Comprehensive Geriatric Assessment for Older Women with Early-Stage (Non-Metastatic) Breast Cancer—An Updated Systematic Review of the Literature
Abstract
:1. Introduction
2. Materials and Methods
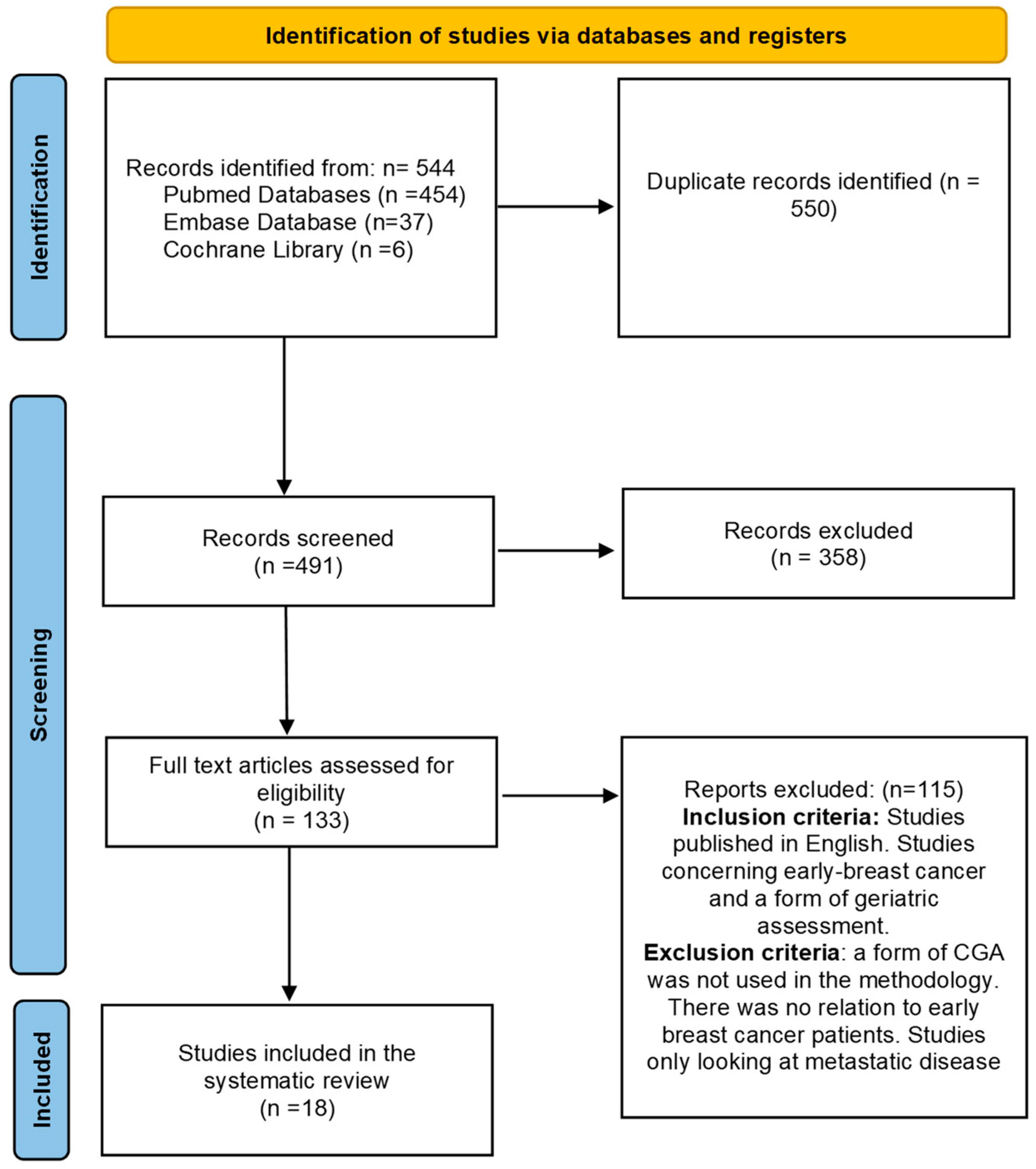
2.1. PRISMA Statement
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Study Selection
2.5. Data Extraction
2.6. Critical Appraisal
3. Results
3.1. General Characteristics
3.2. Level of Evidence
3.3. Findings
3.4. To Determine Factors Influencing Survival or Mortality
3.5. As an Adjunct to Treatment Decision-Making
3.6. Measuring Quality of Life and Functionality
3.7. To Determine Which Tools Should Be Used in CGA
4. Discussion
4.1. Level of Evidence
4.2. Factors Influencing Survival or Mortality
4.3. As an Adjunct to Treatment Decision-Making
4.4. To Measure Quality of Life and Functional State
4.5. To Determine Which Tools Should Be Used in CGA
4.6. Comparisons to Parks RM et al., 2012
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Scheme 1 | Country of Study | Date | Author | Study Type | Aims of Analysis | Level of Evidence | N | Age in Years | Cancer Type | Stage | Tools That Were Used |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | United Kingdom | Mar 2022 | Munir, A. et al. [29] | Single-centre prospective study | Evaluating whether the use of self-administered CGA in older patients with breast cancer patients resulted in a change in their treatment. | 3 | 101 | ≥65 | Breast | Early stage | ADL, iADL, KPS, number of falls, BMOC, MHI-17, MOSS-SS, TUG. |
| 2 | France | May 2021 | Boulahassass, R. et al. [18] | Single-centre retrospective study | Analysing the quality of life and CGA domains within 6 months in older adults receiving accelerating partial breast irradiation. | 3 | 37 | ≥70 | Breast | — | iADL, MMSE, QLQ-BR23, QLQ-C30 and global QOL. |
| 3 | Belgium | Sep 2020 | Quinten, C. et al. [24] | Multicentre prospective study | Assessing the relationship between CGA and health-related quality of life in older patients with breast cancer. | 3 | 109 | ≥75 | Breast | Early stage | ADL, iADL, MMSE, MNA-SF, LOFS, and CCI. |
| 4 | France | Aug 2020 | Liuu, E. et al. [15] | Single-centre prospective cohort study | Assessing the prognostic value of MPI for 1-year mortality in elderly cancer patients. | 3 | 433 | ≥75 | 23% Prostate, 17% skin, 15% colorectal, 12% breast | — | ADL, iADL, MNA-SR, SPMSQ, ESS, CIRS. |
| 5 | Germany, Belgium, U.K. | Nov 2018 | Speigl, L. et al. [19] | Multicentre prospective study | To compare the relationship between patient fitness/frailty status and survival to the local tumour environment in older patients with breast cancer. | 3 | 58 | ≥70 | Breast | — | ADL, iADL, MOB-T, MMSE, GDS-15, MNA-SF, VAS and CCI. |
| 6 | USA | Nov 2018 | Owusu, C. et al. [28] | Cross-sectional study retrospective study. | Examining the racial differences in physical performance amongst older women who have recently been diagnosed with cancer. | 3 | 135 | ≥65 | Breast | Stage 1–3 | LTPA, MMSE, GDS, MET-physical activity. |
| 7 | France | Apr 2018 | Falandry, C. et al. [17] | Multicentre prospective study | Assessing CGA in older patients with breast cancer with multiple treatment options. | 3 | 631 | ≥70 | Breast | — | G8 and VES-13 |
| 8 | U.K. | Sep 2017 | Okonji, D. et al. [20] | Multicentre prospective study. | Evaluating a cohort of older women using CGA to determine whether fitness explained the apparent under-treatment. | 3 | 326 | ≥70 | Breast | Stage 1–3 | ASA, II, ECOG, KPS, ADL, iADL, G8 and CCI |
| 9 | Germany, Belgium, USA | Feb 2017 | Bailur, J.K. et al. [21] | Multicentre prospective study. | To investigate how immune cell biomarkers evolve in older patients with breast cancer. | 3 | 56 | ≥70 | Breast | — | G8 screening tool, LOFS, ADL, iADL, MOB-T, MMSE, GDS-15, MNA-SF and CCI. |
