Personalized Immunotherapy Achieves Complete Response in Metastatic Adenoid Cystic Carcinoma Despite Lack of Conventional Biomarkers
Abstract
1. Introduction
2. Case Presentation
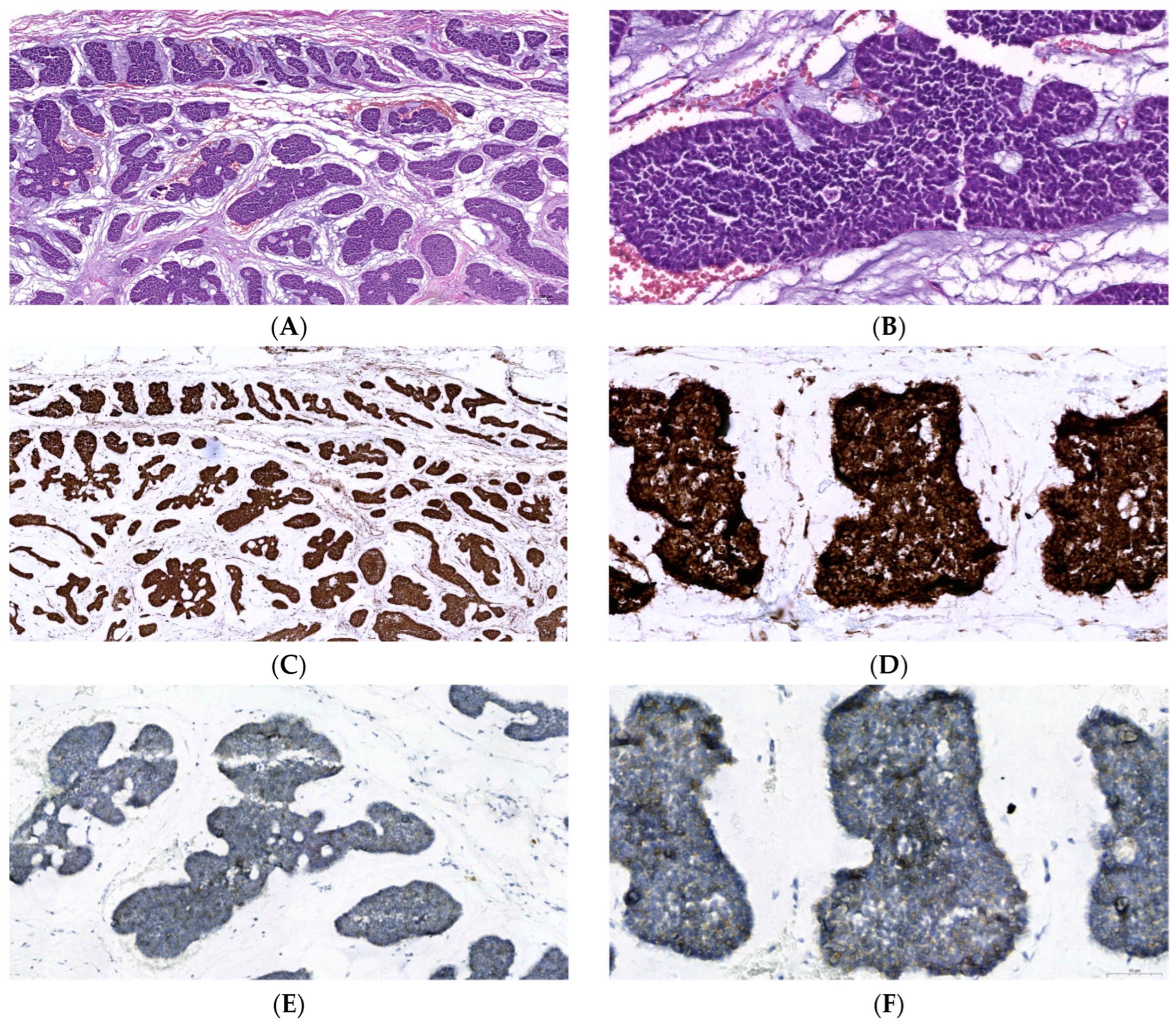
2.1. Diagnosis and Pathology
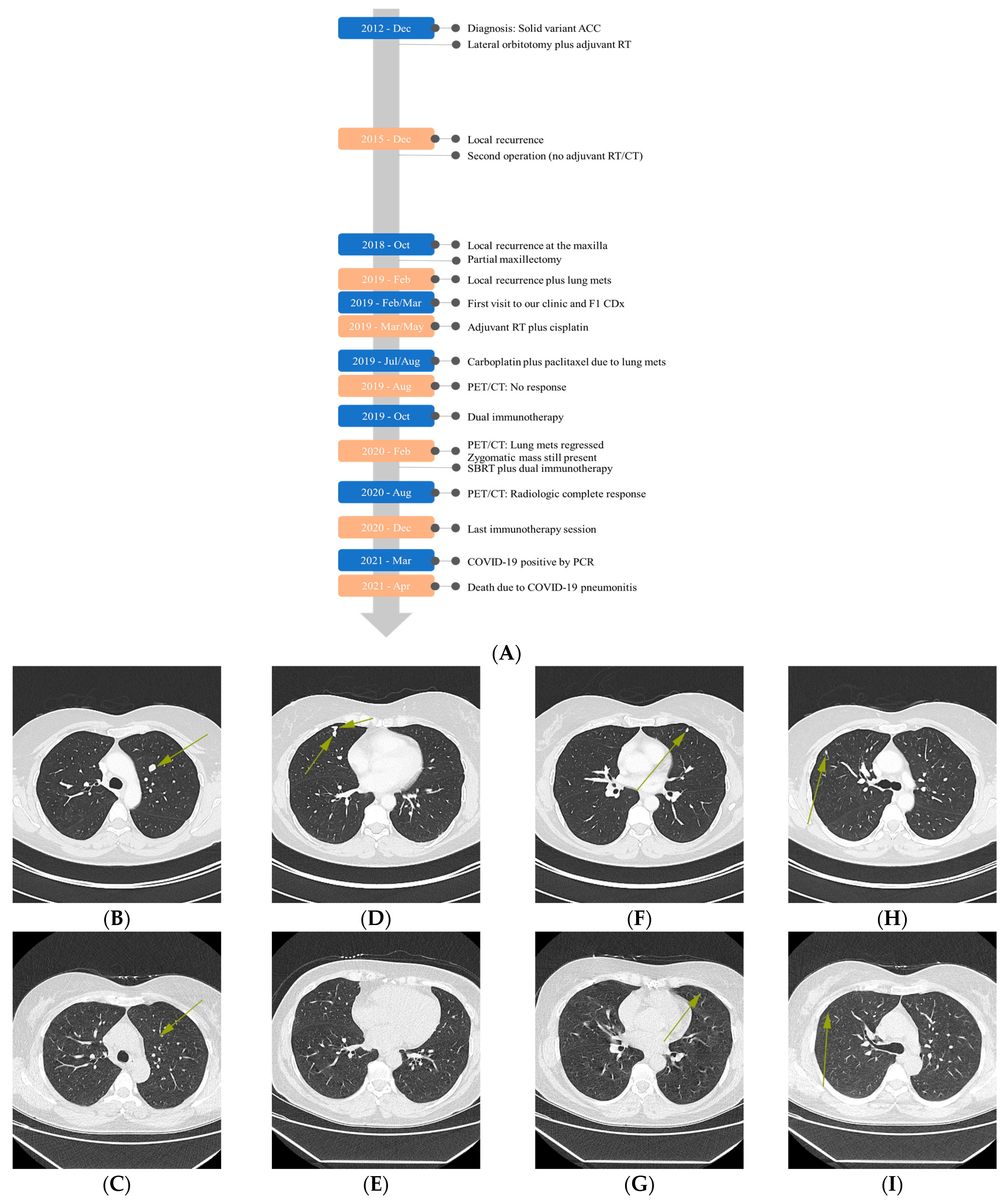
2.2. Treatment
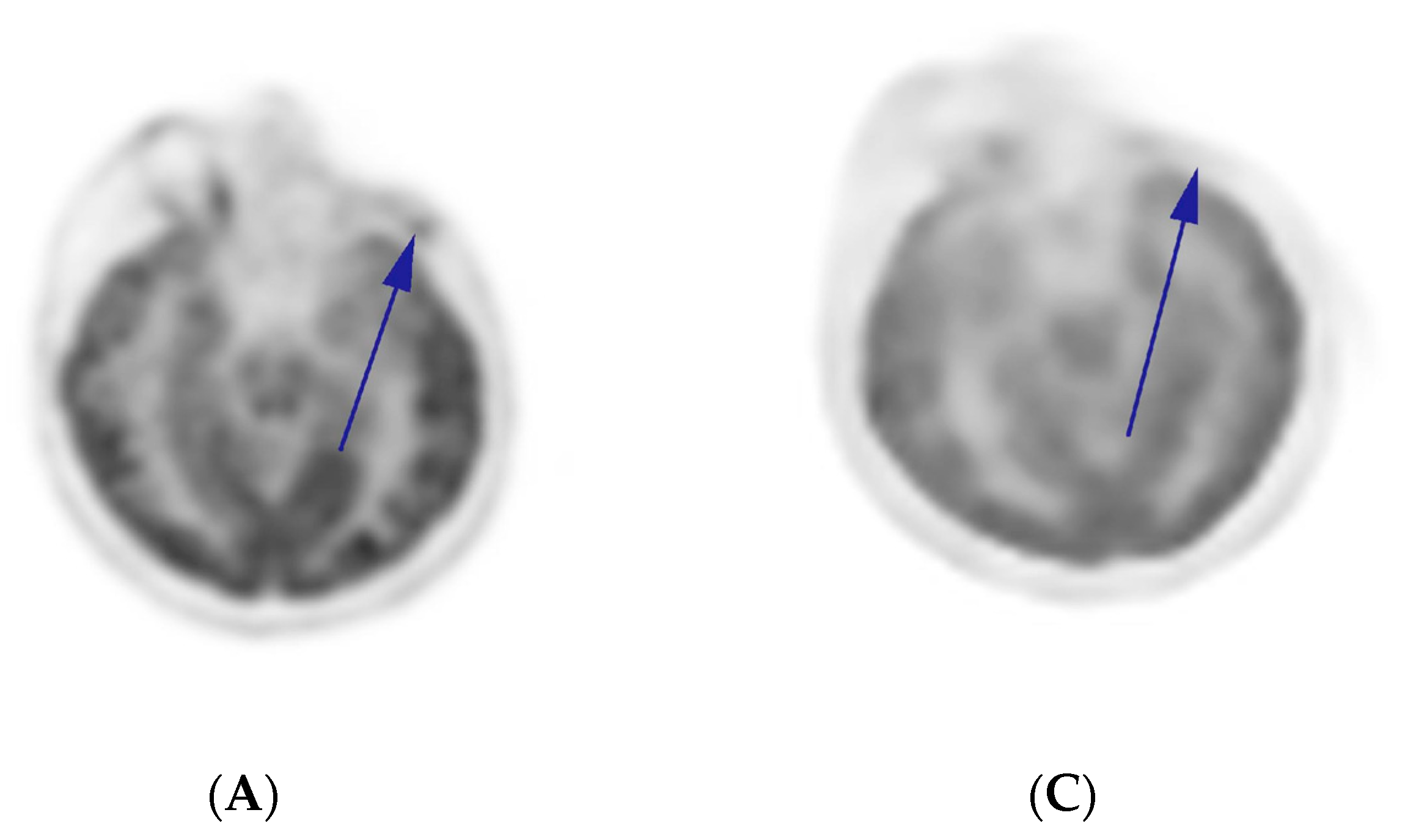
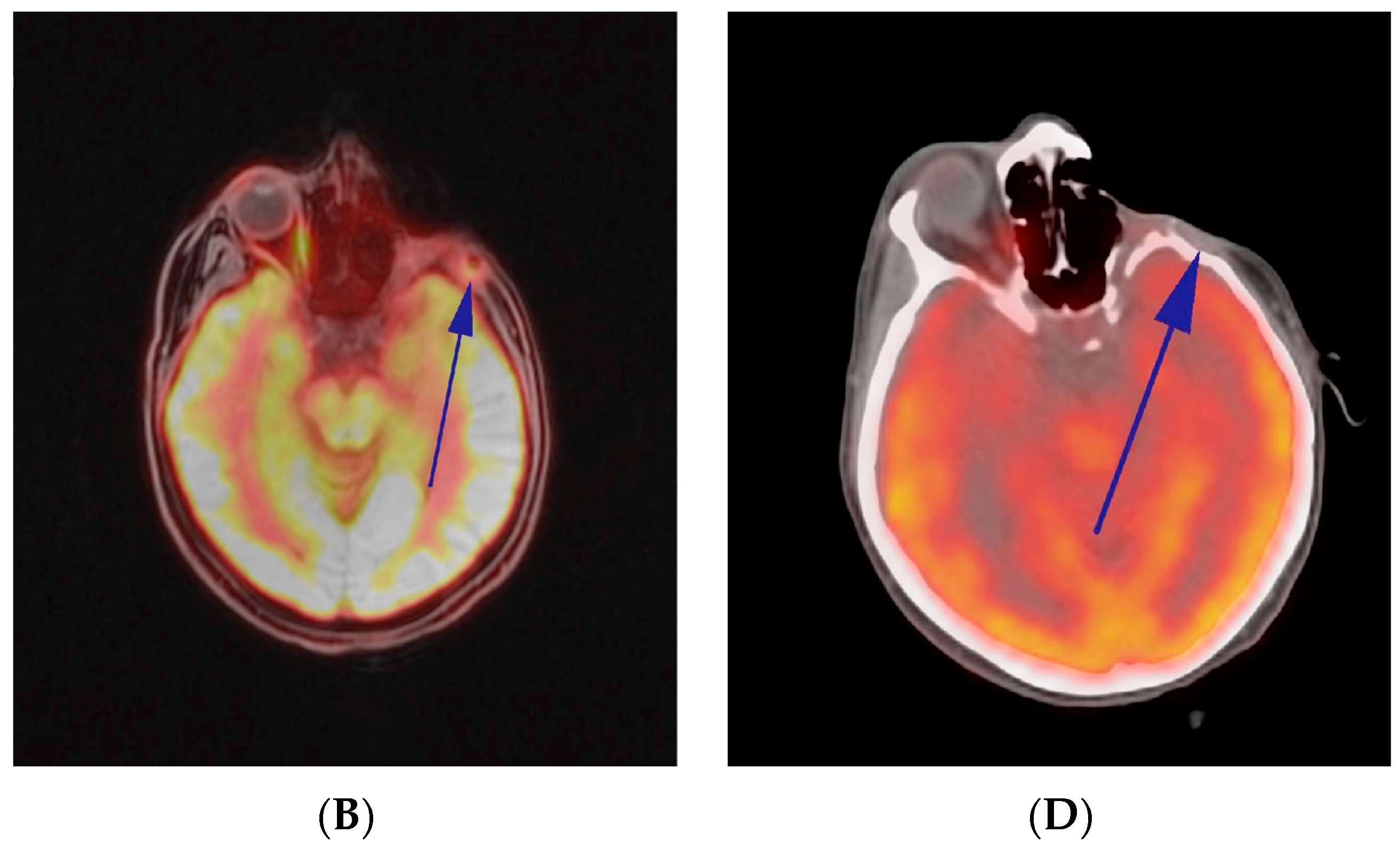
2.3. Comprehensive Genomic Profiling (CGP)-Guided Treatment
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steuer, C.E.; Hanna, G.J.; Viswanathan, K.; Bates, J.E.; Kaka, A.S.; Schmitt, N.C.; Ho, A.L.; Saba, N.F. The evolving landscape of salivary gland tumors. CA Cancer J. Clin. 2023, 73, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xu, L.; Zhao, H.; El-Naggar, A.K.; Sturgis, E.M. A comparison of the demographics, clinical features, and survival of patients with adenoid cystic carcinoma of major and minor salivary glands versus less common sites within the Surveillance, Epidemiology, and End Results registry. Cancer 2012, 118, 3945–3953. [Google Scholar] [CrossRef] [PubMed]
- Lesueur, P.; Rapeaud, E.; De Marzi, L.; Goudjil, F.; Levy, C.; Galatoire, O.; Jacomet, P.V.; Dendale, R.; Calugaru, V. Adenoid Cystic Carcinoma of the Lacrimal Gland: High Dose Adjuvant Proton Therapy to Improve Patients Outcomes. Front. Oncol. 2020, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Emerick, C.; Mariano, F.V.; Vargas, P.A.; Nor, J.E.; Squarize, C.H.; Castilho, R.M. Adenoid Cystic Carcinoma from the salivary and lacrimal glands and the breast: Different clinical outcomes to the same tumor. Crit. Rev. Oncol. Hematol. 2022, 179, 103792. [Google Scholar] [CrossRef] [PubMed]
- Ferrarotto, R.; Mitani, Y.; McGrail, D.J.; Li, K.; Karpinets, T.V.; Bell, D.; Frank, S.J.; Song, X.; Kupferman, M.E.; Liu, B.; et al. Proteogenomic Analysis of Salivary Adenoid Cystic Carcinomas Defines Molecular Subtypes and Identifies Therapeutic Targets. Clin. Cancer Res. 2021, 27, 852–864. [Google Scholar] [CrossRef]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Ho, A.S.; Ochoa, A.; Jayakumaran, G.; Zehir, A.; Valero Mayor, C.; Tepe, J.; Makarov, V.; Dalin, M.G.; He, J.; Bailey, M.; et al. Genetic hallmarks of recurrent/metastatic adenoid cystic carcinoma. J. Clin. Investig. 2019, 129, 4276–4289. [Google Scholar] [CrossRef]
