Abstract
The management of myelodysplastic syndrome (MDS) and chronic myelomonocytic leukemia (CMML) is limited and remains an unmet need. Decitabine/cedazuridine (DEC-C, ASTX727) is Canada’s first and only approved oral hypomethylating agent for MDS and CMML. We characterized the real-world use of DEC-C through a Canadian compassionate use program. Demographic and clinical data from 769 patients enrolled in Taiho Pharma Canada’s Patient Support Program were collected and analyzed. These patients represent a collection period from 10 November 2020 to 31 August 2022 with a median age of 76 years. Among 651 patients who started DEC-C, the median treatment duration was 4.2 cycles. The median overall and progression-free survival were 21.6 and 10.7 months, respectively. Among 427 patients who discontinued treatment, the majority (69.5%) stopped due to death (n = 164) or disease progression (n = 133). Multivariable cox regression showed that age, province of residence, blast counts, antibiotic prophylaxis, and number of dose reductions and delays were not significantly associated with overall and progression-free survival. DEC-C is a promising alternative to parenteral hypomethylating agent therapy, and it likely addresses an important unmet need for effective and convenient therapies in this setting.
1. Introduction
Myelodysplastic syndrome (MDS) encompasses a heterogeneous group of hematopoietic stem cell disorders characterized by ineffective hematopoiesis and dysplastic changes in myeloid, erythroid, and megakaryocytic progenitors [1]. The resulting pancytopenia and increased risk of progression to acute myeloid leukemia (AML) cause significant morbidity and mortality in patients with MDS [2]. Chronic myelomonocytic leukemia (CMML) is an overlapping myelodysplastic/myeloproliferative malignancy [3]. In Canada, the estimated age-standardized incidence of MDS is 3.69 per 100,000 [4]. CMML is less common, with an average incidence rate of 2.45 cases per million individuals annually [5]. While long-term epidemiological studies in Canada remain limited, yearly incidence rates have increased in several countries including Germany, the UK, and the United States [6]. In combination with increasing physician awareness and improved diagnostic classifications, it is likely that MDS in Canada’s aging population will demonstrate similar trends in years to come [4].
With a median age of diagnosis between 70 and 76 years [2,4,7], most MDS patients are elderly with a varied prognosis depending on disease-related factors [8]. Patients with MDS may be classified according to the International Prognostic Scoring System (IPSS), by degree of pre-leukemic blast expansion, response to therapeutic agents, disease outcomes, and prognosis [9]. Based on the IPSS score, patients can be further classified as “lower risk MDS” (LR-MDS), which includes IPSS low risk or intermediate-1, and comprises approximately 70% of patients with a median survival of 3.5–5.7 years. “Higher risk MDS” (HR-MDS) includes IPSS intermediate-2 and high-risk categories with a median survival of 0.4–1.2 years [9]. Newer IPSS-R and IPSS-M classification updates include both cytogenetic and molecular features not collected in this study [10].
LR-MDS is often treated with hematopoietic growth factors, transfusions, and other supportive care measures. HR-MDS is typically treated with hypomethylating agents (HMAs) with the minority of patients undergoing intensive chemotherapy and hematopoietic stem cell transplantation (HSCT) [11]. The management of complications in MDS is critical as more than 80% of patients are anemic (hemoglobin < 100 g/L) at diagnosis and more than 50% of patients become red blood cell transfusion dependent during the course of their disease [12,13]. HSCT remains the only potentially curative therapy for MDS and CMML patients and is generally reserved for patients without significant comorbidity due to a high risk of complications [3,14,15,16]. For patients who are not candidates for HSCT, HMAs are an effective treatment option for those with HR-MDS and as appropriate, LR-MDS [11]. Azacitidine and decitabine are the cytidine analogues most commonly used as HMAs. These single-agent therapies were approved by Health Canada for the treatment of patients with HR-MDS (and CMML for decitabine) who are not candidates for HSCT [17,18,19,20]. Although these drugs have been shown to induce hematologic improvement in approximately one-third of patients, their demanding subcutaneous (SC)/intravenous (IV) infusion schedules prove to be a barrier for treatment continuation [21]. While a Canadian national guideline for the management of MDS remains unavailable, Cancer Care Ontario guidelines acknowledge intravenous HMA’s potential role in transfusion dependence reversal and its use in compassionate palliative settings [22].