| 10 | USA | Nov 2015 | Guerad, E.J. et al. [14] | Single-centre retrospective study | To evaluate oncology providers recognition of and response to falls in older patients with cancer. | 3 | 528 | ≥65 | 62% Breast | — | Karnofsky performance status score, ADL, iADL. |
| 11 | U.K. | Apr 2015 | Stotter, A. et al. [12] | Single-centre retrospective study | To estimate the 3-year survival rate in frail patients with early breast cancer and to inform treatment decisions | 3 | 398 | ≥43 | Breast | — | MMSE, ASA, GDS IV, iADL, BI and Charlson |
| 12 | Germany | Feb 2015 | Denkinger, M.D. et al. [23] | Single-centre retrospective study. | Assessing the value of different assessments for predicting fatigue after radiotherapy in older breast cancer patients. | 3 | 74 | ≥65 | Breast | — | VES-13, KPS, EORTC-QLQ-C30 and cancer-specific CGA |
| 13 | U.K. | Jan 2015 | Parks, R.M. et al. [25] | Single-centre polit study | Assessing CGA for early cancer breast patients ages 70 and over. | 3 | 47 | ≥70 | Breast | Stage 1, stage 2 | EORTC, QLQ-C30, QLQ-BR23 |
| 14 | France | Jan 2014 | Blanc, M. et al. [16] | Single-centre retrospective study. | Evaluating the impact of GCA on the final therapeutic management of cancer in patients >70. | 3 | 191 | ≥75 | Breast 3.9%, lung 10.5%, colon 17.1% | — | MMSE, Mini GDS, MNA, ADL, iADL, Ki, CCI and CIRS-G |
| 15 | Italy | Feb 2013 | Biganzoli, L. et al. [27] | Single-centre prospective study | Evaluating the role of cardiovascular health in predicting the presence of an abnormality with the CGA screening tool. | 3 | 259 | ≥70 | Breast 50%, colorectal 27% | — | ADL, CRIS-G, GDS, iADL, MMSE, and VES-13 |
| 16 | USA | Nov 2013 | Owusu, C. et al. [26] | Cross-sectional retrospective study | To assess racial differences in functional disability amongst older women with non-metastatic breast cancer | 3 | 581 | ≥65 | Breast | Stage 1–3 | ADL, iADL, MMSE, GDS and CCI |
| 17 | USA | Apr 2012 | Clough-Gorr, K.M. et al. [13] | Multicentre retrospective study | To investigate 5- and 10-year survival based on cancer CGA breast cancer patients amongst older women. | 2 | 660 | ≥65 | Breast | 54% stage 1, 48% stage 2–3A | CCI, KPI, mini GDS, MMSE, MNA, ADL, CIRS-G, iADL, MOS-SF-36 |
| 18 | France | Dec 2011 | Freyer, G. et al. [22] | Multicentre retrospective observational study. | To describe the tolerance of women treated with adjuvant chemotherapy in patients aged >70 years. | 3 | 110 | ≥70 | Breast | — | ADL, MMSE, MNA, GDS |
| GA Domain | Tools Used (% of Studies Using) |
|---|---|
| Functionality | iADL (73.7); ADL (63.1); CCI (31.6); CIRS-G (10.5); ECOG (5.3) |
| Mobility/balance | MOB-T(5.3); LOFS (10.5) |
| Physical | MOS-SF-36 (5.3); ASA (10.5); ESS (5.3) |
| Socioeconomic | KPS (15.8) |
| Psychological | GDS-15 (36.6); MMSE (52.3); VAS (5.3); MHI5 (5.3); SPMSQ (5.3) |
| Nutritional status | MNA (15.8); MNA-SF (5.3) |
| Quality of life | QLQ-C30 (10.5); QLQ-BR23 (10.5) |
| Screening tools | VES-13 (15.8); G8 screening tool (10.5); SOAP (5.3) |
| Study | Author | Findings | Statistical Analysis | Future Directions |
|---|---|---|---|---|
| 1 | Munir, et al. [29] | Self-administered frailty assessment may influence treatment recommendations. The results emphasise the importance and potential benefit of treatment choice in older patients with breast cancer. | Data were analysed using the Pearson’s chi-squared test. As the data were not normally distributed, differences were calculated using the Mann–Whitney U test. A multivariate logistic regression was performed to determine associations between CGA domains and change in treatment decision (p < 0.20). | Future research should focus on incorporating GA results to estimate more accurate the benefits of adjuvant chemotherapy in older patients with breast cancer. |
