Accuracy and Outcomes of Sentinel Lymph Node Biopsy in Male with Breast Cancer: A Narrative Review and Expert Opinion
Abstract
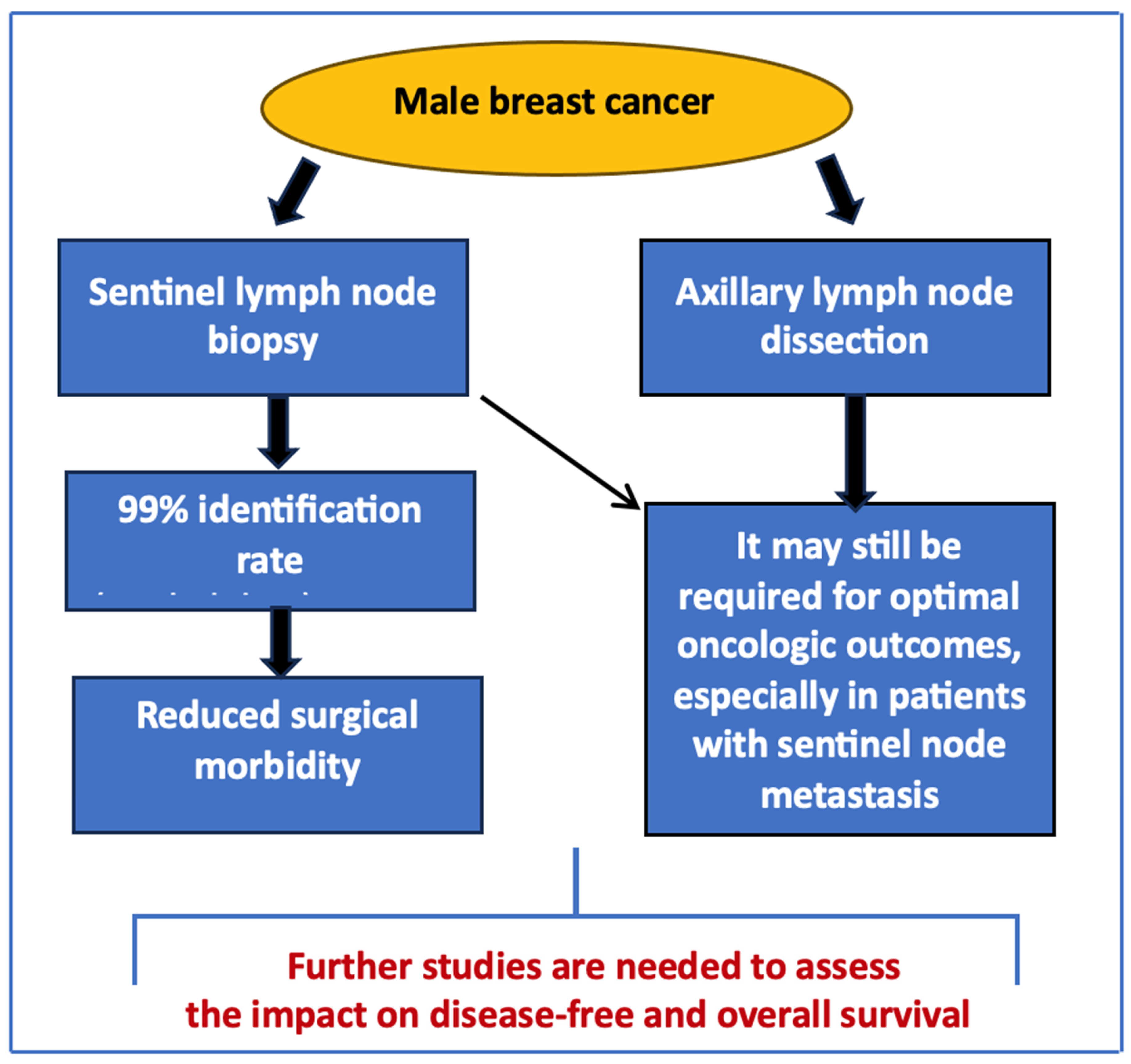
1. Introduction
2. Diagnostic Accuracy of SNB in Men
3. Impact on Surgical and Oncologic Outcomes
4. Expert Opinion and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gucalp, A.; Traina, T.A.; Eisner, J.R.; Parker, J.S.; Selitsky, S.R.; Park, B.H.; Elias, A.D.; Baskin-Bey, E.S.; Cardoso, F. Male breast cancer: A disease distinct from female breast cancer. Breast Cancer Res. Treat. 2019, 173, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Arzanova, E.; Mayrovitz, H.N. Male Breast Cancer: Treatment Trends, Reported Outcomes, and Suggested Recommendations. Cureus 2021, 13, e18337. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.V.; Gupta, S.; Elyash, A.; Teplinsky, E. Male Breast Cancer: A Review on Diagnosis, Treatment, and Survivorship. Curr. Oncol. Rep. 2024, 26, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Hassett, M.J.; Somerfield, M.R.; Giordano, S.H. Management of Male Breast Cancer: ASCO Guideline Summary. JCO Oncol. Pr. 2020, 16, e839–e843. [Google Scholar] [CrossRef]
- Parpex, G.; Ottaviani, M.; Lorphelin, H.; Mezzadri, M.; Marchand, E.; Cahen-Doidy, L.; Benifla, J.L.; Huchon, C.; Mimoun, C. Accuracy of sentinel lymph node biopsy in male breast cancer: Systematic review and meta-analysis. Breast 2024, 75, 103703. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, V.M.; Degnim, A.C.; Sabel, M.S.; Diehl, K.M.; Newman, L.A.; Chang, A.E. Efficacy of sentinel lymph node biopsy in male breast cancer. J. Surg. Oncol. 2004, 86, 74–77. [Google Scholar] [CrossRef]
- Gentilini, O.; Chagas, E.; Zurrida, S.; Intra, M.; De Cicco, C.; Gatti, G.; Silva, L.; Renne, G.; Cassano, E.; Veronesi, U. Sentinel Lymph Node Biopsy in Male Patients with Early Breast Cancer. The Oncologist 2007, 12, 512–515. [Google Scholar] [CrossRef]
- Plichta, J.K.; Ren, Y.; Marks, C.E.; Thomas, S.M.; Greenup, R.A.; Rosenberger, L.H.; Fayanju, O.M.; McDuff, S.G.R.; Hwang, E.S.; Force, J. Surgery for Men with Breast Cancer: Do the Same Data Still Apply? Ann. Surg. Oncol. 2020, 27, 4720–4729. [Google Scholar] [CrossRef]
- Mansel, R.E.; Fallowfield, L.; Kissin, M.; Goyal, A.; Newcombe, R.G.; Dixon, J.M.; Yiangou, C.; Horgan, K.; Bundred, N.; Monypenny, I.; et al. Randomized Multicenter Trial of Sentinel Node Biopsy Versus Standard Axillary Treatment in Operable Breast Cancer: The ALMANAC Trial. JNCI J. Natl. Cancer Inst. 2006, 98, 599–609. [Google Scholar] [CrossRef]
- Reimer, T. Omission of axillary sentinel lymph node biopsy in early invasive breast cancer. Breast 2023, 67, 124–128. [Google Scholar] [CrossRef]
