Managing the Risk of Lung Toxicity with Trastuzumab Deruxtecan (T-DXd): A Canadian Perspective
Abstract
:1. Introduction
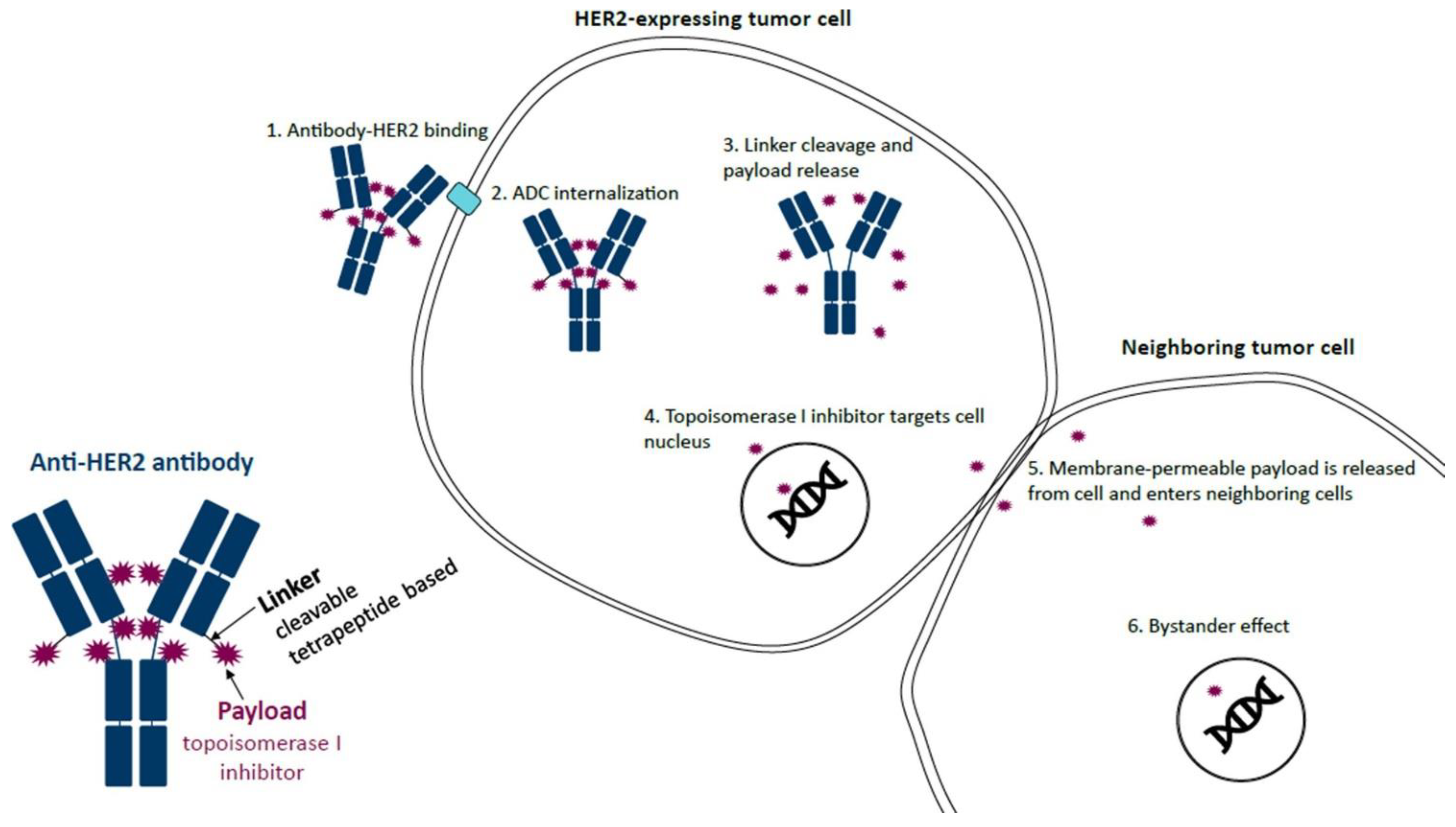
2. Indications for T-DXd in Canada
3. Known Risk Factors for DI-ILD
4. DI-ILD with Specific Anticancer Treatments
5. T-DXd and the Risk of ILD
6. Diagnosis and Monitoring of T-DXd-Related ILD
6.1. Key Investigations
6.1.1. Medical History
6.1.2. Monitoring for Symptoms
6.1.3. Pulmonary Function Testing (PFT)
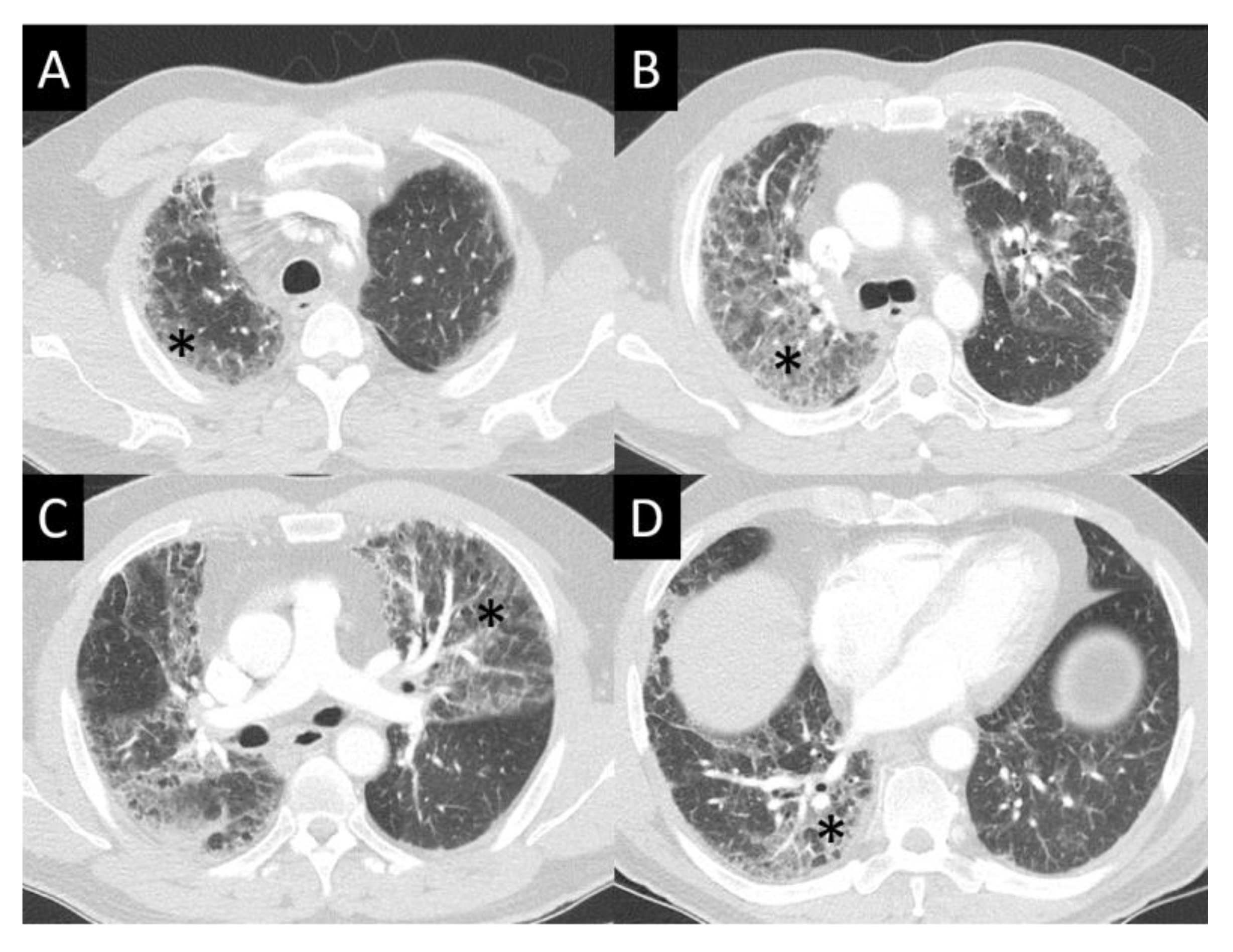
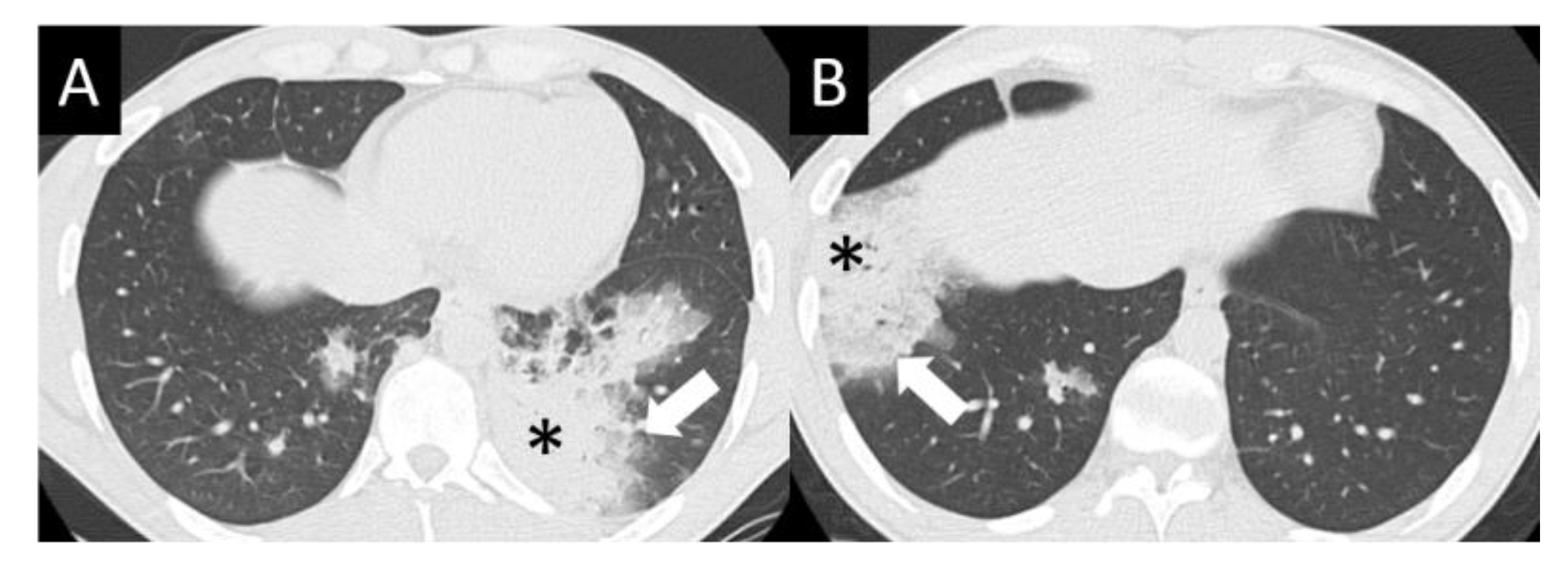
6.1.4. Chest Imaging
6.2. Additional Investigations
- Summary: Key Investigations for Patients Undergoing Treatment with T-DXd
- ●
- Conduct a history and physical examination at baseline with the focus on known T-DXd-related ILD risk factors;
- ●
- Ensure patient undergoes education and regular reminders concerning the potential adverse events associated with T-DXd and the need to immediately report symptoms, e.g., cough (particularly dry cough), shortness of breath/exertional dyspnea, fever, and unexplained fatigue;
- ●
- Pulse oximetry (SpO2) should be performed at each clinic assessment, and an SpO2 < 95% or drop of more than 4% from baseline should prompt a detailed respiratory assessment;
- ●
- Conduct baseline staging chest CT and restaging CT chest surveillance every 9–12 weeks:
- ⚬