Decitabine/cedazuridine (DEC-C, ASTX727, INQOVI®) is an oral fixed-dose combination tablet containing 35 mg of decitabine and 100 mg of cedazuridine. DEC-C was developed by Astex Pharmaceuticals and is distributed by Taiho Pharma Canada. Cedazuridine is an inhibitor of cytidine deaminase in the gut and liver that increases the systemic exposure of decitabine. DEC-C’s recommended dose is one tablet once daily on days 1–5 of each 28-day cycle. It was approved by Health Canada on July 7, 2020 for the treatment of adult patients diagnosed with MDS and CMML based (IPSS intermediate-1, intermediate-2, and high risk) or CMML [23].
DEC-C demonstrated similar pharmacokinetic, pharmacodynamic, and safety profiles of IV decitabine in a 2019 phase 1 study [24]. Subsequent phase 2 and phase 3 studies evaluated daily oral dosing regiments of 5 days in each 28-day cycle. The median treatment duration in the phase 2 study reported seven cycles (range 1–29) with a median follow up of 24.3 months. Of the eighty patients, forty-eight (60%) reported clinical responses including 17 (21%) with complete responses. Of those with baseline red blood cell transfusion dependence (n = 38), 50% (n = 19) became transfusion independent. Half of the 12 patients with baseline platelet transfusion dependence achieved transfusion independence at the end of Phase 2 studies [25]. In the ASCERTAIN phase 3 study, the median number of cycles was 9 and median follow-up was 32 months. In addition, 22% of patients achieved complete response, 26% proceeded to HSCT, and 53% became transfusion-independent for both red blood cells and platelets. Safety findings in both phase 2 and 3 studies were consistent with those anticipated for IV-DEC (related Grade ≥ 3 AEs in more than 5% were thrombocytopenia, neutropenia, anemia, febrile neutropenia, and leukopenia) [26]. Currently, the cost of DEC-C in Canada is comparable to and in some cases lower than generic azacytidine, and all Canadian provinces and territories except for Quebec now fund DEC-C according to the product label.
To our knowledge, the real-world use of oral DEC-C for intermediate-1 to high-risk MDS and CMML in Canada has yet to be studied since approval in 2020. Using data from Canadians enrolled to receive DEC-C through Taiho Pharma Canada’s Patient Support Program (PSP), we characterized demographic and clinical parameters of this patient cohort. As a secondary objective, we assessed both safety and tolerability, treatment duration, and survival outcomes alongside factors that may impact each of the above.
2. Materials and Methods
2.1. Study Design and Population
This was a retrospective cohort study of patients from Canadian provinces receiving oral DEC-C through Taiho Pharma Canada’s PSP. All patients included for analysis were adults (≥18 years of age) diagnosed with IPSS intermediate-1, intermediate-2, or high risk MDS (previously treated or untreated, de novo or secondary) or CMML, and who received treatment with oral DEC-C through the PSP. Patients in our analysis also reported an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less. Approval for this study was obtained from the Health Research Ethics Board of Alberta Cancer Committee.
2.2. Study Data
Study data were collected by Bayshore HealthCare and provided by Taiho Pharma Canada. Demographical and clinical characteristics were collected during patient enrollment, including age at diagnosis, province of residence, and IPSS risk score. Treatment characteristics included enrollment date, treatment status, reason for status, treatment start date, treatment stop date, reimbursement information, and any treatment modification if applicable. At the 6th cycle of oral DEC-C therapy, physicians were sent a re-enrollment form to complete. This form assisted in collecting updated patient characteristics including treatment status, red blood cell and platelet transfusion status, maintenance of blast counts, use of prophylactic antibiotics, and any dose reductions or delays in the last 6 cycles. Our analysis reflects a data collection period from 10 November 2020 to 31 August 2022.
2.3. Statistical Analysis
Descriptive statistics were used to summarize baseline patient and treatment characteristics. Continuous variables were presented as medians with interquartile ranges and means with standard deviations while categorical variables were presented as frequencies and percentages. Subgroup analysis was performed to analyze potential relationships between clinical parameters. Patients were grouped as LR-MDS (IPSS status intermediate-1) or HR-MDS (IPSS status intermediate-2 and high risk) as per IPSS [9]. Further analysis explored parameters based on treatment duration. Subgroups were divided between patients receiving greater than, or equal to, 4 cycles of DEC-C versus less than 4 cycles. Each cycle is defined in 28-day periods as per the product monograph [22]. The 6th cycle re-enrollment data were also utilized to compare the transfusion status against initial enrollment forms to note any change in the last 6 cycles.
Progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan–Meier method and utilizing time-to-event data involving date of enrollment to date of event (i.e., treatment discontinuation due to death or disease progression).
Wilcoxon rank-sum tests were used for continuous variables while Pearson’s Chi-square test or Fischer’s exact test were used for categorical variables. Multivariable Cox regression analysis was performed using likelihood ratio tests to find associations between survival outcomes and select demographic/clinical factors. The significance level of all statistical two-sided tests was defined a priori as <0.05. All analyses were performed using R Studio [27].
3. Results
Across Canadian provinces, 769 patients were enrolled in the Taiho Pharma Canada PSP to receive oral DEC-C for the treatment of MDS and CMML. The median age at enrollment was 76 years (range 21–97 years). Examining patient enrollment by geographical location, the greatest number of patients resided in Ontario (n = 357, 46.4%). Patients from Quebec reflected the second largest proportion of patients (n = 150, 19.5%). The remaining patients were distributed across Western provinces (British Columbia, Alberta, Saskatchewan, Manitoba) (n = 178, 23.1%) and Atlantic provinces (New Brunswick, Newfoundland and Labrador, Nova Scotia, Prince Edward Island) (n = 84, 10.9%). Of patients with recorded IPSS risk scores, the greatest proportion of patients (40.9%, n = 277) had intermediate-1 risk MDS. Patients with intermediate-2 risk totaled 212 (31.3%) while 177 patients (26.1%) had high risk of disease. Of patients with reported transfusion dependence status at enrollment, 60.2% were red blood cell transfusion dependent while 16.1% of patients were platelet transfusion dependent. Six hundred and fifty-one patients (84.7%) started treatment on oral DEC-C with a median time from enrollment to treatment start of 12 days (range 0–273). Median treatment duration for patients who discontinued therapy was 4.2 cycles (range 0.0–22.7). The majority of patients (n = 570, 74.7%) were reimbursed by a compassionate use program funded by Taiho Pharma Canada (Table 1).

Table 1.
Demographic and clinical characteristics of the patient cohort.
Of 769 patients enrolled, 118 (15.3%) never started DEC-C during the data collection period. The vast majority (79.7%) of these patients were discharged from the program for reasons outlined in Figure 1, while others (n = 24, 20.3%) were pending treatment initiation. Of the 651 patients who initiated treatment, 427 patients discontinued treatment during the study period with the most common reasons being death (38.4%), disease progression (33.1%), and physician decision (13.3%). Two hundred and seven patients (26.9%) were still on treatment at the data collection cut-off date (Figure 1).
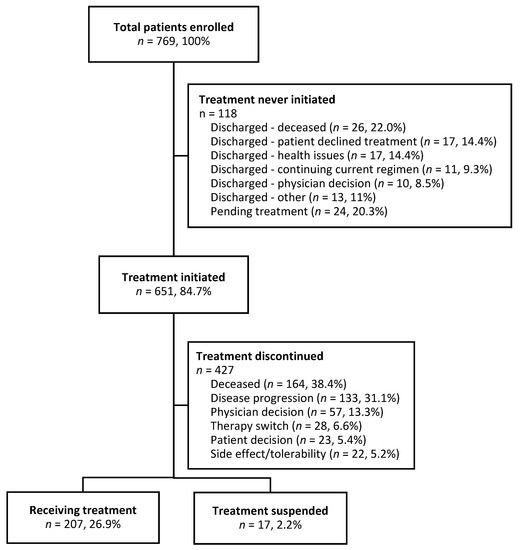
Figure 1.
Flow diagram of patients enrolled to receive DEC-C.
Stratifying patients by IPSS risk group, there were 389 patients with HR-MDS and 277 with LR-MDS. While there were more patients who died in the higher-risk subgroup (n = 97, 44.3%) versus the lower-risk subgroup (n = 49, 32.0%), this is expected, and the difference was not statistically significant. There were no statistically significant differences in treatment duration or red blood cell/platelet transfusion dependence status at enrollment between the HR-MDS and LR-MDS subgroups (Table 2). Of 155 patients who received the 6th cycle re-enrollment form, data from 120 (77%) patients were collected. Of these patients, 108 patients reported both IPSS score and the number of dose reductions in the last six cycles. Fifty-eight patients (53.7%) did not require any dose reduction. Comparing IPSS risk groups, a greater proportion of patients with HR-MDS did not require any dose reduction in the last six cycles compared to those with LR-MDS (60.3% vs. 42.5%, p = 0.03). Eighty-eight patients had both IPSS status and red blood cell transfusion status reported at the 6th cycle. The greatest proportion of patients had maintained red blood cell transfusion independence since enrollment. Of 90 patients with both IPSS scores and platelet transfusion status reported at the 6th cycle, the greatest proportion had maintained platelet transfusion independence since enrollment. Both antibiotic prophylaxis status and IPSS status was reported in 108 patients during the re-enrollment process among which only eight patients (7.8%) received antibiotic prophylaxis in the last 6 cycles (Table 2).