| 2 | Boulahassass, R. et al. [18] | The scores produced by CGA were decreased from the initial assessment compared with the CGA score performed 6 months later post-treatment, indicating a better quality of life for the patients following the treatment. | Statistical comparisons made using Student’s test or Mann–Whitney test for data that were continuous. All analyses were performed at a 5% alpha risk. Global QoL: baseline median 77.56 (SD 19.97); 1-month median 75.64 (SD 13.73), 3-month median 76.28 (SD 13.06); 6-month median 76.00 (SD 14.5). | Replicate the study on a larger patient cohort size to examine if the results could be replicated. |
| 3 | Quinten, C. et al. [24] | The study concludes that there are strong links between a CGA being undertaken and the patient’s quality of life post-treatment in regard to the treatment regimen they were on. | Correlation between EORTC QLQ-C30 functioning scales and GHS scale. GA measures were analysed at 3 time points using Spearman rank correlation coefficient. p-value was set at p < 0.05 calculated from Wald chi-squared test | CGA should be considered alongside the HRQOL regardless of treatment. |
| 4 | Liuu, E. et al. [15] | MPI based on CGA is shown to improve risk prediction of 1 year mortality and aid in cancer treatment intervention. | The time to event was plotted as Kaplan–Meier survival curve depending on the results of the MPI groups; compositions were then made using the log-rank test. Hazard ratio (HR) and 1-year mortality was determined by Cox proportional hazards. Univariate and multivariate models were used, with adjustments for age, sex and tumour site. | Studies focusing on whether MPI can help to predict survival rate of patients with breast cancer. |
| 5 | Speigl, L. et al. [19] | Fluorescence microscopy was used to investigate clinical outcomes compared to CGA evaluation. Those who the CGA determined to be fitter correlated with those who also have a higher abundance of CD3+ infiltrating cells, indicating better survival rates of the patients. | Correlations were assessed suing the non-parametric two-tailed (Spearman) correlation tests. Differences between groups were assessed with Mann–Whitney U tests, survival analysis was performed using the Kaplan–Meier method, and the log-rank test was applied. Significant relationships were considered at p < 0.05. | Further research includes; identifying patients using CGA that might not be appropriate for normal conventional treatment, but within a shorter time frame than the standard CGA timeframe. |
| 6 | Owusu, C. et al. [28] | CGA can be used as an initial indicator for cancer treatment to determine a poor physical performance prior to breast cancer treatment. CGA correctly indicated that those of African American (AA) ethnicity would have a poorer outcome compared to their white counterparts. | The chi-squared test or Fisher’s exact test was used to determine statistically significant results in the distribution between baselines of two groups. Univariate regression was used to examine the relation of race and other variables to determine statistical significance for poor physical status (p < 0.10). | Further research to investigate whether financial toxicity could also play a part in poorer outcomes with those from an AA heritage. |
| 7 | Falandry, C. et al. [17] | The study concludes that there has been an increase in the amount of CGA to aid with the treatment decision-making process. CGA can aid in determining which treatment regime might be optimal for patients over a certain age range. | Data were collected between October 4 and November 8 2011 by either face-face interviews or questionnaires. Qualitative data were presented as percentage and quantitative data were described as averages (mean, median, standard deviation, and range). Chi-squared test was used to compare baseline variables p < 0.05. | Further research is required to investigate the validity of CGA regimens that are being used. |