- Mahmood, U.; Bang, A.; Chen, Y.H.; Mak, R.H.; Lorch, J.H.; Hanna, G.J.; Nishino, M.; Manuszak, C.; Thrash, E.M.; Severgnini, M.; et al. A Randomized Phase 2 Study of Pembrolizumab with or without Radiation in Patients with Recurrent or Metastatic Adenoid Cystic Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 134–144. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- McGranahan, N.; Furness, A.J.; Rosenthal, R.; Ramskov, S.; Lyngaa, R.; Saini, S.K.; Jamal-Hanjani, M.; Wilson, G.A.; Birkbak, N.J.; Hiley, C.T.; et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016, 351, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chong, W.; Teng, C.; Yao, Y.; Wang, X.; Li, X. The immune response-related mutational signatures and driver genes in non-small-cell lung cancer. Cancer Sci. 2019, 110, 2348–2356. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Wang, D.; Wang, A.; Chen, P.; Lin, Y.; Bian, J.; Yang, X.; Zheng, M.; Zhang, H.; Zheng, Y.; et al. A mutation-based gene set predicts survival benefit after immunotherapy across multiple cancers and reveals the immune response landscape. Genome Med. 2022, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.K.; Viveiros, P.; Lopes, G.; Sukhadia, B.; Sheikh, M.M.; Saravia, D.; Florou, V.; Sokol, E.S.; Frampton, G.M.; Chalmers, Z.R.; et al. Clinical and Immunological Implications of Frameshift Mutations in Lung Cancer. J. Thorac. Oncol. 2019, 14, 1807–1817. [Google Scholar] [CrossRef]
- Cormedi, M.C.V.; Van Allen, E.M.; Colli, L.M. Predicting immunotherapy response through genomics. Curr. Opin. Genet. Dev. 2021, 66, 1–9. [Google Scholar] [CrossRef]
- Wei, S.C.; Anang, N.A.S.; Sharma, R.; Andrews, M.C.; Reuben, A.; Levine, J.H.; Cogdill, A.P.; Mancuso, J.J.; Wargo, J.A.; Pe’er, D.; et al. Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc. Natl. Acad. Sci. USA 2019, 116, 22699–22709. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Magri, A.; Germano, G.; Lorenzato, A.; Lamba, S.; Chila, R.; Montone, M.; Amodio, V.; Ceruti, T.; Sassi, F.; Arena, S.; et al. High-dose vitamin C enhances cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaay8707. [Google Scholar] [CrossRef]
- Hoffer, L.J.; Levine, M.; Assouline, S.; Melnychuk, D.; Padayatty, S.J.; Rosadiuk, K.; Rousseau, C.; Robitaille, L.; Miller, W.H., Jr. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann. Oncol. 2008, 19, 1969–1974. [Google Scholar] [CrossRef]
- Peng, D.; He, A.; He, S.; Ge, G.; Wang, S.; Ci, W.; Li, X.; Xia, D.; Zhou, L. Ascorbic acid induced TET2 enzyme activation enhances cancer immunotherapy efficacy in renal cell carcinoma. Int. J. Biol. Sci. 2022, 18, 995–1007. [Google Scholar] [CrossRef]