- Giammarile, F.; Vidal-Sicart, S.; Paez, D.; Pellet, O.; Enrique, E.-L.; Mikhail-Lette, M.; Morozova, O.; Camila, N.M.M.; Ivonne, R.S.D.; Bolton, R.C.D.; et al. Sentinel Lymph Node Methods in Breast Cancer. Semin. Nucl. Med. 2022, 52, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Popa-Nimigean, V.; Ahmed, M. Current state of surgical management for male breast cancer. Transl. Cancer Res. 2019, 8, S457–S462. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.P.; Huang, T.-W.; Tam, K.-W. Treatment of male breast cancer: Meta-analysis of real-world evidence. Br. J. Surg. 2021, 108, 1034–1042. [Google Scholar] [CrossRef]
- Leone, J.P.; Leone, J.; Zwenger, A.O.; Iturbe, J.; Leone, B.A.; Vallejo, C.T. Locoregional treatment and overall survival of men with T1a,b,cN0M0 breast cancer: A population-based study. Eur. J. Cancer 2017, 71, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; de Geus, S.W.L.; Shewmaker, G.; Romatoski, K.S.; Drake, F.T.; Ko, N.Y.; Merrill, A.L.; Hirsch, A.E.; Tseng, J.F.; Sachs, T.E.; et al. Axillary Lymph Node Dissection is Associated with Improved Survival Among Men with Invasive Breast Cancer and Sentinel Node Metastasis. Ann. Surg. Oncol. 2023, 30, 5610–5618. [Google Scholar] [CrossRef]
- Shang, Q.; Feng, K.; Wei, Y.; Wang, K.; Yang, C.; Zhao, S.; Liu, J.; Meng, X.; Li, Y.; Du, C.; et al. Evaluation of Male Breast Cancer and the Application of Sentinel Lymph Node Biopsy: A Multicenter Retrospective Study. The Oncologist 2023, 28, e1170–e1178. [Google Scholar] [CrossRef]
- Carter, M.; Reyna, C.; Shaughnessy, E.; Hanseman, D.; Meier, T.; Barrord, M.; Lewis, J.D. Trends and Outcomes Associated with Axillary Management of Males with Clinical N0 Breast Cancer–An NCDB Analysis. J. Surg. Res. 2021, 268, 97–104. [Google Scholar] [CrossRef]
- Chen, W.; Wang, H.; Yang, B.; Zhang, M.; Chen, J. Nomogram for predicting preoperative axillary lymph node status in male breast carcinoma: A SEER population-based study. Transl. Cancer Res. 2023, 12, 793–803. [Google Scholar] [CrossRef]
- Pratt, C.G.; Whitrock, J.N.; Carter, M.M.; Long, S.-A.; Lewis, J.D.; Heelan, A.A. Implementation of Choosing Wisely® Recommendations for Lymph Node Surgery in Male Breast Cancer. Ann. Surg. Oncol. 2024, 31, 7641–7653. [Google Scholar] [CrossRef]
- Willems, B.; Bracke, P. Participants, Physicians or Programmes: Participants’ Educational Level and Initiative in Cancer Screening. Health Policy 2018, 122, 422–430. [Google Scholar] [CrossRef]
- D’angelo, A.; Portaluri, A.; Caprini, F.; Sofia, C.; Ferrara, F.; Condorelli, E.; Iaccarino, L.; Catanzariti, F.; Mancino, M.; Trombadori, C.M.L.; et al. Male Breast: A Review of the Literature and Current State of the Art of Diagnostic Imaging Work-Up. Diagnostics 2023, 13, 3620. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.G.; O’flaherty, C.; Cleere, E.F.; Nohilly, A.; Phelan, J.; Ronane, E.; Lowery, A.J.; Kerin, M.J. Sentinel lymph node biopsy in patients with ductal carcinoma in situ: Systematic review and meta-analysis. BJS Open 2022, 6, zrac022. [Google Scholar] [CrossRef] [PubMed]