- Notify the radiologist to read the CT for both assessment of tumor response, as well as screening for ILD;
- ⚬
- Conduct an HRCT promptly to confirm the diagnosis if ILD is suspected on the restaging CT but not confidently diagnosed or if ILD is clinically suspected but restaging is not required.
- ●
- Patients with infectious/inflammatory opacities on CT scan should be considered for further evaluation to elucidate the cause and severity of these abnormalities:
- ⚬
- Sputum for routine culture, acid-fast bacilli, and fungus; blood work to look for markers of inflammation and infection; beta-natriuretic peptide, echocardiogram, and PFT; consultation with infectious diseases or respirology; and bronchoscopy should all be considered in the appropriate clinical scenario.
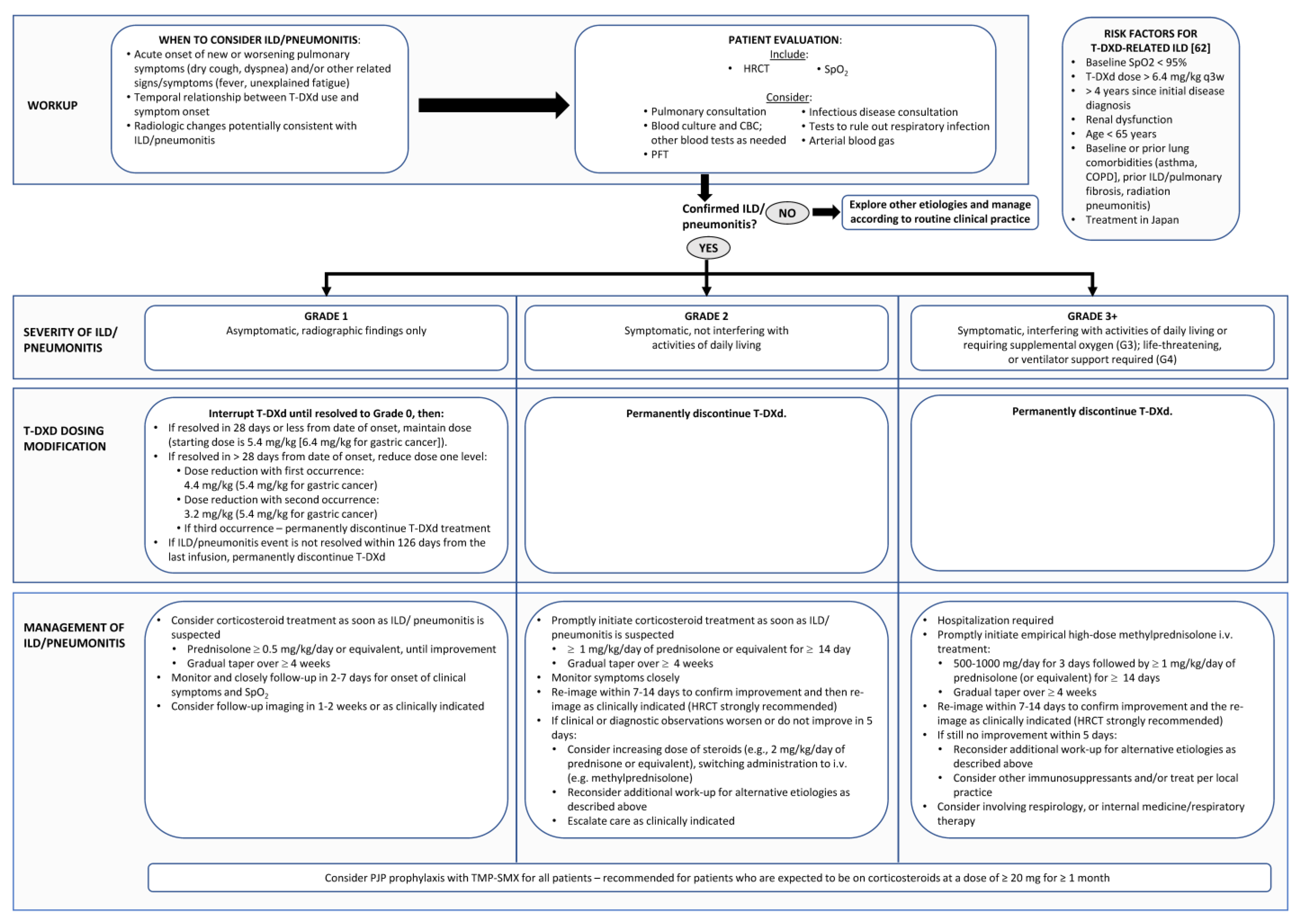
7. Management of T-DXd—Related ILD
7.1. Grade 1/Asymptomatic ILD
7.2. Grade 2 ILD
7.3. Grade 3 or 4 ILD
- Summary: Management of T-DXd-Related DI-ILD
- Grade 1 DI-ILD
- ●
- Interrupt T-DXd until resolved to grade 0 (resolution of CT abnormalities), then:
- ⚬
- If resolved in ≤28 days from date of onset, maintain dose (starting dose is 5.4 mg/kg (6.4 mg/kg for gastric cancer));
- ⚬
- If resolved in >28 days from date of onset, reduce dose one level:
- ●
- Dose reduction with first occurrence: 4.4 mg/kg (5.4 mg/kg for gastric cancer);
- ●
- Dose reduction with second occurrence: 3.2 mg/kg (4.4 mg/kg for gastric cancer).
