Monitoring the Response of Cyclin-Dependent Kinase 4/6 Inhibitors with Mean Corpuscular Volume
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
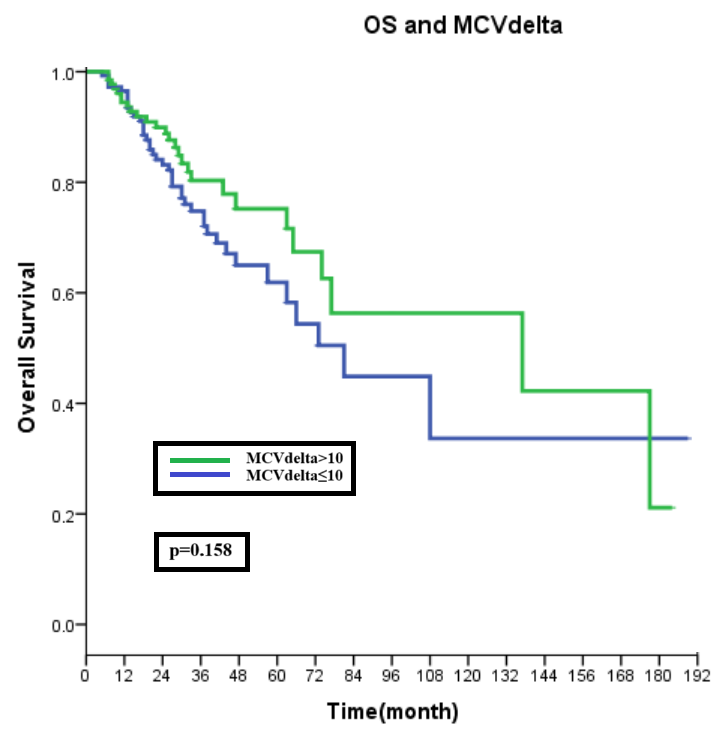
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.-A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541–1547. [Google Scholar] [CrossRef]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.-C.; Manso, L. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J. clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Im, S.-A.; Lu, Y.-S.; Colleoni, M.; Franke, F.A.; Bardia, A.; Harbeck, N.; Chow, L.; Sohn, J.; Lee, K.S. Phase III MONALEESA-7 trial of premenopausal patients with HR+/HER2− advanced breast cancer (ABC) treated with endocrine therapy ± ribociclib: Overall survival (OS) results. J. Clin. Oncol. 2019, 37. [Google Scholar] [CrossRef]
- Antonarelli, G.; Salimbeni, B.T.; Marra, A.; Esposito, A.; Locatelli, M.A.; Trapani, D.; Pescia, C.; Fusco, N.; Curigliano, G.; Criscitiello, C. The CDK4/6 Inhibitors Biomarker Landscape: The most Relevant Biomarkers of Response or Resistance for Further Research and Potential Clinical Utility. Crit. Rev. Oncol. Hematol. 2023, 192, 104148. [Google Scholar] [CrossRef]
- Asghar, U.S.; Kanani, R.; Roylance, R.; Mittnacht, S. Systematic review of molecular biomarkers predictive of resistance to CDK4/6 inhibition in metastatic breast cancer. JCO Precis. Oncol. 2022, 6, e2100002. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, I.-M.; Parosanu, A.I.; Orlov-Slavu, C.; Iaciu, I.C.; Popa, A.M.; Olaru, C.M.; Pirlog, C.F.; Vrabie, R.C.; Nitipir, C. Mechanisms of Resistance to CDK4/6 Inhibitors and Predictive Biomarkers of Response in HR+/HER2-Metastatic Breast Cancer—A Review of the Literature. Diagnostics 2023, 13, 987. [Google Scholar] [CrossRef]
- Witkiewicz, A.K.; Schultz, E.; Wang, J.; Hamilton, D.; Levine, E.; O’Connor, T.; Knudsen, E.S. Determinants of response to CDK4/6 inhibitors in the real-world setting. NPJ Precis. Oncol. 2023, 7, 90. [Google Scholar] [CrossRef]
- Futamura, M.; Nakayama, T.; Yoshinami, T.; Oshiro, C.; Ishihara, M.; Morita, M.; Watanabe, A.; Tanigichi, A.; Tsukabe, M.; Shimoda, M. Detection of high-risk patients resistant to CDK4/6 inhibitors with hormone receptor-positive HER2-negative advanced and metastatic breast cancer in Japan (KBCSG-TR-1316). Breast Cancer 2023, 30, 943–951. [Google Scholar] [CrossRef]
- Moukas, S.I.; Kasimir-Bauer, S.; Tewes, M.; Kolberg, H.-C.; Hoffmann, O.; Kimmig, R.; Keup, C. Ratios of monocytes and neutrophils to lymphocytes in the blood predict benefit of CDK4/6 inhibitor treatment in metastatic breast cancer. Sci. Rep. 2023, 13, 21262. [Google Scholar] [CrossRef]
- Anampa, J.; Haque, T.; Murakhovskaya, I.; Wang, Y.; Bachiashvili, K.; Papazoglu, C.; Pradhan, K.; Steidl, U.G.; Sparano, J.A.; Verma, A. Macrocytosis and dysplastic anemia is associated with the cyclin-dependent kinase 4/6 inhibitor palbociclib in metastatic breast cancer. Haematologica 2018, 103, e98. [Google Scholar] [CrossRef] [PubMed]