| 8 | Okonji, D. et al. [20] | CGA used to assess high risk of elderly women who would normally be in receipt of adjuvant chemotherapy, but were offered primary surgery, as the CGA predicted a better survival rate and outcome. | Two tailed p-values were calculated using Fisher’s exact test and p < 0.05 was considered significant. p-values as follows: breast surgery 0.0002, axillary surgery 0.0340, adjuvant radiotherapy 0.8195, chemotherapy 0.0001, HER2 positive (trastuzumab) 0.7451, ER-positive 0.7451. | Investigating the survival rate of women over 70 years old, who undergo primary surgery but do not receive chemotherapy. |
| 9 | Bailur, J.K. et al. [21] | CGA can be used as a fragility marker to measure the patient’s progression over the course of their treatment. | The Kruskal–Wallis test was used to compare biomarkers between more than two groups. The Mann–Whitney U test was used to compare data between two groups and Fisher’s exact test was used to determine the association between CMV and other variables. The study was a hypothesising study, and as such did not necessitate correction for multiple testing. All p-values were exploratory. | The immune biomarkers identified could be used in future research to better guide therapeutic management of patients with breast cancer. |
| 10 | Guerad, E.J. et al. [14] | There is a need to increase awareness of falls prevalence and consequences among oncology providers in order to provide timely interventions to reduce the risks associated with falls. | Percentages and frequencies were reported: 10% of patients had falls documented, 20% of patients had their gait assessed, 6% were referred for further assessment and 17% had vitamin D levels measured. | To increase oncologist awareness of the greater chance of falls for older patients with cancer. Implementing a falls assessment within the CGA. |
| 11 | Stotter, A. et al. [12] | CGA was shown to indicate good survival rates. Poor CGA was associated with a reduced survival score. CGA was recommended to complete before treatment commences and to aid with therapeutic choice. | The study’s characteristics were described using mean, median, range and percentages. The risk score was derived using logistic regression, by calculating probability of death within 3 years from the intercept and β-coefficients from all elements of the CGA. The Charlson index was used to develop the final risk score. | A larger prospective patient cohort on CGA should be conducted to help improve the assistance of treatment decision-making. |
| 12 | Denkinger, M.D. et al. [23] | CGA and frailty score were better indicators at predicting fatigue in a group compared to other variables in women with primary breast cancer. | Descriptive baseline statistics were analysed. Variables that were included in the models were chosen depending on their univariate correlation with both outcomes. | Further research to compare the current assessment to different outcomes, different time points, and different populations with alternative functional states. |
| 13 | Parks, R.M. et al. [25] | The study confirmed the feasibility of using CGA in a research setting. | Categorical data were described using percentages and frequency. Chi-squared test used to compare patient characteristics. Fisher’s exact test was used for smaller samples. T test was used for normal data distributed around the mean or Mann–Whitney test for data not normally distributed. All tests considered p < 0.05 to be significant. | More data will need to be gathered to definitively determine whether the components are required for a CGA. |
| 14 | Blanc, M. et al. [16] | CGA should be used alongside the oncologist to aid treatment regime. | Qualitative variables were described as percentages. Quantitative variables were described as means (SD), medians and ranges. Comparative analyses were determined with chi-squared test, Fisher’s exact test or Cochran–Armitage trend test. Relationship between mortality and comorbidity was assessed using univariate Cox regression analysis. | Conducting a prospective multicentre randomised studies to determine the impact of CGA to aid in potential treatment options. |