- ⚬
- Permanently discontinue T-DXd if there is a third recurrence;
- ⚬
- Permanently discontinue T-DXd if the grade 1 ILD/pneumonitis event has not resolved within 18 weeks (126 days) from the last infusion.
- ●
- Consider prednisolone ≥ 0.5 mg/kg/day or equivalent with a gradual taper over ≥4 weeks, until improvement; *
- ●
- Monitor and closely follow up in 2–7 days for onset of clinical symptoms and SpO2;
- ●
- Consider follow-up imaging in 1–2 weeks or as clinically indicated.
- Grade 2 DI-ILD
- ●
- Permanently discontinue T-DXd;
- ●
- Promptly initiate corticosteroid treatment as soon as ILD/pneumonitis is suspected: *
- ⚬
- A total of 1 mg/kg/day of prednisolone or equivalent for ≥14 days;
- ⚬
- Gradually taper over ≥4 weeks.
- ●
- Monitor symptoms closely;
- ●
- Re-image with HRCT within 7–14 days to confirm improvement and then re-image as clinically indicated;
- ●
- If clinical or radiographic worsening or still no improvement (especially within 5 days):
- ⚬
- Consider increasing dose of steroids (e.g., 2 mg/kg/day of prednisolone or equivalent), switching administration to i.v. (e.g., methylprednisolone); *
- ⚬
- Reconsider additional work-up for alternative etiologies, as described above;
- ⚬
- Escalate care as clinically indicated.
- Grade 3+ DI-ILD
- ●
- Permanently discontinue T-DXd;
- ●
- Hospitalization required;
- ●
- Promptly initiate empirical high-dose methylprednisolone IV treatment: *
- ⚬
- Give 500–1000 mg/day for 3 days followed by ≥1 mg/kg/day of prednisolone (or equivalent) for ≥14 days;
- ⚬
- Gradually taper over ≥4 weeks
- ●
- Re-image with HRCT within 7–14 days to confirm improvement and then re-image as clinically indicated;
- ●
- If clinical or radiographic worsening or still no improvement (especially within 5 days):
- ⚬
- Reconsider additional work-up for alternative etiologies, as described above;
- ⚬
- Consider other immunosuppressants and/or treat per local practice.
- ●
- Consider involvement of respirology or internal medicine.
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. DESTINY-Breast01 Investigators. Trastuzumab deruxtecan in previously treated Her2-positive breast cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- André, F.; Hee Park, Y.; Kim, S.B.; Takano, T.; Im, S.A.; Borges, G.; Lima, J.P.; Aksoy, S.; Gavila Gregori, J.; De Laurentiis, M.; et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 401, 1773–1785. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Hegg, R.; Chung, W.P.; Im, S.A.; Jacot, W.; Ganju, V.; Chiu, J.W.Y.; Xu, B.; Hamilton, E.; Madhusudan, S.; et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: Updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 2023, 401, 105–117. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. DESTINY-Breast04 Trial Investigators. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Swain, S.M.; Nishino, M.; Lancaster, L.H.; Li, B.T.; Nicholson, A.G.; Bartholmai, B.J.; Naidoo, J.; Schumacher-Wulf, E.; Shitara, K.; Tsurutani, J.; et al. Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)-related interstitial lung disease/pneumonitis-Focus on proactive monitoring, diagnosis, and management. Cancer Treat. Rev. 2022, 106, 102378. [Google Scholar] [CrossRef]
- Rugo, H.S.; Bianchini, G.; Cortes, J.; Henning, J.W.; Untch, M. Optimizing treatment management of trastuzumab deruxtecan in clinical practice of breast cancer. ESMO Open 2022, 7, 100553. [Google Scholar] [CrossRef]
- Enhertu (Trastuzumab Deruxtecan for Injection) Product Monograph; AstraZeneca Canada Inc.: Mississauga, ON, Canada, 2023.
- Manich, S.C.; Modi, S.; Krop, I.; Park, Y.H.; Kim, S.; Tamura, K.; André, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab deruxtecan (T-DXd) in patients with HER2-positive metastatic breast cancer (MBC): Updated survival results from a phase II trial (DESTINY-Breast01). Ann. Oncol. 2021, 32 (Suppl. S5), S486. [Google Scholar]
- Shitara, K.; Bang, Y.J.; Iwasa, S.; Sugimoto, N.; Ryu, M.H.; Sakai, D.; Chung, H.C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. DESTINY-Gastric01 Investigators. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef]
- Ku, G.Y.; Di Bartolomeo, M.; Smyth, E.; Chau, I.; Park, H.; Siena, S.; Lonardi, S.; Wainberg, Z.A.; Ajani, J.A.; Chao, J.; et al. 1205MO Updated analysis of DESTINY-Gastric02: A phase II single-arm trial of trastuzumab deruxtecan (T-DXd) in western patients (Pts) with HER2-positive (HER2+) unresectable/metastatic gastric/gastroesophageal junction (GEJ) cancer who progressed on or after trastuzumab-containing regimen. Ann. Oncol. 2022, 33, S1100. [Google Scholar]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. DESTINY-Lung01 Trial Investigators. Trastuzumab deruxtecan in Her2-mutant non-small-cell lung cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef]
- Smit, E.F.; Nakagawa, K.; Nagasaka, M.; Felip, E.; Goto, Y.; Li, B.T.; Pacheco, J.M.; Murakami, H.; Barlesi, F.; Saltos, A.N.; et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2-mutated metastatic non-small cell lung cancer (NSCLC): Interim results of DESTINY-Lung01. J. Clin. Oncol. 2020, 38 (Suppl. S15), 9504. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Trastuzumab Deruxtecan in Participants with HER2-Mutated Metastatic Non-Small Cell Lung Cancer (NSCLC) (DESTINY-LUNG02). Available online: https://clinicaltrials.gov/ct2/show/NCT04644237 (accessed on 12 May 2023).