- Choong, G.M.Y.; Leon-Ferre, R.A.; O’Sullivan, C.C.; Ruddy, K.J.; Haddad, T.C.; Hobday, T.J.; Peethambaram, P.P.; Loprinizi, C.L.; Liu, M.C.; Suman, V.J. Abstract P1-19-42: Evaluation of mean corpuscular volume (MCV) as a pharmacodynamic predictive biomarker in patients receiving CDK4/6 inhibitors for metastatic breast cancer (MBC). Cancer Res. 2020, 80, P1-19-42. [Google Scholar] [CrossRef]
- Jayapal, S.R.; Wang, C.Q.; Bisteau, X.; Caldez, M.J.; Lim, S.; Tergaonkar, V.; Osato, M.; Kaldis, P. Hematopoiesis specific loss of Cdk2 and Cdk4 results in increased erythrocyte size and delayed platelet recovery following stress. Haematologica 2015, 100, 431. [Google Scholar] [CrossRef]
- Haque, T.; Anampa, J.; Murakhovskaya, I.; Wang, Y.; Bachiashvili, K.; Papazoglu, C.; Pradhan, K.; Steidl, U.G.; Verma, A. First Report of Reversible Macrocytic Anemia with Dysplastic Features Associated with Palbociclib Use in Patients with Metastatic Breast Cancer. Blood 2017, 130, 2207. [Google Scholar]
- Kamboj, J.; Chalhoub, E.; Friedell, P.E. Reversible Macrocytosis with Cyclin Dependent Kinase Inhibitors. Blood 2018, 132, 4882. [Google Scholar] [CrossRef]
- Kahraman, S.; Erul, E.; Seyyar, M.; Gumusay, O.; Bayram, E.; Demirel, B.C.; Acar, O.; Aksoy, S.; Baytemur, N.K.; Sahin, E. Treatment efficacy of ribociclib or palbociclib plus letrozole in hormone receptor-positive/HER2-negative metastatic breast cancer. Future Oncol. 2023, 19, 727–736. [Google Scholar] [CrossRef]
- Rugo, H.; Finn, R.; Diéras, V.; Ettl, J.; Lipatov, O.; Joy, A.; Harbeck, N.; Castrellon, A.; Iyer, S.; Lu, D. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res. Treat. 2019, 174, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.; Martin, M.; Di Leo, A.; Im, S.-A.; Awada, A.; Forrester, T.; Frenzel, M.; Hardebeck, M.C.; Cox, J.; Barriga, S. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019, 5, 5. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar]
- Bilić-Pavlinović, M.; Rumora, T.; Barta, L.; Cahun, E.; Mutić, T.F.; Silovski, T.; Plavetić, N.D. Mijelodisplastični sindrom dijagnosticiran tijekom liječenja ribociklibom hormonski pozitivnog, Her2 negativnog metastatskog raka dojke–prikaz slučaja i pregled literature. Lijec. Vjesn. 2023, 145, 100. [Google Scholar]
- Hoffmann, J.J.; Nabbe, K.C.; van den Broek, N.M. Effect of age and gender on reference intervals of red blood cell distribution width (RDW) and mean red cell volume (MCV). Clin. Chem. Lab. Med. (CCLM) 2015, 53, 2015–2019. [Google Scholar] [CrossRef] [PubMed]

| Variable | Total | MCV ≤ Delta10fl | MCV > Delta10fl | p-Value |
|---|---|---|---|---|
| n | 275 | 145 | 130 | |
| Age (mean ± std) | 56.1 ± 12.1 | 53.1 ± 12.3 | 59.5 ± 10.9 | <0.001 * |
| ECOG (n%) | ||||
| 0–1 | 262 (95.3) | 137 (94.5) | 125 (96.2) | 0.514 |
| >1 | 13 (4.7) | 8 (5.5) | 5 (3.8) | |
| Comorbidity | ||||
| Yes | 143 (52) | 72 (49.7) | 71 (54.6) | 0.568 |
| No | 132 (48) | 73 (51.3) | 59 (45.4) | |
| Menopausal status (n%) | ||||
| Post-menopausal | 189 (68.7) | 83 (57.2) | 106 (81.5) | |
| Pre-menopausal | 86 (31.3) | 62 (42.8) | 24 (18.5) | <0.001 * |
