: A Case Report of Misdiagnosed Brain Metastasis and Literature Review. Current Oncology, 31(12), 7555-7565. https://doi.org/10.3390/curroncol31120556