- ClinicalTrials.gov. A Phase 2 Study of T-DXd in Patients With Selected HER2 Expressing Tumors (DPT02). Available online: https://clinicaltrials.gov/ct2/show/NCT04482309 (accessed on 9 February 2023).
- ClinicalTrials.gov. Trastuzumab Deruxtecan in Participants with HER2-Overexpressing Advanced or Metastatic Colorectal Cancer (DESTINY-CRC02). Available online: https://clinicaltrials.gov/ct2/show/NCT04744831 (accessed on 9 February 2023).
- Kreuter, M.; Herth, F.J.; Wacker, M.; Leidl, R.; Hellmann, A.; Pfeifer, M.; Behr, J.; Witt, S.; Kauschka, D.; Mall, M.; et al. Exploring clinical and epidemiological characteristics of interstitial lung diseases: Rationale, aims, and design of a nationwide prospective registry—The EXCITING-ILD Registry. Biomed. Res. Int. 2015, 2015, 123876. [Google Scholar] [CrossRef]
- Camus, P.; Bonniaud, P.; Fanton, A.; Camus, C.; Baudaun, N.; Foucher, P. Drug-induced and iatrogenic infiltrative lung disease. Clin. Chest Med. 2004, 25, 479–519. [Google Scholar] [CrossRef] [PubMed]
- Skeoch, S.; Weatherley, N.; Swift, A.J.; Oldroyd, A.; Johns, C.; Hayton, C.; Giollo, A.; Wild, J.M.; Waterton, J.C.; Buch, M.; et al. Drug-induced interstitial lung disease: A systematic review. J. Clin. Med. 2018, 7, 356. [Google Scholar] [CrossRef] [PubMed]
- Osawa, M.; Kudoh, S.; Sakai, F.; Endo, M.; Hamaguchi, T.; Ogino, Y.; Yoneoka, M.; Sakaguchi, M.; Nishimoto, H.; Gemma, A. Clinical features and risk factors of panitumumab-induced interstitial lung disease: A postmarketing all-case surveillance study. Int. J. Clin. Oncol. 2015, 20, 1063–1071. [Google Scholar] [CrossRef]
- Sakurada, T.; Kakiuchi, S.; Tajima, S.; Horinouchi, Y.; Okada, N.; Nishisako, H.; Nakamura, T.; Teraoka, K.; Kawazoe, K.; Yanagawa, H.; et al. Characteristics of and risk factors for interstitial lung disease induced by chemotherapy for lung cancer. Ann. Pharmacother. 2015, 49, 398–404. [Google Scholar] [CrossRef]
- Schwaiblmair, M.; Behr, W.; Haeckel, T.; Märkl, B.; Foerg, W.; Berghaus, T. Drug induced interstitial lung disease. Open Respir. Med. J. 2012, 6, 63–74. [Google Scholar] [CrossRef]
- Yonemori, K.; Hirakawa, A.; Kawachi, A.; Kinoshita, F.; Okuma, H.; Nishikawa, T.; Tamura, K.; Fujiwara, Y.; Takebe, N. Drug induced interstitial lung disease in oncology phase I trials. Cancer Sci. 2016, 107, 1830–1836. [Google Scholar] [CrossRef]
- Vansteenkiste, J. Nivolumab for NSCLC in Japanese patients: Similar benefits, but beware of pneumonitis. ESMO Open 2017, 2 (Suppl. S1), e000119. [Google Scholar] [CrossRef]
- Vial-Dupuy, A.; Sanchez, O.; Douvry, B.; Guetta, L.; Juvin, K.; Wermert, D.; Guérot, E.; Israël-Biet, D. Outcome of patients with interstitial lung disease admitted to the intensive care unit. Sarcoidosis Vasc. Diffuse Lung Dis. 2013, 30, 134–142. [Google Scholar]
- Park, S.Y.; Lim, S.Y.; Um, S.W.; Koh, W.J.; Chung, M.P.; Kim, H.; Kwon, O.J.; Park, H.K.; Kim, S.J.; Im, Y.H.; et al. Outcome and predictors of mortality in patients requiring invasive mechanical ventilation due to acute respiratory failure while undergoing ambulatory chemotherapy for solid cancers. Support. Care Cancer 2013, 21, 1647–1653. [Google Scholar] [CrossRef]
- Chikura, B.; Lane, S.; Dawson, J.K. Clinical expression of leflunomide-induced pneumonitis. Rheumatology 2009, 48, 1065–1068. [Google Scholar] [CrossRef]
- Mankikian, J.; Favelle, O.; Guillon, A.; Guilleminault, L.; Cormier, B.; Jonville-Béra, A.P.; Perrotin, D.; Diot, P.; Marchand-Adam, S. Initial characteristics and outcome of hospitalized patients with amiodarone pulmonary toxicity. Respir. Med. 2014, 108, 638–646. [Google Scholar] [CrossRef]
- Sato, T.; Inokuma, S.; Sagawa, A.; Matsuda, T.; Takemura, T.; Otsuka, T.; Saeki, Y.; Takeuchi, T.; Sawada, T.; Study Committee for Leflunomide-induced Lung Injury; et al. Factors associated with fatal outcome of leflunomide-induced lung injury in Japanese patients with rheumatoid arthritis. Rheumatology 2009, 48, 1265–1268. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, N.; Suzuki, T.; Nagashima, M.; Kon, A.; Kakihata, K.; Gemma, A. Clarification of clinical features of interstitial lung disease induced by irinotecan based on postmarketing surveillance data and spontaneous reports. Anti-Cancer Drugs 2011, 22, 563–568. [Google Scholar] [CrossRef]
- Kubo, K.; Azuma, A.; Kanazawa, M.; Kameda, H.; Kusumoto, M.; Genma, A.; Saijo, Y.; Sakai, F.; Sugiyama, Y.; Tatsumi, K.; et al. Japanese Respiratory Society Committee for formulation of Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respir. Investig. 2013, 51, 260–277. [Google Scholar] [CrossRef] [PubMed]