Table 2.
(a) Patient characteristics by IPSS risk group, overall. (b) Patient characteristics by IPSS risk group, 6th cycle follow-up.
Subgroup analyses based on treatment duration revealed that a significantly greater proportion of patients who received <4 cycles stopped treatment due to death, patient decision, or side effect compared to those who received ≥4 cycles (p < 0.001). Conversely, treatment discontinuation due to disease progression, physician decision, or therapy switch was more common in patients who received ≥ 4 cycles. No significant differences between treatment duration subgroups were shown for age, province, or time to treatment initiation (Table 3).

Table 3.
Baseline demographics and treatment characteristics by treatment duration, patients who initiated and discontinued treatment only.
At the time of the data cut-off, the median OS among all patients who received DEC-C was 21.6 months (95% confidence interval (CI) 18.5-inf). The median PFS overall was 10.7 months (95% CI 9.2–13.3). Differences in OS between IPSS risk groups were statistically significant (p = 0.03), while differences in PFS were not statistically significant (p = 0.45). Survival estimates are further summarized in Figure 2 and Figure 3.
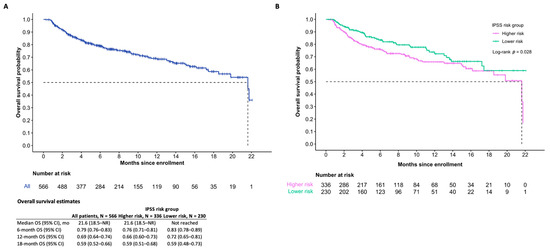
Figure 2.
Overall survival of patients (A) and IPSS risk subgroups (B) of those who initiated treatment.
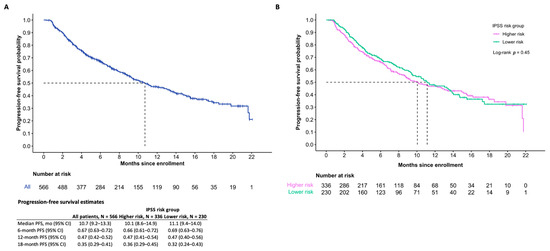
Figure 3.
Progression-free survival (A) and IPSS risk subgroups (B) of those who initiated treatment.
Multivariable cox regression analysis revealed no significant associations between OS, PFS and various demographic and clinical factors (i.e., patient age at enrollment, patient province, IPSS risk group, time to treatment initiation, blast count status, antibiotic prophylaxis, dose reductions, and dose delays) (Table 4).

Table 4.
Multivariable cox regression models, patients who initiated treatment only.
4. Discussion
This study examines real-world oral DEC-C use for the treatment of MDS and CMML through the Taiho Pharma Canada PSP. To our knowledge, the use of DEC-C in the Canadian real-world setting has yet to be described in the literature since its approval by Health Canada. There were 769 patients who enrolled into the PSP during our study period, which represents a notable uptake of this novel oral therapeutic. Interest and preference toward oral formulations over IV/SC chemotherapy is well documented in the literature. Indeed, a recent 2022 study analyzing the online survey data from MDS patients revealed both a preference and perceived personal benefit from improved quality of life from receiving oral DEC-C in comparison to IV/SC treatment [21]. The distribution of IPSS risk subgroups within patients with MDS in our cohort was similar to that of representative patient samples in the existing literature, where the greatest proportion of patients was INT-1 followed by INT-2 and then high risk [14,26]. The median age of our cohort was 76 years old (Range: 21–97). This is similar to the Canadian median age of diagnosis of 75 years for both MDS and CMML [4,5], and it is consistent with epidemiological studies characterizing both MDS and CMML to be diseases of older age [28,29]. The median age of our cohort was approximately five years older than that of previous clinical trials exploring oral DEC-C for MDS and CMML [24,25,26]. This difference may be explained by the more extensive eligibility criteria that trials use, which builds study cohorts that may not represent the diverse population of patients for whom DEC-C may be prescribed in the real world.