- Luchtel, R.A.; Bhagat, T.; Pradhan, K.; Jacobs, W.R., Jr.; Levine, M.; Verma, A.; Shenoy, N. High-dose ascorbic acid synergizes with anti-PD1 in a lymphoma mouse model. Proc. Natl. Acad. Sci. USA 2020, 117, 1666–1677. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Sa, G. Curcumin as an Adjuvant to Cancer Immunotherapy. Front. Oncol. 2021, 11, 675923. [Google Scholar] [CrossRef] [PubMed]
- Demiray, M.; Sahinbas, H.; Atahan, S.; Demiray, H.; Selcuk, D.; Yildirim, I.; Atayoglu, A.T. Successful treatment of c-kit-positive metastatic Adenoid Cystic Carcinoma (ACC) with a combination of curcumin plus imatinib: A case report. Complement. Ther. Med. 2016, 27, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef]
- Laurie, S.A.; Ho, A.L.; Fury, M.G.; Sherman, E.; Pfister, D.G. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: A systematic review. Lancet Oncol. 2011, 12, 815–824. [Google Scholar] [CrossRef]
- Ho, A.S.; Kannan, K.; Roy, D.M.; Morris, L.G.; Ganly, I.; Katabi, N.; Ramaswami, D.; Walsh, L.A.; Eng, S.; Huse, J.T.; et al. The mutational landscape of adenoid cystic carcinoma. Nat. Genet. 2013, 45, 791–798. [Google Scholar] [CrossRef]
- Sahara, S.; Herzog, A.E.; Nor, J.E. Systemic therapies for salivary gland adenoid cystic carcinoma. Am. J. Cancer Res. 2021, 11, 4092–4110. [Google Scholar]
- Valkenburg, K.C.; de Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018, 15, 366–381. [Google Scholar] [CrossRef]
- Schlauch, D.; Fu, X.; Jones, S.F.; Burris, H.A., 3rd; Spigel, D.R.; Reeves, J.; McKenzie, A.J. Tumor-Specific and Tumor-Agnostic Molecular Signatures Associated with Response to Immune Checkpoint Inhibitors. JCO Precis. Oncol. 2021, 5, 1625–1638. [Google Scholar] [CrossRef]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef]
- Boyiadzis, M.M.; Kirkwood, J.M.; Marshall, J.L.; Pritchard, C.C.; Azad, N.S.; Gulley, J.L. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J. Immunother. Cancer 2018, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Massi, D.; Mandala, M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2016, 100, 88–98. [Google Scholar] [CrossRef] [PubMed]
- McGrail, D.J.; Pilie, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Lim, B.; et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Samstein, R.M.; Lee, C.H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Gay, L.M.; Wang, K.; Vergilio, J.A.; Suh, J.; Ramkissoon, S.; Somerset, H.; Johnson, J.M.; Russell, J.; Ali, S.; et al. Comprehensive genomic profiles of metastatic and relapsed salivary gland carcinomas are associated with tumor type and reveal new routes to targeted therapies. Ann. Oncol. 2017, 28, 2539–2546. [Google Scholar] [CrossRef]