- Sleijfer, S. Bleomycin-induced pneumonitis. Chest 2001, 120, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Dabydeen, D.A.; Jagannathan, J.P.; Ramaiya, N.; Krajewski, K.; Schutz, F.A.; Cho, D.C.; Pedrosa, I.; Choueiri, T.K. Pneumonitis associated with mTOR inhibitors therapy in patients with metastatic renal cell carcinoma: Incidence, radiographic findings and correlation with clinical outcome. Eur. J. Cancer 2012, 48, 1519–1524. [Google Scholar] [CrossRef]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A., 3rd; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef]
- Akamatsu, H.; Inoue, A.; Mitsudomi, T.; Kobayashi, K.; Nakagawa, K.; Mori, K.; Nukiwa, T.; Nakanishi, Y.; Yamamoto, N. Interstitial lung disease associated with gefitinib in Japanese patients with EGFR-mutated non-small-cell lung cancer: Combined analysis of two phase III trials (NEJ 002 and WJTOG 3405). Jpn. J. Clin. Oncol. 2013, 43, 664–668. [Google Scholar] [CrossRef]
- Hotta, K.; Kiura, K.; Takigawa, N.; Yoshioka, H.; Harita, S.; Kuyama, S.; Yonei, T.; Fujiwara, K.; Maeda, T.; Aoe, K.; et al. Comparison of the incidence and pattern of interstitial lung disease during erlotinib and gefitinib treatment in Japanese Patients with non-small cell lung cancer: The Okayama Lung Cancer Study Group experience. J. Thorac. Oncol. 2010, 5, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Kudoh, S.; Ohe, Y.; Johkoh, T.; Ando, M.; Yamazaki, N.; Seki, A.; Takemoto, S.; Fukuoka, M. Postmarketing surveillance study of erlotinib in Japanese patients with non-small-cell lung cancer (NSCLC): An interim analysis of 3488 patients (POLARSTAR). J. Thorac. Oncol. 2012, 7, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, B.; Facchinetti, F.; Bordi, P.; Silva, M.; Gnetti, L.; Tiseo, M. Lung toxicity in non-small-cell lung cancer patients exposed to ALK inhibitors: Report of a peculiar case and systematic review of the literature. Clin. Lung Cancer 2018, 19, e151–e161. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Seto, A.; Sasaki, T.; Shimbashi, W.; Fukushima, H.; Yonekawa, H.; Mitani, H.; Takahashi, S. Incidence and risk factors of interstitial lung disease of patients with head and neck cancer treated with cetuximab. Head Neck 2019, 41, 2574–2580. [Google Scholar] [CrossRef]
- Gemma, A.; Kusumoto, M.; Sakai, F.; Endo, M.; Kato, T.; Saito, Y.; Baba, T.; Sata, M.; Yamaguchi, O.; Yabuki, Y.; et al. Real-world evaluation of factors for interstitial lung disease incidence and radiologic characteristics in patients with EGFR T790M-positive NSCLC treated with osimertinib in Japan. J. Thorac. Oncol. 2020, 15, 1893–1906. [Google Scholar] [CrossRef]
- Suh, C.H.; Kim, K.W.; Pyo, J.; Hatabu, H.; Nishino, M. The incidence of ALK inhibitor-related pneumonitis in advanced non-small-cell lung cancer patients: A systematic review and meta-analysis. Lung Cancer 2019, 132, 79–86. [Google Scholar] [CrossRef]
- Suh, C.H.; Park, H.S.; Kim, K.W.; Pyo, J.; Hatabu, H.; Nishino, M. Pneumonitis in advanced non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitor: Meta-analysis of 153 cohorts with 15,713 patients: Meta-analysis of incidence and risk factors of EGFR-TKI pneumonitis in NSCLC. Lung Cancer 2018, 123, 60–69. [Google Scholar] [CrossRef]
- Hackshaw, M.D.; Danysh, H.E.; Singh, J.; Ritchey, M.E.; Ladner, A.; Taitt, C.; Camidge, D.R.; Iwata, H.; Powell, C.A. Incidence of pneumonitis/interstitial lung disease induced by HER2-targeting therapy for HER2-positive metastatic breast cancer. Breast Cancer Res. Treat. 2020, 183, 23–39. [Google Scholar] [CrossRef]
- Banerji, U.; van Herpen, C.M.L.; Saura, C.; Thistlethwaite, F.; Lord, S.; Moreno, V.; Macpherson, I.R.; Boni, V.; Rolfo, C.; de Vries, E.G.E.; et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019, 20, 1124–1135. [Google Scholar] [CrossRef]
- Manich, C.S.; O’Shaughnessy, J.; Aftimos, P.G.; van den Tweel, E.; Oesterholt, M.; Escrivá-de-Romaní, S.I.; Quenel Tueux, N.; Tan, T.J.; Lim, J.S.; Ladoire, S.; et al. Primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann. Oncol. 2021, 32, S1288. [Google Scholar]
- Zhang, Y.; Qiu, M.; Wang, J.; Zhang, Y.Q.; Shen, A.; Yuan, X.L.; Zhang, T.; Wei, X.L.; Zhao, H.Y.; Wang, D.S.; et al. A phase 1 multicenter, dose expansion study of ARX788 as monotherapy in patients with HER2-positive advanced gastric and gastroesophageal junction adenocarcinoma (ACE-Gastric-01). J. Clin. Oncol. 2021, 39, e16059. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Park, H.; Frentzas, S.; Shannon, C.M.; Cuff, K.; Eek, R.W.; Budd, G.T.; McCartney, A.; O’Shaughnessy, J.; Lu, J.M.; et al. Safety and unique pharmacokinetic profile of ARX788, a site-specific ADC, in heavily pretreated patients with HER2-overexpresing solid tumors: Results from two phase 1 clinical trials. J. Clin. Oncol. 2021, 39, 1038. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Robert, C.; Hersey, P.; Nathan, P.; Garbe, C.; Milhem, M.; Demidov, L.V.; Hassel, J.C.; Rutkowski, P.; Mohr, P.; et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. M. Engl. J. Med. 2012, 367, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Verzanio (Abemaciclib) Product Monograph; Eli Lilly Canada: Toronto, ON, Canada, 2019.