| 15 | Biganzoli, L. et al. [27] | Evaluating whether CHS can be used in the GCA. The use of CHS for CGA may be limited due to the stage of the disease of patients with breast cancer. | Patient characteristics were described using percentages and frequencies. 250 patients were recruited to provide a two-sided 95% confidence interval for accuracy of estimates with an equal width of 0.15 or closer. This was assuming the prevalence of impairment was equal to 60%. | Investigating whether CHS can replace VES-13 screening tool in a CGA. |
| 16 | Owusu, C. et al. [26] | Functional disability in older women with early breast cancer is highly prevalent in African American women compared to women of other races. | Bivariate analysis of all variables by race. The chi-squared test or Fisher’s exact test was used to determine statistically significant differences between the two groups. All p-values calculated were two-sided. | Investigating whether interventions to optimise functional status of at-risk groups (African American women) during and after cancer treatment is needed to help improve treatment tolerance and overall survival. |
| 17 | Clough-Gorr, K.M. et al. [13] | 5- and 10-year survival rates can be indicated by a CGA being conducted. CGA should be used to aid in treatment regime regardless of age and stage of disease. | Descriptive statistics on all study variables were calculated. Bivariate distributions were evaluated between independent and mortality outcomes using Spearman correlations, chi-squared, log-rank test and Cochran–Armitage test. Five- and ten-year survival was analysed using Kaplan–Meier. Cox proportional hazards were used to predict five- and ten-year all-cause and breast-cancer-specific mortality. | Investigating the survival rate using C-SGA in various populations of older adults with different cancer types. |
| 18 | Freyer, G. et al. [22] | CGA is a useful tool that can help predict chemotherapy toxicities; however, performing CGA can be limited to geriatricians being available, specifically when treating older patients. | Data were descriptive in nature (mean and standard deviation for continuous data). Frequencies and percentages were calculated for categorical data. 95% confidence intervals were calculated when relevant. | Investigating the collaborative effect of performing CGA alongside the oncologist and geriatrician. |
References
- Office for National Statistics. Cancer Registration Statistics, England: 2017. 2017. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancerregistrationstatisticsengland/2017 (accessed on 29 July 2021).
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef]
- National Institute for Health Care and Excellence. Early and Locally Advanced Breast Cancer: Diagnosis and Management. Available online: https://www.nice.org.uk/guidance/ng101 (accessed on 1 April 2022).
- Biganzoli, L.; Battisti, N.M.L.; Wildiers, H.; McCaartney, A.; Colloca, G.; Kunkler, I.H.; Cardosos, M.J.; Cheung, K.L.; Aafke de Glass, N.; Trimboli, R.M.; et al. Updated recommendations regarding the management of older patients with breast cancer: A joint paper from the European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG). Lancet Oncol. 2021, 22, e327–e340. [Google Scholar] [CrossRef]
- National Audit of Breast Cancer in Older Patients. Part of the National Clinical Audit and Patients Outcomes Programme. 2020 Annual Report; The National Audit of Breast Cancer in Older Patients, Clinical Effectiveness Unit, The Royal College of Surgeons of England: London, UK.
- Taira, N.; Sawaki, M.; Takahashi, M.; Shimozuma, K. Comprehensive geriatric assessment in elderly breast cancer patients. Breast Cancer 2010, 17, 183–189. [Google Scholar] [CrossRef]
- International Society of Geriatric Oncology. Comprehensive Geriatric Assessment (CGA) of the Older Patient with Cancer. Available online: https://www.siog.org/content/comprehensive-geriatric-assessment-cga-older-patient-cancer (accessed on 5 September 2021).