- Davis, A.A.; Patel, V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef]
- Chen, W.; Fung, A.S.; McIntyre, J.B.; Simpson, R.; Afzal, A.R.; Hao, D.; Lau, H. Assessment Of Tumour Infiltrating Lymphocytes And Pd-l1 Expression In Adenoid Cystic Carcinoma Of The Salivary Gland. Clin. Investig. Med. 2021, 44, E38–E41. [Google Scholar] [CrossRef]
- Sridharan, V.; Gjini, E.; Liao, X.; Chau, N.G.; Haddad, R.I.; Severgnini, M.; Hammerman, P.; El-Naggar, A.; Freeman, G.J.; Hodi, F.S.; et al. Immune Profiling of Adenoid Cystic Carcinoma: PD-L2 Expression and Associations with Tumor-Infiltrating Lymphocytes. Cancer Immunol. Res. 2016, 4, 679–687. [Google Scholar] [CrossRef]
- Oliva, M.; Spreafico, A.; Taberna, M.; Alemany, L.; Coburn, B.; Mesia, R.; Siu, L.L. Immune biomarkers of response to immune-checkpoint inhibitors in head and neck squamous cell carcinoma. Ann. Oncol. 2019, 30, 57–67. [Google Scholar] [CrossRef]
- Sridharan, V.; Liao, X.; Chau, N.G.; Haddad, R.; El-Naggar, A.; Freeman, G.J.; Hodi, F.S.; Rodig, S.J.; Dranoff, G.; Schoenfeld, J.D. Immunologic profiling of adenoid cystic carcinoma (acc). J. Immunother. Cancer 2015, 3, P105. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Du, J.; Gao, Z.; Sun, H.; Mei, M.; Wang, Y.; Ren, Y.; Zhou, X. Evolving landscape of PD-L2: Bring new light to checkpoint immunotherapy. Br. J. Cancer 2023, 128, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Yearley, J.H.; Gibson, C.; Yu, N.; Moon, C.; Murphy, E.; Juco, J.; Lunceford, J.; Cheng, J.; Chow, L.Q.M.; Seiwert, T.Y.; et al. PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clin. Cancer Res. 2017, 23, 3158–3167. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.B.; Delord, J.P.; Doi, T.; Piha-Paul, S.A.; Liu, S.V.; Gilbert, J.; Algazi, A.P.; Damian, S.; Hong, R.L.; Le Tourneau, C.; et al. Pembrolizumab for the Treatment of Advanced Salivary Gland Carcinoma: Findings of the Phase 1b KEYNOTE-028 Study. Am. J. Clin. Oncol. 2018, 41, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Fayette, J.; Even, C.; Digue, L.; Geoffrois, L.; Rolland, D.; Guigay, J.; Tourneau, C.L.; Dillies, A.F.; Zanetta, S.; Bozec, L.; et al. NISCAHN: A phase II trial of nivolumab in patients with salivary gland carcinoma (Unicancer ORL-08). BMJ Oncol. 2023, 2, e000065. [Google Scholar] [CrossRef]
- Vos, J.L.; Burman, B.; Jain, S.; Fitzgerald, C.W.R.; Sherman, E.J.; Dunn, L.A.; Fetten, J.V.; Michel, L.S.; Kriplani, A.; Ng, K.K.; et al. Nivolumab plus ipilimumab in advanced salivary gland cancer: A phase 2 trial. Nat. Med. 2023, 29, 3077–3089. [Google Scholar] [CrossRef]