- Finn, R.S.; Rugo, H.S.; Gelmon, K.A.; Cristofanilli, M.; Colleoni, M.; Loi, S.; Schnell, P.; Lu, D.R.; Theall, K.P.; Mori, A.; et al. Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: Updated analysis with up to 5 years of follow-up. Oncologist 2021, 26, e749–e755. [Google Scholar] [CrossRef] [PubMed]
- Kisqali (Ribociclib) Product Monograph; Novartis Pharmaceuticals Canada Inc.: Dorval, QC, Canada, 2012.
- Ma, Z.; Sun, X.; Zhao, Z.; Lu, W.; Guo, Q.; Wang, S.; You, J.; Zhang, Y.; Liu, L. Risk of pneumonitis in cancer patients treated with PARP inhibitors: A meta-analysis of randomized controlled trials and a pharmacovigilance study of the FAERS database. Gynecol. Oncol. 2021, 162, 496–505. [Google Scholar] [CrossRef]
- Okada, N.; Matsuoka, R.; Sakurada, T.; Goda, M.; Chuma, M.; Yagi, K.; Zamami, Y.; Nishioka, Y.; Ishizawa, K. Risk factors of immune checkpoint inhibitor-related interstitial lung disease in patients with lung cancer: A single-institution retrospective study. Sci. Rep. 2020, 10, 13773. [Google Scholar] [CrossRef] [PubMed]
- Khunger, M.; Rakshit, S.; Pasupuleti, V.; Hernandez, A.V.; Mazzone, P.; Stevenson, J.; Pennell, N.A.; Velcheti, V. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: A systematic review and meta-analysis of trials. Chest 2017, 152, 271–281. [Google Scholar] [CrossRef]
- Suzuki, Y.; Karayama, M.; Uto, T.; Fujii, M.; Matsui, T.; Asada, K.; Kusagaya, H.; Kato, M.; Matsuda, H.; Matsuura, S.; et al. Assessment of immune-related interstitial lung disease in patients with NSCLC treated with immune checkpoint inhibitors: A multicenter prospective study. J. Thorac. Oncol. 2020, 15, 1317–1327. [Google Scholar] [CrossRef]
- Kato, T.; Masuda, N.; Nakanishi, Y.; Takahashi, M.; Hida, T.; Sakai, H.; Atagi, S.; Fujita, S.; Tanaka, H.; Takeda, K.; et al. Nivolumab-induced interstitial lung disease analysis of two phase II studies patients with recurrent or advanced non-small-cell lung cancer. Lung Cancer 2017, 104, 111–118. [Google Scholar] [CrossRef]
- Nishino, M.; Giobbie-Hurder, A.; Hatabu, H.; Ramaiya, N.H.; Hodi, F.S. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: A systematic review and meta-analysis. JAMA Oncol. 2016, 2, 1607–1616. [Google Scholar] [CrossRef]
- Delaunay, M.; Cadranel, J.; Lusque, A.; Meyer, N.; Gounant, V.; Moro-Sibilot, D.; Michot, J.M.; Raimbourg, J.; Girard, N.; Guisier, F.; et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur. Respir. J. 2017, 50, 1700050. [Google Scholar] [CrossRef] [PubMed]
- Barlési, F.; Villani, P.; Doddoli, C.; Gimenez, C.; Kleisbauer, J.P. Gemcitabine-induced severe pulmonary toxicity. Fundam. Clin. Pharmacol. 2004, 18, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Singavi, A.K.; Ramalingam, V.; George, B. Etanercept for treatment of taxane-induced pneumonitis. J. Oncol. Pract. 2019, 15, 556–557. [Google Scholar] [CrossRef] [PubMed]
- Suthar, K.H.; Al Mutar, S.; Venkatesan, R. Oxaliplatin-induced pulmonary toxicity: A rare but serious complication. Cureus 2020, 12, e7483. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, O. Drug-induced interstitial lung disease: Mechanisms and best diagnostic approaches. Respir. Res. 2012, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.A.; Modi, S.; Iwata, H.; Takahashi, S.; Smit, E.F.; Siena, S.; Chang, D.Y.; Macpherson, E.; Qin, A.; Singh, J.; et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open 2022, 7, 100554. [Google Scholar] [CrossRef]
- Conte, P.; Ascierto, P.A.; Patelli, G.; Danesi, R.; Vanzulli, A.; Sandomenico, F.; Tarsia, P.; Cattelan, A.; Comes, A.; De Laurentiis, M.; et al. Drug-induced interstitial lung disease during cancer therapies: Expert opinion on diagnosis and treatment. ESMO Open 2022, 7, 100404. [Google Scholar] [CrossRef]
- Kumagai, K.; Aida, T.; Tsuchiya, Y.; Kishino, Y.; Kai, K.; Mori, K. Interstitial pneumonitis related to trastuzumab deruxtecan, a human epidermal growth factor receptor 2-targeting Ab-drug conjugate, in monkeys. Cancer Sci. 2020, 111, 4636–4645. [Google Scholar] [CrossRef]
- Müller, N.L.; White, D.A.; Jiang, H.; Gemma, A. Diagnosis and management of drug-associated interstitial lung disease. Br. J. Cancer 2004, 91 (Suppl. S2), S24–S30. [Google Scholar] [CrossRef]
- Hague, C.; McInnis, M.; Souza, C. High Resolution CT of the Chest Recommended Technique. February 2020. Available online: https://car.ca/wp-content/uploads/2020/02/High-Resolution-CT-of-the-Chest-Recommended-Technique-2020.pdf (accessed on 6 February 2023).