The median OS of patients who received oral DEC-C was 21.6 months. This was significantly less than the median OS in the phase 3 study ASCERTAIN which demonstrated a median OS of 31.7 months [30]. A large proportion of patients in our study stopped treatment due to death (n = 164, 38.4%) or disease progression (n = 133, 33.1%). In comparison, the ASCERTAIN phase 3 study reported treatment discontinuation for disease progression in only six patients (4.5%) while reporting no deaths while on treatment. These discrepancies may be explained by ASCERTAIN’s more stringent enrollment criteria which included eligibility for IV decitabine administration, life expectancy of three or more months, adequate organ function, ECOG performance status of 0–1, and fewer than 1 prior cycle of azacitidine or decitabine [31]. As such, patients enrolled in the phase 3 ASCERTAIN study were likely healthier at baseline and at lower risk of earlier death or disease progression compared to the present study cohort, which comprised all for whom DEC-C was indicated. Additionally, the data collection period reflects a unique timeframe in society where the COVID-19 pandemic brought unprecedented challenges to both the individual patient and healthcare systems at large. MDS patients, by the nature of their mean age and disease, were more susceptible to downstream effects of the virus. From treatment delays, scarcity of transfusion products, to death from COVID-19 virus itself, MDS patients have been well described in the literature to have suffered multiple negative consequences [32,33]. Future studies may reveal the true extent of COVID-19 on survival outcomes data during this time.
Our study revealed a median treatment duration of 4.2 cycles (range 0.0–22.7) for patients who received therapy and similar durations between LR- and HR-MDS. This median duration is in keeping with one other study exploring real-world data of DEC-C in the United States [34]. However, the median treatment duration for patients in ASCERTAIN was significantly longer at nine cycles [30]. Research has demonstrated that the longer treatment duration of HMA is associated with improved clinical outcomes, including higher reported response rates, reduced AML transformation, and improved OS [35,36,37,38,39,40]. Specifically, clinical trial evidence has suggested that patients require four to six cycles of HMA therapy to achieve a clinical response [36,41,42,43]. However, prior real-world data suggest many patients do not persist with HMA treatment after initiation, receiving less than four or six cycles or having gaps over 90 days between cycles [41]. This is consistent with Canadian data from the national MDS Registry, with one study finding 33% of patients with HR-MDS receive <4 cycles of subcutaneous hypomethylation therapy with short overall survival [44]. Delayed response to hypomethylation therapy is not unusual; hence, for patients with stable disease or those who achieve clinical response from HMA, the continuation of therapy remains closely associated with improved survival [45]. This is reflected in current guidelines from the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) [14,15]. Consistent with the evidence that longer treatment duration is associated with superior survival outcomes, our study found that a significantly greater proportion of patients who received <4 cycles stopped treatment due to death versus those who received ≥4 cycles (49.3% vs. 28.4%, p < 0.001).
Reasons for early HMA discontinuation can be clinical in nature (e.g., patient mortality, disease progression, adverse effects) and/or non-clinical (e.g., logistical challenges, provider inexperience, socioeconomic barriers). In this study, oral DEC-C is shown to be a well-tolerated drug with 5.2% of patients (n = 22) stopping therapy due to side effects/tolerability issues. DEC-C has a tolerability profile consistent with IV decitabine [46] but is not impacted by the complex logistics inherent with IV HMA treatment which may result in early discontinuation or dose delay [47]. Of the 119 patients in this study who were followed up at 6 cycles, 55 patients (46.2%) reported no dose delays in their therapy, 30 patients (25.2%) received 1 dose delay, 14 patients (11.8%) received 2 dose delays, while 20 patients (16.8%) received 3 or more dose delays. Although most patients experienced one dose delay or less, reasons for dose delay may have been of a clinical or non-clinical nature and will require further investigation.
Transfusion dependence is not uncommon among MDS and CMML patients [12]. Approximately 50–90% of patients will require red blood cell transfusions with 30–50% requiring ≥1 platelet transfusion [48]. Most patients (60.2%) in our study were red blood cell transfusion dependent at enrollment with some (16.1%) who were platelet transfusion dependent. Transfusion dependence is associated with poorer quality of life and survival outcomes in MDS. As HMAs have been shown to promote transfusion independence in both LR-MDS [49] and HR-MDS [50], reporting any change in transfusion status is valuable in assessing the real-world performance of DEC-C. Of patients receiving ≥6 cycles of DEC-C, 19.8% achieved a change in transfusion status from being red blood cell transfusion dependent at enrollment to independent at the 6th cycle versus 9.3% who shifted from red blood cell transfusion independent to dependent. Red blood cell transfusion independence was maintained in 40.7% of patients after 6 cycles.