- Fumet, J.D.; Truntzer, C.; Yarchoan, M.; Ghiringhelli, F. Tumour mutational burden as a biomarker for immunotherapy: Current data and emerging concepts. Eur. J. Cancer 2020, 131, 40–50. [Google Scholar] [CrossRef]
- Wolf, Y.; Bartok, O.; Patkar, S.; Eli, G.B.; Cohen, S.; Litchfield, K.; Levy, R.; Jimenez-Sanchez, A.; Trabish, S.; Lee, J.S.; et al. UVB-Induced Tumor Heterogeneity Diminishes Immune Response in Melanoma. Cell 2019, 179, 219–235.e21. [Google Scholar] [CrossRef]
- Litchfield, K.; Reading, J.L.; Puttick, C.; Thakkar, K.; Abbosh, C.; Bentham, R.; Watkins, T.B.K.; Rosenthal, R.; Biswas, D.; Rowan, A.; et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell 2021, 184, 596–614.e514. [Google Scholar] [CrossRef]
- Gajic, Z.Z.; Deshpande, A.; Legut, M.; Imielinski, M.; Sanjana, N.E. Recurrent somatic mutations as predictors of immunotherapy response. Nat. Commun. 2022, 13, 3938. [Google Scholar] [CrossRef]
- Martinez-Jimenez, F.; Priestley, P.; Shale, C.; Baber, J.; Rozemuller, E.; Cuppen, E. Genetic immune escape landscape in primary and metastatic cancer. Nat. Genet. 2023, 55, 820–831. [Google Scholar] [CrossRef]
- Zhang, K.; Hong, X.; Song, Z.; Xu, Y.; Li, C.; Wang, G.; Zhang, Y.; Zhao, X.; Zhao, Z.; Zhao, J.; et al. Identification of Deleterious NOTCH Mutation as Novel Predictor to Efficacious Immunotherapy in NSCLC. Clin. Cancer Res. 2020, 26, 3649–3661. [Google Scholar] [CrossRef]
- Wang, F.; Long, J.; Li, L.; Zhao, Z.B.; Wei, F.; Yao, Y.; Qiu, W.J.; Wu, Z.X.; Luo, Q.Q.; Liu, W.; et al. Mutations in the notch signalling pathway are associated with enhanced anti-tumour immunity in colorectal cancer. J. Cell Mol. Med. 2020, 24, 12176–12187. [Google Scholar] [CrossRef] [PubMed]
- Roper, N.; Velez, M.J.; Chiappori, A.; Kim, Y.S.; Wei, J.S.; Sindiri, S.; Takahashi, N.; Mulford, D.; Kumar, S.; Ylaya, K.; et al. Notch signaling and efficacy of PD-1/PD-L1 blockade in relapsed small cell lung cancer. Nat. Commun. 2021, 12, 3880. [Google Scholar] [CrossRef]
- Qiu, H.; Zmina, P.M.; Huang, A.Y.; Askew, D.; Bedogni, B. Inhibiting Notch1 enhances immunotherapy efficacy in melanoma by preventing Notch1 dependent immune suppressive properties. Cancer Lett. 2018, 434, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Reyes, K.R.; Zhang, L.; Zhu, X.; Jindal, T.; Deshmukh, P.; Kouchkovsky, I.D.; Koshkin, V.S. Association of biomarkers and outcomes in patients (pts) with metastatic urothelial carcinoma (mUC) treated with immune checkpoint inhibitors (ICIs). J. Clin. Oncol. 2023, 41, 532. [Google Scholar] [CrossRef]