- US Food and Drug Administration; Center for Drug Evaluation and Research. NDA/BLA Multi-Disciplinary Review and Evaluation {BLA 761139} ENHERTU (Fam-Trastuzumab Deruxtecan-Nxki); US Food and Drug Administration: Silver Spring, MD, USA; Center for Drug Evaluation and Research: Silver Spring, MD, USA, 2019. [Google Scholar]
- Gemma, A.; Kudoh, S.; Ando, M.; Ohe, Y.; Nakagawa, K.; Johkoh, T.; Yamazaki, N.; Arakawa, H.; Inoue, Y.; Ebina, M.; et al. Final safety and efficacy of erlotinib in the phase 4 POLARSTAR surveillance study of 10,708 Japanese patients with non-small-cell lung cancer. Cancer Sci. 2014, 105, 1584–1590. [Google Scholar] [CrossRef]
- Johkoh, T.; Lee, K.S.; Nishino, M.; Travis, W.D.; Ryu, J.H.; Lee, H.Y.; Ryerson, C.J.; Franquet, T.; Bankier, A.A.; Brown, K.K.; et al. Chest CT diagnosis and clinical management of drug-related pneumonitis in patients receiving molecular targeting agents and immune checkpoint inhibitors: A position paper from the Fleischner Society. Chest 2021, 159, 1107–1125. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef] [PubMed]
- Kohno, N.; Awaya, Y.; Oyama, T.; Yamakido, M.; Akiyama, M.; Inoue, Y.; Yokoyama, A.; Hamada, H.; Fujioka, S.; Hiwada, K. KL-6, a mucin-like glycoprotein, in bronchoalveolar lavage fluid from patients with interstitial lung disease. Am. Rev. Respir. Dis. 1993, 148, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, H.; Yokoyama, A.; Yasuhara, Y.; Watanabe, A.; Naka, T.; Hamada, H.; Abe, M.; Nishimura, K.; Higaki, J.; Ikezoe, J.; et al. Circulating KL-6 levels in patients with drug induced pneumonitis. Thorax 2003, 58, 872–875. [Google Scholar] [CrossRef]
- Kondo, A. Drug-induced pneumonitis. Kekkaku 1999, 74, 33–41. [Google Scholar]
- Limper, A.H.; Knox, K.S.; Sarosi, G.A.; Ampel, N.M.; Bennett, J.E.; Catanzaro, A.; Davies, S.F.; Dismukes, W.E.; Hage, C.A.; Marr, K.A.; et al. American Thoracic Society Fungal Working Group. An official American Thoracic Society statement: Treatment of fungal infections in adult pulmonary and critical care patients. Am. J. Respir. Crit. Care Med. 2011, 183, 96–128. [Google Scholar] [CrossRef]
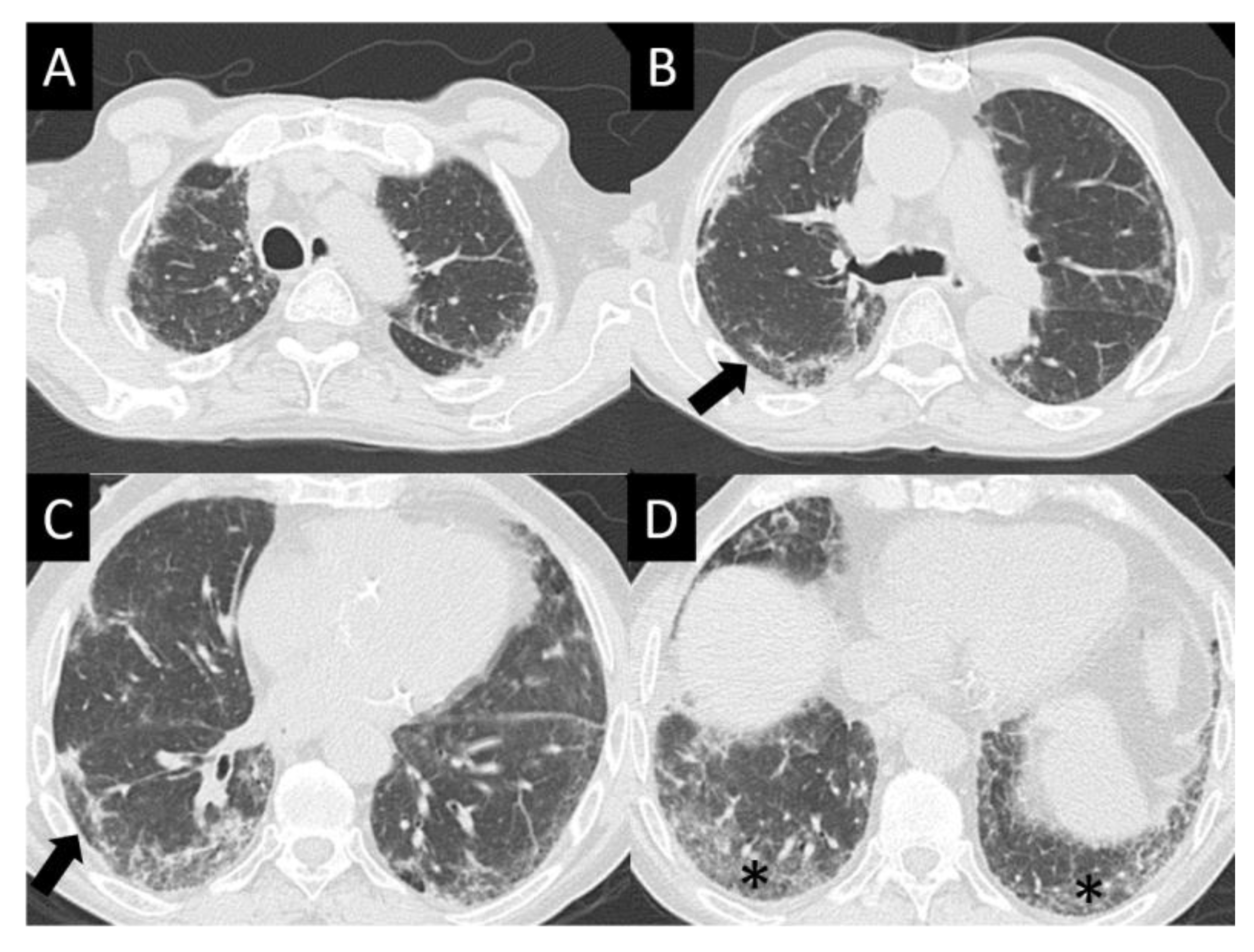
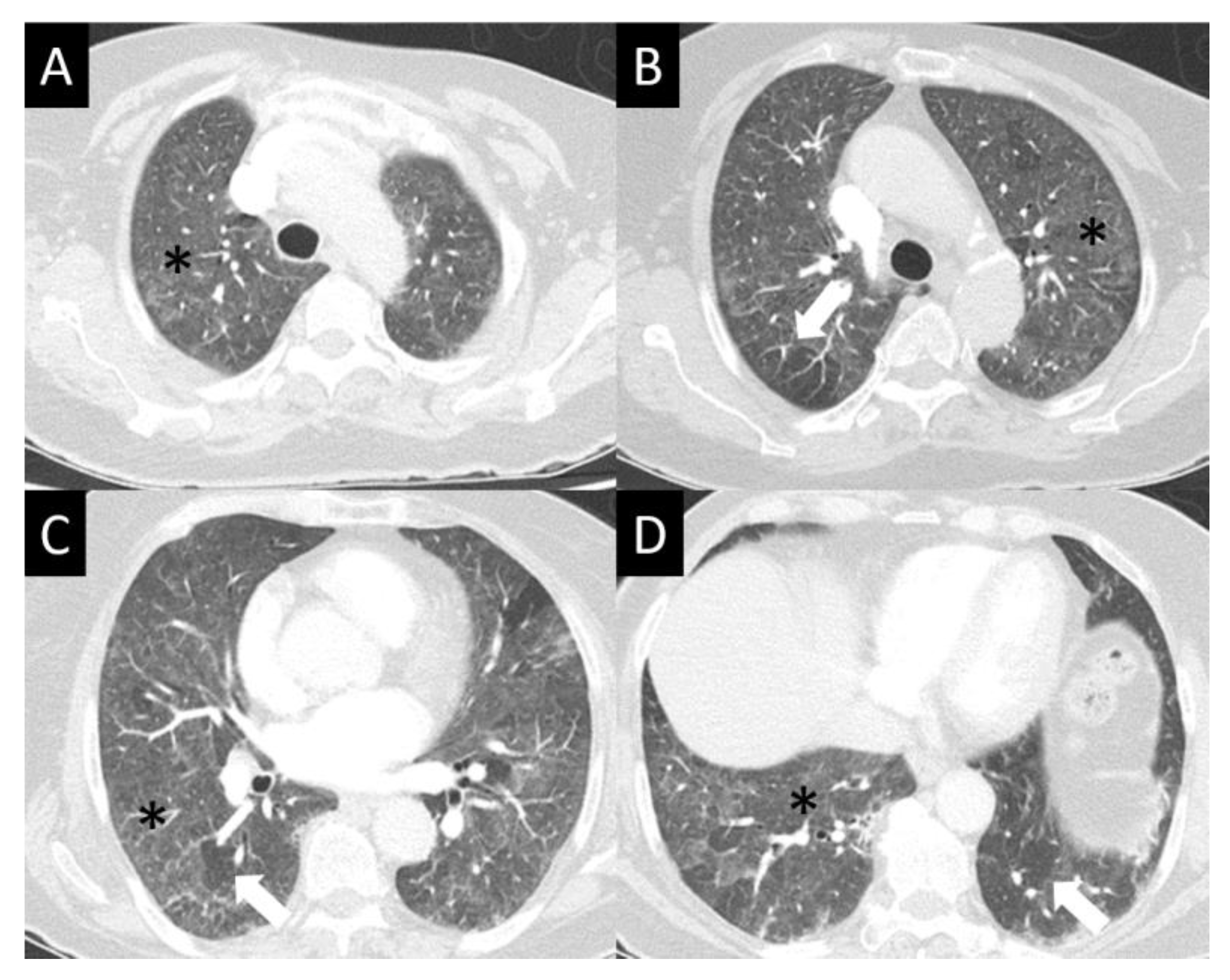
| Study | n | Population | Design | Median PFS, Months | Median OS, Months | Response Rate (RR), % | Duration of Response, Months |
|---|---|---|---|---|---|---|---|
| DESTINY-Breast01 [1,8] | 184 | HER2-positive metastatic breast cancer with previous treatment with trastuzumab emtansine | Open-label Phase 2 Single arm | 16.4 | 29.1 | Overall RR 60.9 | 14.8 |
| DESTINY-Breast02 [2] | 608 | HER2-positive metastatic breast cancer with previous treatment with trastuzumab emtansine | Open label Phase 3 Randomized (T-DXd vs. investigator’s choice of treatment) | 17.8 T-DXd vs. 6.9 investigator’s choice | 39.2 T-DXd vs. 26.5 investigator’s choice | Objective RR 69.7 T-DXd vs. 29.2 investigator’s choice | 19.6 T-DXd vs. 8.3 investigator’s choice |
| DESTINY-Breast03 [3] | 524 | HER2-positive metastatic breast cancer previously treated with trastuzumab and a taxane | Open label Phase 3 Randomized (T-DXd vs. T-DM1) | 28.8 T-DXd vs. 6.8 T-DM1 | Not reached | Overall RR 79 T-DXd vs. 35 T-DM1 | 36.6 T-DXd vs. 23.8 T-DM1 |
| DESTINY-Breast04 [4] | 557 | HER2-low * metastatic breast cancer patients who received one or two previous lines of chemotherapy | Open label Phase 3 Randomized (T-DXd vs. physician’s choice of chemotherapy) | 9.9 T-DXd vs. 5.1 physician’s choice of chemotherapy | 23.4 T-DXd vs. 16.8 physician’s choice of chemotherapy | Objective RR 52.3 T-DXd vs. 16.3 physician’s choice of chemotherapy | 10.7 T-DXd vs. 6.8 physician’s choice of chemotherapy |
| DESTINY-Gastric01 [9] | 187 | HER2-positive advanced gastric cancer | Open label Phase 2 Randomized (T-DXd vs. physician’s choice of chemotherapy) | 5.6 T-DXd vs. 3.6 physician’s choice of chemotherapy | 12.5 T-DXd vs. 8.4 physician’s choice of chemotherapy | Objective RR 51 T-DXd vs. 14 physician’s choice of chemotherapy | 11.3 T-DXd vs. 3.9 physician’s choice of chemotherapy |
| DESTINY-Gastric02 [10] | 79 | HER2-positive unresectable or metastatic gastric/GEJ cancer | Phase 2 Single arm | 5.6 | 12.1 | Objective RR 41.8 | 8.1 |
| DESTINY-Lung01 [11] | 91 | HER2-mutant unresectable or metastatic NSCLC | Phase 2 Single arm | 8.2 | 17.8 | Objective RR 55 | 9.3 |
| Treatment | Tumor Types | Number of Studies (Number of Patients) | Any Grade ILD, n (%) | Grade 5 ILD, n (%) |
|---|---|---|---|---|
| Anti-HER2 | ||||
| Trastuzumab [42] | HER2-positive advanced or unresectable/metastatic breast cancer | 8 (1642) | 162 (9.9) | 3 (0.2) |
| Lapatinib [42] | HER2-positive advanced or metastatic breast cancer | 4 (4470) | 8 (0.2) | 0 |