The cytopenic complications of MDS and CMML and the associated predisposition to infections warrant the consideration of antibiotic prophylaxis. Current guidelines recommend prophylactic antibiotics use for patients at high risk of febrile neutropenia, profound protracted neutropenia, and patients on hematopoietic stem cell therapy [51]. Additional recommendations include anti-viral and anti-fungal coverage in specific clinical scenarios. In this study, 57.0% of patients were reported as maintaining baseline blast counts from enrollment to cycle 6. This is important to quantify, as hypomethylating agents may cause transient neutropenia especially during initial cycles of therapy and increasing risks of infection [35,37,52,53]. Multivariable cox regression models revealed no statistically significant impact of blast count on OS or PFS. During DEC-C treatment, 8.7% reported being on prophylactic antibiotics.
This study contains inherent limitations which should be considered. Due to the nature of data collection and necessary privacy, we were not permitted to link other data sources; thus, we lacked access to data points not captured within the PSP. These may include prior treatment history, leukemic transformation prior to therapy, and specifics on antibiotic prophylaxis. Another limitation was the inability to identify specific reasons for patients’ decisions not to receive DEC-C nor the rationales for physician decisions to stop DEC-C. Parsing these decisions in future studies may allow for more thorough assessments of non-persistence due to logistical factors, patient preferences, or provider inexperience. Furthermore, while the PSP was effective in facilitating early access to oral DEC-C to Canadians, this dataset may not be fully representative in the setting of wider access involving public drug funding plans and private reimbursement. Finally, due to the retrospective nature of our study analysis, the associations explored in our analysis are susceptible to unmeasured confounding variables.
Our study highlights the role that PSP and compassionate use programs serve in providing access and financial support to Canadians who require novel therapeutics like HMAs. Most patients in this study (64.4%, n = 496) were reimbursed through Taiho Pharma Canada’s compassionate support program. By enabling early patient access to oral DEC-C in the context of MDS and CMML, the program facilitates foundational research which may allow for more comprehensive decision making regarding public drug funding. Additionally, despite data supporting the use of HMAs in the treatment of HR-MDS and CMML, several studies have suggested that approximately half of patients with HR-MDS do not receive HMA therapy at all [41,53,54,55,56,57]. As real-world data on oral DEC-C’s performance in the management of MDS and CMML are limited, the PSP enables timely research to help address this knowledge gap and optimize HMA use.
Author Contributions
Conceptualization, J.P.Y., P.Q.D., A.D. and W.Y.C.; methodology, J.P.Y., P.Q.D., A.D. and W.Y.C.; software, P.Q.D.; validation, J.P.Y., P.Q.D., A.D. and W.Y.C.; formal analysis, J.P.Y. and P.Q.D.; investigation, J.P.Y., P.Q.D.,A.D. and W.Y.C.; resources, J.P.Y., P.Q.D. and A.D.; data curation, J.P.Y., P.Q.D. and A.D.; writing—original draft preparation, J.P.Y. and P.Q.D.; writing—review and editing, J.P.Y., P.Q.D., A.D. and W.Y.C.; visualization, J.P.Y., P.Q.D., A.D. and W.Y.C.; supervision, A.D. and W.Y.C.; project administration, A.D. and W.Y.C.; funding acquisition, W.Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this study was provided by Taiho Pharma Canada, Inc. as an unrestricted research grant under project ID 22-0007.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Health Research Ethics Board of Alberta Cancer Committee (HREBA-22-0007) on (28 January 2022).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of this study.
Data Availability Statement
Data will not be shared due to patient confidentiality, according to ethics approval for this study.
Acknowledgments
We thank Mary Lisa Sheridan and Jane Craik from Taiho Pharma Canada, Inc., Karen Rowley, Emil Markaryan, and Devi Gopalan from Bayshore HealthCare, and Chantelle Carbonell from Oncology Outcomes for their support throughout this study.
Conflicts of Interest
W.Y.C. received research funding from Taiho Pharma Canada. A.D. is an employee of Taiho Pharma Canada. J.P.Y. and P.Q.D. declare no conflict of interest.