- Jindal, T.; Zhu, X.; Zhang, L.; Reyes, K.R.; Deshmukh, P.; Kouchkovsky, I.D.; Koshkin, V.S. Association of biomarkers and response to immune checkpoint inhibitors (ICIs) in patients with metastatic urothelial carcinoma (mUC) with high and low tumor mutation burden (TMB). J. Clin. Oncol. 2023, 41, 534. [Google Scholar] [CrossRef]
- Bedhiafi, T.; Inchakalody, V.P.; Fernandes, Q.; Mestiri, S.; Billa, N.; Uddin, S.; Merhi, M.; Dermime, S. The potential role of vitamin C in empowering cancer immunotherapy. Biomed. Pharmacother. 2022, 146, 112553. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Amatu, A.; Nicolantonio, F.D.; Troiani, T.; Tosi, F.; Napolitano, S.; Martini, G.; Bencardino, K.; Bonazzina, E.; Carnevali, P.; et al. High-dose vitamin C to enhance neoadjuvant immune checkpoint therapy in mismatch repair proficient colon cancer: The ALFEO pilot study. J. Clin. Oncol. 2023, 41, TPS2675. [Google Scholar] [CrossRef]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef]
- Adibi, A.; Tokat, U.M.; Ozgu, E.; Aydin, E.; Demiray, I.; Demiray, M. PARP inhibitor combinations with high-dose vitamin C in the treatment of Ewing sarcoma: Two case reports and mechanistic overview. Ther. Adv. Med. Oncol. 2023, 15, 17588359231213841. [Google Scholar] [CrossRef]
- Adibi, A.; Tokat, U.M.; Ozgu, E.; Mamyrov, N.; Aydin, E.; Bilgic, S.N.; Tutar, O.; Demiray, M. Durable radiologic and molecular complete response following nivolumab in an HNSCC patient with UV signature and HIV. Oral. Oncol. 2024, 154, 106859. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Ji, C.; Mayfield, J.E.; Goel, A.; Xiao, J.; Dixon, J.E.; Guo, X. Ancient drug curcumin impedes 26S proteasome activity by direct inhibition of dual-specificity tyrosine-regulated kinase 2. Proc. Natl. Acad. Sci. USA 2018, 115, 8155–8160. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.J.; Chen, G.; Zhang, W.; Hu, X.; Liu, Y.; Zhou, Q.; Zhu, L.X.; Zhao, Y.F. Curcumin dually inhibits both mammalian target of rapamycin and nuclear factor-kappaB pathways through a crossed phosphatidylinositol 3-kinase/Akt/IkappaB kinase complex signaling axis in adenoid cystic carcinoma. Mol. Pharmacol. 