- Andersson, Y.; Bergkvist, L.; Frisell, J.; de Boniface, J. Omitting completion axillary lymph node dissection after detection of sentinel node micrometastases in breast cancer: First results from the prospective SENOMIC trial. Br. J. Surg. 2021, 108, 1105–1111. [Google Scholar] [CrossRef]
- Galimberti, V.; Cole, B.F.; Zurrida, S.; Viale, G.; Luini, A.; Veronesi, P.; Baratella, P.; Chifu, C.; Sargenti, M.; Intra, M.; et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): A phase 3 randomised controlled trial. Lancet Oncol. 2013, 14, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, V.; Cole, B.F.; Viale, G.; Veronesi, P.; Vicini, E.; Intra, M.; Mazzarol, G.; Massarut, S.; Zgajnar, J.; Taffurelli, M.; et al. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol. 2018, 19, 1385–1393. [Google Scholar] [CrossRef]
- Solá, M.; Alberro, J.A.; Fraile, M.; Santesteban, P.; Ramos, M.; Fabregas, R.; Moral, A.; Ballester, B.; Vidal, S. Complete Axillary Lymph Node Dissection Versus Clinical Follow-up in Breast Cancer Patients with Sentinel Node Micrometastasis: Final Results from the Multicenter Clinical Trial AATRM 048/13/2000. Ann. Surg. Oncol. 2012, 20, 120–127. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of Axillary Dissection vs. No Axillary Dissection on 10-Year Overall Survival Among Women with Invasive Breast Cancer and Sentinel Node Metastasis. JAMA 2017, 318, 918–926. [Google Scholar] [CrossRef]
- Huang, T.-W.; Su, C.-M.; Tam, K.-W. Axillary Management in Women with Early Breast Cancer and Limited Sentinel Node Metastasis: A Systematic Review and Metaanalysis of Real-World Evidence in the Post-ACOSOG Z0011 Era. Ann. Surg. Oncol. 2020, 28, 920–929. [Google Scholar] [CrossRef]
- Tinterri, C.; Gentile, D.; Gatzemeier, W.; Sagona, A.; Barbieri, E.; Testori, A.; Errico, V.; Bottini, A.; Marrazzo, E.; Dani, C.; et al. Preservation of Axillary Lymph Nodes Compared with Complete Dissection in T1–2 Breast Cancer Patients Presenting One or Two Metastatic Sentinel Lymph Nodes: The SINODAR-ONE Multicenter Randomized Clinical Trial. Ann. Surg. Oncol. 2022, 29, 5732–5744. [Google Scholar] [CrossRef]
- Tee, S.R.; Devane, L.A.; Evoy, D.; Rothwell, J.; Geraghty, J.; Prichard, R.S.; McDermott, E.W. Meta-analysis of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with initial biopsy-proven node-positive breast cancer. Br. J. Surg. 2018, 105, 1541–1552. [Google Scholar] [CrossRef]
- Lu, W.; Chen, X.; Ho, D.C.W.; Wang, H. Analysis of the construction waste management performance in Hong Kong: The public and private sectors compared using big data. J. Clean Prod. 2016, 112, 521–531. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Q.; Wang, L.; Zhao, X.; Feng, G. Digitalization and third-party logistics performance: Exploring the roles of customer collaboration and government. Int. J. Phys. Distrib. Logist. Manag. 2023, 53, 467–488. [Google Scholar] [CrossRef]