- Wildiers, H.; Heeran, P.; Puts, M.; Topinkova, E.; Janssen-Heijen, M.L.G.; Extermann, M.; Falandry, C.; Artz, A.; Brain, E.; Colloca, G.; et al. International society of geriatric oncology consensus on geriatric assessment in older patients with cancer. J. Clin. Oncol. 2014, 32, 2595–2603. [Google Scholar] [CrossRef]
- Transparent Reporting of Systematic Reviews and Meta-Analyses. Available online: http://www.prisma-statement.org (accessed on 10 October 2021).
- Parks, R.M.; Lakshamanan, R.; Winterbottom, L.; Morgan, D.A.L.; Cox, K.; Cheung, K.L. Comprehensive geriatric assessment for older women with early breast cancer—A systematic review of literature. World J. Surg. Oncol. 2012, 10, 88. [Google Scholar] [CrossRef]
- Biganzoli, L.; Marotti, L.; Hart, C.D.; Cataliotti, L.; Cutuli, B.; Kukn, T.; Mansel, R.E.; Pointi, A.; Poormans, P.; Regitnig, P.; et al. Quality indicators in breast cancer care: An update from the EUSOMA working group. Eur. J. Cancer 2017, 86, 59–81. [Google Scholar] [CrossRef]
- Stotter, A.; Reed, M.W.; Gray, L.J.; Moor, N.; Robinson, T.G. Comprehensive Geriatric Assessment and predicted 3-year survival in treatment planning for frail patients with early breast cancer. Br. J. Surg. 2015, 102, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Clough-Gorr, K.M.; Thwin, S.S.; Stuck, A.E.; Silliman, R.A. Examining five- and ten-year survival in older women with rbeast cancer using cancer-sepciifc geriatric assessmeant (CGA). Eur. J. Cancer 2012, 48, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Guerard, E.J.; Deal, A.M.; Williams, G.R.; Jolly, T.A.; Nyrop, K.A.; Muss, H.B. Falls in Older Adults with Cancer: Evaluation by Oncology Providers. J. Oncol. Pract. 2015, 11, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Liuu, E.; Hu, C.; Valero, S.; Brunet, T.; Jamet, A.; Bureau, M.L.; Pilotto, A.; Saulnier, P.J.; Paccalin, M. Comprehensive geriatric assessment in older patients with cancer: An external validation of the multidimensional prognostic index in a French prospective cohort study. BMJ Geriatr. 2020, 18, 295. [Google Scholar] [CrossRef] [PubMed]
- Blanc, M.; Dialla, O.; Manckoundia, P.; Arveux, P.; Dabakuyo, S.; Quipourt, V. Influence of the geriatric oncology consolation on the final therapeutic decision in elderly subjects with cancer: Analysis of the 191 patients. J. Nutr. Health Aging 2014, 18, 76–82. [Google Scholar] [CrossRef]
- Falandry, C.; Krakowski, I.; Cure, H.; Carola, E.; Soubeyran, P.; Guerin, O.; Gaudin, H.; Freyer, G. Impact of geriatric assessment for the therapeutic decision-making of breast cancer: Results of a French survey. AFSOS and SOFOG collaborative work. Breast Cancer Res. Treat. 2018, 168, 433–441. [Google Scholar] [CrossRef]
- Boulahassass, R.; Chand, M.E.; Cal, J.; Dittlot, C.; Schiappa, R.; Rambaud, C.; Gonfrier, S.; Guerin, O.; Hannoun-Levi, J.M. Quality of life and comprehensive geriatric assessment (CGA) in older adults receiving acerlated partial breat irradiation (APBI) using a single fraction of multi0catherter interstitial high dose-rate brachytherapy (MIB). The SiFEBI phase I/II trial. J. Geriatr. Oncol. 2021, 12, 1085–1091. [Google Scholar] [CrossRef]
- Speigl, L.; Grieb, A.; Janssen, N.; Hatse, S.; Brouwers, B.; Smeets, A.; Floris, G.; Bailur, J.K.; Kenis, C.; Neven, P.; et al. Low levels of intra-tumoural T cells in breast cancer identify clinically frail patients with shorter disease-specific survival. J. Geriatr. Oncol. 2018, 9, 606–612. [Google Scholar] [CrossRef]
- Okonji, D.O.; Sinha, R.; Philips, I.; Fats, D.; Ring, A. Comprehensive geriatric assessment in 326 older women with early breast cancer. Br. J. Cancer 2017, 117, 925–931. [Google Scholar] [CrossRef]