References
- Platzbecker, U. Treatment of MDS. Blood 2019, 133, 1096–1107. [Google Scholar] [PubMed]
- Steensma, D.P. Myelodysplastic syndromes current treatment algorithm 2018. Blood Cancer J. 2018, 8, 47. [Google Scholar] [PubMed]
- Patnaik, M.M.; Tefferi, A. Chronic Myelomonocytic leukemia: 2020 update on diagnosis, risk stratification and management. Am. J. Hematol. 2020, 95, 97–115. [Google Scholar]
- Slack, J.; Nguyen, L.; Naugler, C.; Rashid-Kolvear, F. Incidence of Myelodysplastic Syndromes in a Major Canadian Metropolitan Area. J. Appl. Lab. Med. 2018, 3, 378–383. [Google Scholar] [CrossRef]
- Le, M.; Ghazawi, F.; Popradi, G.; Glassman, S.; Sasseville, D.; Litvinov, I. Epidemiology and geographic trends for chronic myelomonocytic leukemia in Canada. J. Am. Acad. Dermatol. 2018, 79, AB130. [Google Scholar]
- Cogle, C.R. Incidence and Burden of the Myelodysplastic Syndromes. Curr. Hematol. Malig. Rep. 2015, 10, 272–281. [Google Scholar]
- Sekeres, M.A.; Taylor, J. Diagnosis and Treatment of Myelodysplastic Syndromes: A Review. JAMA 2022, 328, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, A.; Nachtkamp, K.; Gattermann, N.; Germing, U. Assessing the Prognosis of Patients with Myelodysplastic Syndromes (MDS). Cancers 2022, 14, 1941. [Google Scholar] [CrossRef]
- Greenberg, P.; Cox, C.; LeBeau, M.M.; Fenaux, P.; Morel, P.; Sanz, G.; Sanz, M.; Vallespi, T.; Hamblin, T.; Oscier, D.; et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997, 89, 2079–2088. [Google Scholar]
- Wu, J.; Zhang, Y.; Qin, T.; Xu, Z.; Qu, S.; Pan, L.; Li, B.; Jia, Y.; Li, C.; Wang, H.; et al. IPSS-M has greater survival predictive accuracy compared with IPSS-R in persons ≥ 60 years with myelodysplastic syndromes. Exp. Hematol. Oncol. 2022, 11, 73. [Google Scholar]
- Palacios-Berraquero, M.L.; Alfonso-Piérola, A. Current Therapy of the Patients with MDS: Walking towards Personalized Therapy. J. Clin. Med. 2021, 10, 2107. [Google Scholar] [CrossRef] [PubMed]
- Balducci, L. Transfusion independence in patients with myelodysplastic syndromes: Impact on outcomes and quality of life. Cancer 2006, 106, 2087–2094. [Google Scholar]
- de Swart, L.; Smith, A.; Johnston, T.W.; Haase, D.; Droste, J.; Fenaux, P.; Symeonidis, A.; Sanz, G.; Hellström-Lindberg, E.; Cermák, J.; et al. Validation of the revised international prognostic scoring system (IPSS-R) in patients with lower-risk myelodysplastic syndromes: A report from the prospective European LeukaemiaNet MDS (EUMDS) registry. Br. J. Haematol. 2015, 170, 372–383. [Google Scholar]
- Greenberg, P.L.; Stone, R.M.; Al-Kali, A.; Barta, S.K.; Bejar, R.; Bennett, J.M.; Carraway, H.; De Castro, C.M.; Deeg, H.J.; DeZern, A.E.; et al. Myelodysplastic Syndromes, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 60–87. [Google Scholar]
- Fenaux, P.; Haase, D.; Santini, V.; Sanz, G.F.; Platzbecker, U.; Mey, U. Myelodysplastic syndromes: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 142–156. [Google Scholar] [PubMed]
- de Witte, T.; Bowen, D.; Robin, M.; Malcovati, L.; Niederwieser, D.; Yakoub-Agha, I.; Mufti, G.J.; Fenaux, P.; Sanz, G.; Martino, R.; et al. Allogeneic hematopoietic stem cell transplantation for MDS and CMML: Recommendations from an international expert panel. Blood 2017, 129, 1753–1762. [Google Scholar]
- DACOGEN (Decitabine) for Injection [Product Monograph]; Otsuka Canada Pharmaceutical Inc.: Saint-Laurent, QC, USA, 2019.
- DEMYLOCAN (Decitabine) for Injection [Product Monograph]; PENDOPHARM, Division of Pharmascience Inc.: Montreal, QC, USA, 2019.