- Zheng, G.; Leone, J.P. Male Breast Cancer: An Updated Review of Epidemiology, Clinicopathology, and Treatment. J. Oncol. 2022, 2022, 1734049. [Google Scholar] [CrossRef] [PubMed]
| Study | Design | Population | Key Results | Key Findings |
|---|---|---|---|---|
| Shang et al. (2023) [16] | Multicenter retrospective study | 92 men with early-stage MBC and clinically negative nodes |
| SLNB showed non-inferiority to ALND for DFS and OS in men with clinically negative axillary nodes, with fewer complications in the SLNB group |
| Chung et al. (2023) [15] | Retrospective using the NCDB | 1203 men with T1–T2 breast cancer and 1–2 positive sentinel nodes |
| ALND was associated with superior survival compared to SLNB alone in men with sentinel node metastasis, suggesting SLNB alone may not be sufficient |
| Leone et al. (2017) [14] | Population-based using the SEER database | 1263 men with early-stage (T1abcN0M0) breast cancer |
| SLNB may be adequate for node-negative patients, reducing unnecessary morbidity without compromising survival outcomes |
| Carter et al. (2021) [17] | Retrospective using NCDB | 2646 men with clinically node-negative (cN0) breast cancer |
| SLNB use has increased, but patients in the later cohort (2012–2016) had worse survival, possibly due to more advanced disease and reduced chemotherapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cipolla, C.; Gebbia, V.; D’Agati, E.; Greco, M.; Mesi, C.; Scandurra, G.; Sambataro, D.; Valerio, M.R. Accuracy and Outcomes of Sentinel Lymph Node Biopsy in Male with Breast Cancer: A Narrative Review and Expert Opinion. Curr. Oncol. 2024, 31, 7566-7574. https://doi.org/10.3390/curroncol31120557
Cipolla C, Gebbia V, D’Agati E, Greco M, Mesi C, Scandurra G, Sambataro D, Valerio MR. Accuracy and Outcomes of Sentinel Lymph Node Biopsy in Male with Breast Cancer: A Narrative Review and Expert Opinion. Current Oncology. 2024; 31(12):7566-7574. https://doi.org/10.3390/curroncol31120557
Chicago/Turabian StyleCipolla, Calogero, Vittorio Gebbia, Eleonora D’Agati, Martina Greco, Chiara Mesi, Giuseppa Scandurra, Daniela Sambataro, and Maria Rosaria Valerio. 2024. "Accuracy and Outcomes of Sentinel Lymph Node Biopsy in Male with Breast Cancer: A Narrative Review and Expert Opinion" Current Oncology 31, no. 12: 7566-7574. https://doi.org/10.3390/curroncol31120557
APA StyleCipolla, C., Gebbia, V., D’Agati, E., Greco, M., Mesi, C., Scandurra, G., Sambataro, D., & Valerio, M. R. (2024). Accuracy and Outcomes of Sentinel Lymph Node Biopsy in Male with Breast Cancer: A Narrative Review and Expert Opinion. Current Oncology, 31(12), 7566-7574. https://doi.org/10.3390/curroncol31120557