2011, 79, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.J.; Chen, G.; Hu, X.; Zhang, W.; Liu, Y.; Zhu, L.X.; Zhou, Q.; Zhao, Y.F. Activation of PI3K/Akt/IKK-alpha/NF-kappaB signaling pathway is required for the apoptosis-evasion in human salivary adenoid cystic carcinoma: Its inhibition by quercetin. Apoptosis 2010, 15, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Phuchareon, J.; van Zante, A.; Overdevest, J.B.; McCormick, F.; Eisele, D.W.; Tetsu, O. c-Kit Expression is Rate-Limiting for Stem Cell Factor-Mediated Disease Progression in Adenoid Cystic Carcinoma of the Salivary Glands. Transl. Oncol. 2014, 7, 537–545. [Google Scholar] [CrossRef]
- Argiris, A.; Ghebremichael, M.; Burtness, B.; Axelrod, R.S.; Deconti, R.C.; Forastiere, A.A. A phase 2 trial of bortezomib followed by the addition of doxorubicin at progression in patients with recurrent or metastatic adenoid cystic carcinoma of the head and neck: A trial of the Eastern Cooperative Oncology Group (E1303). Cancer 2011, 117, 3374–3382. [Google Scholar] [CrossRef]
- Turajlic, S.; Litchfield, K.; Xu, H.; Rosenthal, R.; McGranahan, N.; Reading, J.L.; Wong, Y.N.S.; Rowan, A.; Kanu, N.; Al Bakir, M.; et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: A pan-cancer analysis. Lancet Oncol. 2017, 18, 1009–1021. [Google Scholar] [CrossRef]
- Schwitalle, Y.; Kloor, M.; Eiermann, S.; Linnebacher, M.; Kienle, P.; Knaebel, H.P.; Tariverdian, M.; Benner, A.; von Knebel Doeberitz, M. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology 2008, 134, 988–997. [Google Scholar] [CrossRef]
- Ballhausen, A.; Przybilla, M.J.; Jendrusch, M.; Haupt, S.; Pfaffendorf, E.; Seidler, F.; Witt, J.; Hernandez Sanchez, A.; Urban, K.; Draxlbauer, M.; et al. The shared frameshift mutation landscape of microsatellite-unstable cancers suggests immunoediting during tumor evolution. Nat. Commun. 2020, 11, 4740. [Google Scholar] [CrossRef]
- Roudko, V.; Bozkus, C.C.; Orfanelli, T.; McClain, C.B.; Carr, C.; O’Donnell, T.; Chakraborty, L.; Samstein, R.; Huang, K.L.; Blank, S.V.; et al. Shared Immunogenic Poly-Epitope Frameshift Mutations in Microsatellite Unstable Tumors. Cell 2020, 183, 1634–1649.e17. [Google Scholar] [CrossRef]
- Florou, V.; Floudas, C.S.; Maoz, A.; Naqash, A.R.; Norton, C.; Tan, A.C.; Sokol, E.S.; Frampton, G.; Soares, H.P.; Puri, S.; et al. Real-world pan-cancer landscape of frameshift mutations and their role in predicting responses to immune checkpoint inhibitors in cancers with low tumor mutational burden. J. Immunother. Cancer 2023, 11, e007440. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Asmann, Y.; Thomas, M.; Knutson, K. Integrating INDEL Mutations into Neoantigen Prediction in Lung Cancer: Developing Personalized Cancer Vaccines. J. Thorac. Oncol. 2017, 12, 1814–1815. [Google Scholar] [CrossRef]