| T-DM1 [42] | HER2-positive advanced or metastatic breast cancer | 3 (3290) | 15 (0.5) | 6 (0.2) |
| SYD985 [42,43] a | HER2-expressing b locally advanced or metastatic breast, gastric, urothelial, or endometrial cancer | 1 (185) | 4 (2.2) | 1 (0.5) |
| SYD985 [44] | HER2-positive locally advanced or metastatic breast cancer | 1 (291) | NR (7.6) | 2 (0.7) |
| ARX788 [45] | HER2-positive advanced gastric and gastroesophageal junction cancer | 1 (23) | NR | 0 |
| ARX788 [46] c | HER2-positive advanced breast cancer | 1 (69) | NR (4.3) c | NR |
| ARX788 [46] c | HER2-positive advanced solid tumors | 1 (34) | NR (2.9) c | NR |
| TKI and/or EGFR inhibitor | ||||
| Gefitinib [34] d | EGFR-mutated NSCLC | 2 (201) | 10 (5.0) | 2 (1.0) |
| Gefitinib [35] d | NSCLC | 1 (330) | 8 (2.4) | 6 (1.8) |
| Erlotinib [36] d | Recurrent/advanced NSCLC | NA (3488) | 158 (4.5) | 55 (1.6) |
| Crizotinib, ceritinib, alectinib, brigatinib, lorlatinib, TSR-011, ASP3026, or ensartinib [37] | NSCLC | NA (4943) | 37 (0.7) | 5 (0.1) e |
| Cetuximab [38] d | Head and neck squamous cancer | NA (201) | 9 (4.5) | 1 (0.5) |
| Osimertinib [39] d | EGFR-mutated inoperable or recurrent NSCLC | NA (3578) | 231 (6.5) f | 29 (0.8) |
| Alectinib, ceritinib, crizotinib, or brigatinib [40] g | Advanced NSCLC | 18 (2261) | NR (2.14) | NR (0.22) |
| Erlotinib, gefitinib, afatinib, or osimertinib [41] g | Advanced NSCLC | 144 (15,713) | NR (1.12) | NR (0.20) |
| Immune checkpoint inhibitor | ||||
| Nivolumab, pembrolizumab, atezolizumab, or durvalumab [52] d | Lung cancer | NA (102) | 19 (18.6) | 4 (3.9) |
| Nivolumab or pembrolizumab (PD-1 inhibitors) [53] a,g | NSCLC | 12 (3232) | NR (3.6) | 7 (NR) |
| Atezolizumab, durvalumab, or avelumab (PD-L1 inhibitors) [53] a,g | NSCLC | 7 (1806) | NR (1.3) | 0 |
| Anti-PD-1 monotherapy [54] d | NSCLC | NA (138) | 20 (14.5) | 3 (2.2) |
| Nivolumab [55] d | Recurrent or advanced NSCLC | 2 (111) | 8 (7.2) | 1 (0.9) |
| PD-L1 inhibitors [56] a,g | Melanoma, NSCLC, or renal cell carcinoma | 20 (4496) | NR (2.7) | NR |
| CTLA-4, PD-1, or PD-L1 inhibitors [57] | NSCLC, melanoma, cavum, Hodgkin’s lymphoma, or UCNT | NR (1862) | 64 (3.5) | 6 (0.3) |
| CDK4/6 Inhibitors | ||||
| Abemaciclib [48] | Metastatic breast cancer | 3 (900) | NR (3.2) | NR (0.4) |
| Palbociclib [49] | HR-positive, HER2-negative advanced breast cancer | 3 (872) | 6 (0.69) | 0 (0) |
| Ribociclib [50] | HR-positive, HER2-negative advanced breast cancer | 3 (1153) | NR (1.6) | NR (0.1) |
| Study | n * | Population | ILD Incidence (T-DXd Arm), % | ||
|---|---|---|---|---|---|
| Any Grade | Grade 1 or 2 | Grade 5 | |||
| DESTINY-Breast01 [1] | 184 | HER2-positive metastatic breast cancer with previous treatment with trastuzumab emtansine | 13.6 | 10.9 | 2.2 |
| DESTINY-Breast02 [2] | 404 | HER2-positive metastatic breast cancer with previous treatment with trastuzumab emtansine | 10.4 | 9.2 | 0.5 |
| DESTINY-Breast03 [3] | 257 | HER2-positive metastatic breast cancer previously treated with trastuzumab and a taxane | 15.2 | 4.3 | 0 |
| DESTINY-Breast04 [4] | 371 | HER2-low † metastatic breast cancer patients who received one or two previous lines of chemotherapy | 12.1 | 10 | 0.8 |
| DESTINY-Gastric01 [9] | 125 | HER2-positive advanced gastric cancer | 9.6 | 7.2 | 0 |
| DESTINY-Gastric02 [10] | 79 | HER2-positive unresectable or metastatic gastric/GEJ cancer | 10.1 | 7.6 | 2.5 |
| DESTINY-Lung01 [11] | 91 | HER2-mutant unresectable or metastatic NSCLC | 26.4 | 19.8 | 2.2 |
| Medical Oncologist | Radiologist | Respirologist |
|---|---|---|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henning, J.-W.; Brezden-Masley, C.; Gelmon, K.; Chia, S.; Shapera, S.; McInnis, M.; Rayson, D.; Asselah, J. Managing the Risk of Lung Toxicity with Trastuzumab Deruxtecan (T-DXd): A Canadian Perspective. Curr. Oncol. 2023, 30, 8019-8038. https://doi.org/10.3390/curroncol30090582
Henning J-W, Brezden-Masley C, Gelmon K, Chia S, Shapera S, McInnis M, Rayson D, Asselah J. Managing the Risk of Lung Toxicity with Trastuzumab Deruxtecan (T-DXd): A Canadian Perspective. Current Oncology. 2023; 30(9):8019-8038. https://doi.org/10.3390/curroncol30090582
Chicago/Turabian StyleHenning, Jan-Willem, Christine Brezden-Masley, Karen Gelmon, Stephen Chia, Shane Shapera, Micheal McInnis, Daniel Rayson, and Jamil Asselah. 2023. "Managing the Risk of Lung Toxicity with Trastuzumab Deruxtecan (T-DXd): A Canadian Perspective" Current Oncology 30, no. 9: 8019-8038. https://doi.org/10.3390/curroncol30090582
APA StyleHenning, J.-W., Brezden-Masley, C., Gelmon, K., Chia, S., Shapera, S., McInnis, M., Rayson, D., & Asselah, J. (2023). Managing the Risk of Lung Toxicity with Trastuzumab Deruxtecan (T-DXd): A Canadian Perspective. Current Oncology, 30(9), 8019-8038. https://doi.org/10.3390/curroncol30090582