| Metastatic status (n%) | ||||
| De novo | 123 (44.7) | 60 (41.4) | 63 (48.5) | |
| Recurrent | 152 (55.3) | 85 (58.6) | 67 (51.5) | 0.238 |
| Metastatic site | ||||
| Non-visceral | 170 (61.8) | 94 (64.8) | 76 (58.5) | 0.278 |
| Visceral | 105 (38.2) | 51 (35.2) | 54 (41.5) | |
| CDK4/6 treatment line | ||||
| First-line | 200 (72.7) | 105 (72.4) | 95 (73.1) | |
| Second-line | 75 (27.3) | 40 (27.6) | 35 (26.9) | 0.902 |
| CDK4/6 Treatment option | ||||
| Ribociclib | 173 (62.9) | 90 (62.1) | 83 (63.8) | |
| Palbociclib | 102 (37.1) | 55 (37.9) | 47 (36.2) | 0.761 |
| CDK4/6 combination (n%) | ||||
| AI | 194 (70.5) | 101 (69.7) | 93 (71.5) | |
| Fluvestrant | 81 (29.5) | 44 (30.3) | 37 (28.5) | 0.732 |
| Variables | Univariate Analyses | Multivariate Analyses | ||||
|---|---|---|---|---|---|---|
| HR | CI 95% | p Value | HR | CI 95% | p Value | |
| Age | 1 | 0.99–1.01 | 0.592 | |||
| ECOG | 2.48 | 1.30–4.75 | 0.006 * | 2.97 | 1.53–5.74 | 0.01 * |
| 0–1 | ||||||
| >1 | ||||||
| Comorbidity | 0.81 | 0.58–1.14 | 0.229 | |||
| Yes | ||||||
| No | ||||||
| Unknown | ||||||
| Menopausal status | 1.08 | 0.75–1.55 | 0.683 | |||
| Post-menopausal | ||||||
| Pre-menopausal | ||||||
| Metastatic status | 1.17 | 0.83–1.65 | 0.361 | |||
| De novo | ||||||
| Recurrent | ||||||
| Metastatic site | 0.92 | 0.78–1.10 | 0.369 | |||
| Non-visceral | ||||||
| Visceral | ||||||
| CDK4/6 treatment line | 1.88 | 1.32–2.66 | 0.001 * | 1.75 | 1.19–2.55 | 0.04 * |
| First-line | ||||||
| Second-line | ||||||
| CDK4/6 Treatment option | 1.41 | 1–1.98 | 0.050 | 1.19 | 0.84–1.69 | 0.329 |
| Ribociclib | ||||||
| Palbociclib | ||||||
| CDK4/6 combination | 1.76 | 1.23–2.53 | 0.002 * | 1.52 | 1.04–2.23 | 0.032 * |
| AI | ||||||
| Fluvestrant | ||||||
| MCV delta (fL) | 0.69 | 0.49–0.97 | 0.031 * | 0.66 | 0.46–0.93 | 0.017 * |
| <10 | ||||||
| ≥10 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurt İnci, B.; Kubilay Tolunay, P.; Öztekin, Ş.; Aydemir, E.; Öner, İ.; Ateş, Ö.; Karaçin, C. Monitoring the Response of Cyclin-Dependent Kinase 4/6 Inhibitors with Mean Corpuscular Volume. Curr. Oncol. 2024, 31, 5722-5729. https://doi.org/10.3390/curroncol31100424
Kurt İnci B, Kubilay Tolunay P, Öztekin Ş, Aydemir E, Öner İ, Ateş Ö, Karaçin C. Monitoring the Response of Cyclin-Dependent Kinase 4/6 Inhibitors with Mean Corpuscular Volume. Current Oncology. 2024; 31(10):5722-5729. https://doi.org/10.3390/curroncol31100424
Chicago/Turabian StyleKurt İnci, Bediz, Pınar Kubilay Tolunay, Şura Öztekin, Ergin Aydemir, İrem Öner, Öztürk Ateş, and Cengiz Karaçin. 2024. "Monitoring the Response of Cyclin-Dependent Kinase 4/6 Inhibitors with Mean Corpuscular Volume" Current Oncology 31, no. 10: 5722-5729. https://doi.org/10.3390/curroncol31100424
APA StyleKurt İnci, B., Kubilay Tolunay, P., Öztekin, Ş., Aydemir, E., Öner, İ., Ateş, Ö., & Karaçin, C. (2024). Monitoring the Response of Cyclin-Dependent Kinase 4/6 Inhibitors with Mean Corpuscular Volume. Current Oncology, 31(10), 5722-5729. https://doi.org/10.3390/curroncol31100424