- Gebert, J.; Gelincik, O.; Oezcan-Wahlbrink, M.; Marshall, J.D.; Hernandez-Sanchez, A.; Urban, K.; Long, M.; Cortes, E.; Tosti, E.; Katzenmaier, E.M.; et al. Recurrent Frameshift Neoantigen Vaccine Elicits Protective Immunity With Reduced Tumor Burden and Improved Overall Survival in a Lynch Syndrome Mouse Model. Gastroenterology 2021, 161, 1288–1302.e13. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.J.; Lizotte, P.; Cavanaugh, M.; Kuo, F.C.; Shivdasani, P.; Frieden, A.; Chau, N.G.; Schoenfeld, J.D.; Lorch, J.H.; Uppaluri, R.; et al. Frameshift events predict anti-PD-1/L1 response in head and neck cancer. JCI Insight 2018, 3, e98811. [Google Scholar] [CrossRef]
- Jayasinghe, R.G.; Cao, S.; Gao, Q.; Wendl, M.C.; Vo, N.S.; Reynolds, S.M.; Zhao, Y.; Climente-Gonzalez, H.; Chai, S.; Wang, F.; et al. Systematic Analysis of Splice-Site-Creating Mutations in Cancer. Cell Rep. 2018, 23, 270–281.e3. [Google Scholar] [CrossRef]
- Maleki Vareki, S. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J. Immunother. Cancer 2018, 6, 157. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokat, Ü.M.; Adibi, A.; Aydın, E.; Özgü, E.; Bilgiç, Ş.N.; Tutar, O.; Özbek Doğançay, M.; Demiray, İ.; Demiray, M. Personalized Immunotherapy Achieves Complete Response in Metastatic Adenoid Cystic Carcinoma Despite Lack of Conventional Biomarkers. Curr. Oncol. 2024, 31, 5838-5849. https://doi.org/10.3390/curroncol31100434
Tokat ÜM, Adibi A, Aydın E, Özgü E, Bilgiç ŞN, Tutar O, Özbek Doğançay M, Demiray İ, Demiray M. Personalized Immunotherapy Achieves Complete Response in Metastatic Adenoid Cystic Carcinoma Despite Lack of Conventional Biomarkers. Current Oncology. 2024; 31(10):5838-5849. https://doi.org/10.3390/curroncol31100434
Chicago/Turabian StyleTokat, Ünal Metin, Ashkan Adibi, Esranur Aydın, Eylül Özgü, Şevval Nur Bilgiç, Onur Tutar, Merve Özbek Doğançay, İrem Demiray, and Mutlu Demiray. 2024. "Personalized Immunotherapy Achieves Complete Response in Metastatic Adenoid Cystic Carcinoma Despite Lack of Conventional Biomarkers" Current Oncology 31, no. 10: 5838-5849. https://doi.org/10.3390/curroncol31100434
APA StyleTokat, Ü. M., Adibi, A., Aydın, E., Özgü, E., Bilgiç, Ş. N., Tutar, O., Özbek Doğançay, M., Demiray, İ., & Demiray, M. (2024). Personalized Immunotherapy Achieves Complete Response in Metastatic Adenoid Cystic Carcinoma Despite Lack of Conventional Biomarkers. Current Oncology, 31(10), 5838-5849. https://doi.org/10.3390/curroncol31100434