- Bailur, J.K.; Pawelec, G.; Hatse, S.; Brouwer, B.; Smeets, A.; Neven, P.; Laenen, A.; Wildiers, H.; Shipp, C. Immune profiles of elderly breast cancer patients are altered by chemotherapy and relate to clinical frailty. Breast Cancer Res. 2017, 19, 20. [Google Scholar] [CrossRef]
- Freyer, G.; Campone, M.; Peron, J.; Facchini, T.; Terret, C.; Berdah, J.F.; Jacquin, J.P.; Coeffic, D.; de Saint Hilaire, P.; Falandry, C. Adjacent docetaxel/cyclophosphamide in breast cancer patients over the age of 70: Results of an observational study. Crit. Rev. Oncol. Hematol. 2011, 80, 466–473. [Google Scholar] [CrossRef]
- Denkinger, M.D.; Hasch, M.; Gerstmayer, A.; Kreienberg, R.; Nikolaus, T.; Hancke, K. Predicting fatigure in older breast cancer patients recieging radiotherapy. A head-to head- comparison of established assessments. Z. Gerontol. Geriatr. 2015, 48, 128–134. [Google Scholar] [CrossRef]
- Quinten, C.; Kenis, C.; Hamaker, M.; Coolbrandt, A.; Brouwers, B.; Dal Lago, L.; Neven, P.; Vuylsteke, P.; Debrock, G.; van Den Bulck, H.; et al. The added value of geriatric assessment in evaluating a patient’s Health-Related Quality-of-Life: A study in ≥70-year-old early-stage invasive breast cancer patients. Eur. J. Cancer Care 2020, 29, e13278. [Google Scholar] [CrossRef]
- Parks, R.M.; Hall, L.; Tnag, S.W.; Howard, P.; Lakshmanan, R.; Winterbottom, L.; Morgan, D.A.; Porock, D.; Cox, K.; Cheung, K.L. The potential value of comprehensive geriatric assessment in evaluation older women with primary operable breast cancer undergoing surgery or non-operative treatment—A pilot study. J. Geriatr. Oncol. 2015, 6, 46–51. [Google Scholar] [CrossRef]
- Owusu, C.; Schluchter, M.; Koroukian, S.M.; Mazhuvanchery, S.; Berger, N.A. Racial Disparities in Functional Disability among Older Women with Newly Diagnosed Non-metastatic Breast Cancer. Cancers 2013, 119, 3839–3846. [Google Scholar] [CrossRef]
- Biganzoli, L.; Boni, L.; Becheri, D.; Zafarana, E.; Biaginoni, C.; Cappadona, S.; Bianchini, E.; Oakman, C.; Magnolia, S.U.; di Leo, A.; et al. Evaluation of the cardiovascular health study (CHS) instrument and the vulnerable elders sruvery-13 (VES-13) in elderly cancer patients. Are we still missing the right screening tool? Ann. Oncol. 2013, 24, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Owusu, C.; Schluchter, M.; Koroukian, S.M.; Schmitz, K.H.; Berger, N.A. Black-white disparity in physical performance among older women with newly diagnosed non-metastatic breast cancer: Exploring the role of inflammation and physical activity. J. Geriatr. Oncol. 2018, 9, 613–619. [Google Scholar] [CrossRef]
- Munir, A.; Huws, A.; Khan, S.; Sharaiha, Y.; Holt, S.; Khawaja, S. Geriatric assessment tool application in treatment recommendations for older women with breast cancer. Breast 2022, 63, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.A.; Parks, R.M.; Cheung, K.L. The impact of breast cancer surgery on functional status in older women—A systematic review of the literature. Eur. J. Surg. Oncol. 2021, 47, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Chia, Z.; Parks, R.M.; Cheung, K.L. Does breast cancer surgery impact functional status and independence in older patients? A narrative review. Oncol. Ther. 2021, 9, 373–383. [Google Scholar] [CrossRef]
- Pieratoni, F.; Basso, U.; Maruzzo, M.; Lamberti, E.; Bimbatti, D.; Tierno, G.; Bergo, E.; Brunello, A.; Zagonel, V. Comprehensive geriatric assessment is an independent prognostic factor in older patients with metastatic renal cell cancer treated with first-line Sunitinib or Pazopanib: A single center experience. J. Geriatr. Oncol. 2021, 12, 290–297. [Google Scholar] [CrossRef]
- Bai, J.F.; Han, H.X.; Li, J.T.; Feng, R.; Wang, T.; Zhang, C.L.; Liu, H. Comprehensive Geriatric Assessment (CGA): A Simple Tool for Guiding the Treatment of Older Adults with Diffuse Large B-Cell Lymphoma in China. Oncologist 2020, 25, e1202–e1208. [Google Scholar] [CrossRef] [PubMed]
- Sourdet, S.; Brechemier, D.; Steinmeye, Z.; Gerad, S.; Balardy, L. Impact of the comprehensive geriatric assessment on treatment deicion in geriatric oncology. BMC Cancer 2020, 20, 384. [Google Scholar] [CrossRef]
- Dura-Ferrandis, E.; Mandelblatt, J.S.; Clapp, J.; Luta, G.; Faul, L.; Kimmick, G.; Cohen, H.J.; Young, R.L.; Hurria, A. Personality, coping, and social support as predictors of long-term quality-of-life trajectories in older breast cancer survivors: CALGB protocol 369901 (Alliance). Psychooncology 2017, 26, 1914–1921. [Google Scholar] [CrossRef]
- Mandelblatt, J.S.; Cai, L.; Luta, G.; Kimmick, G.; Clapp, J.; Isaacs, C.; Pitcher, B.; Barry, W.; Winer, E.; Sugarman, S.; et al. Frailty and long-term mortality of older breast cancer patients: CALGB 369901 (Alliance). Breast Cancer Res. Treat. 2017, 164, 107–117. [Google Scholar] [CrossRef]
- Lawhon, V.M.; England, R.E.; Wallace, A.S.; Williams, C.P.; Williams, B.R.; Niranjan, S.J.; Ingram, S.A.; Roque, G.B. “It’s important to me”: A qualitative analysis on shared decision-making and patient preferences in older adults with early-stage breast cancer. Psychooncology 2021, 30, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.; Kowalski, T.L.; Chang, C.H. Quality of life assessment in women with breast cancer: Benefits, acceptability and utilization. Health Qual. Life Outcomes 2007, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Torres-Hernadez, C.; Hsu, T. Comprehensive Geriatric Assessment in the Older Adult with Cancer: A Review. Eur. Urol. Focus 2017, 3, 330–339. [Google Scholar] [CrossRef]
- Liuu, E.; Calliet, P.; Cure, H.; Anfasi, N.; de Decker, L.; Pamoukdijan, F.; Canoui-Portrine, R.; Soubeyran, P.; Paillaud, E. Comprehensive geriatric assessment (CGA) in elderly with cancer: For whom? Rev. Med. Interne 2016, 37, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Kenig, J.; Szabat, K.; Mitus, J.; Mitus-Kenig, M.; Kreszowiak, J. Usefulness of eight screening tools for predicting frailty and postoperative short- and long-term outcomes among older patients with cancer who qualify for abdominal surgery. Eur. J. Surg. Oncol. 2020, 46, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reid-Agboola, C.; Klukowska, A.; Malcolm, F.L.; Harrison, C.; Parks, R.M.; Cheung, K.-L. Comprehensive Geriatric Assessment for Older Women with Early-Stage (Non-Metastatic) Breast Cancer—An Updated Systematic Review of the Literature. Curr. Oncol. 2023, 30, 8294-8309. https://doi.org/10.3390/curroncol30090602
Reid-Agboola C, Klukowska A, Malcolm FL, Harrison C, Parks RM, Cheung K-L. Comprehensive Geriatric Assessment for Older Women with Early-Stage (Non-Metastatic) Breast Cancer—An Updated Systematic Review of the Literature. Current Oncology. 2023; 30(9):8294-8309. https://doi.org/10.3390/curroncol30090602
Chicago/Turabian StyleReid-Agboola, Chantae, Anita Klukowska, Francesca L. Malcolm, Cora Harrison, Ruth M. Parks, and Kwok-Leung Cheung. 2023. "Comprehensive Geriatric Assessment for Older Women with Early-Stage (Non-Metastatic) Breast Cancer—An Updated Systematic Review of the Literature" Current Oncology 30, no. 9: 8294-8309. https://doi.org/10.3390/curroncol30090602
APA StyleReid-Agboola, C., Klukowska, A., Malcolm, F. L., Harrison, C., Parks, R. M., & Cheung, K.-L. (2023). Comprehensive Geriatric Assessment for Older Women with Early-Stage (Non-Metastatic) Breast Cancer—An Updated Systematic Review of the Literature. Current Oncology, 30(9), 8294-8309. https://doi.org/10.3390/curroncol30090602