- VIDAZA (Azacitidine) for Injection [Product Monograph]; Celgene Inc.: Mississauga, ON, USA, 2018.
- Reddy-Azacitidine (Azacitidine) for Injection [Product Monograph]; Dr. Reddy’s Laboratories Limited.: Mississauga, ON, USA, 2017.
- Zeidan, A.M.; Tsai, J.H.; Karimi, M.; Schmier, J.; Jayade, S.; Zormpas, E.; Hassan, A.; Ruiters, D.; Anthony, C.; Hill, K.; et al. Patient Preferences for Benefits, Risks, and Administration Route of Hypomethylating Agents in Myelodysplastic Syndromes. Clin. Lymphoma Myeloma Leuk. 2022, 22, e853–e866. [Google Scholar]
- Buckstein, R.; Baldassarre, F.; Maze, D.; Schuh, A.; Cheung, M.; the Myelodysplastic Syndrome Guideline Development Group. Systemic Therapy for the Treatment of Adult Patients with Low-Risk Myelodysplastic Syndromes; Program in Evidence-Based Care Guideline No.: 6–13; Cancer Care Ontario: Toronto, ON, USA, 5 March 2018. [Google Scholar]
- INQOVI (Decitabine and Cedazuridine) Tablets [Product Monograph]; Taiho Pharma Canada, Inc.: Oakville, ON, USA, 2022.
- Savona, M.R.; Odenike, O.; Amrein, P.C.; Steensma, D.P.; DeZern, A.E.; Michaelis, L.C.; Faderl, S.; Harb, W.; Kantarjian, H.; Lowder, J.; et al. An oral fixed-dose combination of decitabine and cedazuridine in myelodysplastic syndromes: A multicentre, open-label, dose-escalation, phase 1 study. Lancet Haematol. 2019, 6, e194–e203. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manero, G.; Griffiths, E.A.; Steensma, D.P.; Roboz, G.J.; Wells, R.; McCloskey, J.; Odenike, O.; DeZern, A.E.; Yee, K.; Busque, L.; et al. Oral cedazuridine/decitabine for MDS and CMML: A phase 2 pharmacokinetic/pharmacodynamic randomized crossover study. Blood 2020, 136, 674–683. [Google Scholar]
- Garcia-Manero, G.; McCloskey, J.; Griffiths, E.A.; Yee, K.W.L.; Zeidan, A.M.; Al-Kali, A.; Dao, K.-H.; Deeg, H.J.; Patel, P.A.; Sabloff, M.; et al. Pharmacokinetic Exposure Equivalence and Preliminary Efficacy and Safety from a Randomized Cross over Phase 3 Study (ASCERTAIN study) of an Oral Hypomethylating Agent ASTX727 (cedazuridine/decitabine) Compared to IV Decitabine. Blood 2019, 134, 846. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R. RStudio, PBC, Boston, MA URL. 2020. Available online: http://www.rstudio.com/ (accessed on 8 June 2023).
- Ma, X. Epidemiology of myelodysplastic syndromes. Am. J. Med. 2012, 125, S2–S5. [Google Scholar] [PubMed]
- Sekeres, M.A. Epidemiology, natural history, and practice patterns of patients with myelodysplastic syndromes in 2010. J. Natl. Compr. Cancer Netw. 2011, 9, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Astex Pharmaceuticals. Astex Pharmaceuticals Presents Overall Survival Data from ASCERTAIN Phase 3 Study of Oral Hypomethylating Agent INQOVI (Decitabine and Cedazuridine) in MDS and CMML at International Congress on Myelodysplastic Syndromes; News Release; Astex Pharmaceuticals: Cambridge, UK, 2021. [Google Scholar]
- Study of ASTX727 vs IV Decitabine in MDS, CMML, and AML. Available online: https://www.clinicaltrials.gov/study/NCT03306264 (accessed on 8 June 2023).
- Miskeen, E.; Omer Yahia, A.I.; Eljack, T.B.; Karar, H.K. The Impact of COVID-19 Pandemic on Blood Transfusion Services: A Perspective from Health Professionals and Donors. J. Multidiscip. Healthc. 2021, 14, 3063–3071. [Google Scholar] [PubMed]
- Feld, J.; Demakos, E.P.; Odchimar-Reissig, R.; Tremblay, D.; Alli, S.; Sewah, D.; Navada, S.C.; Silverman, L.R. Myelodysplastic Syndromes (MDS) & COVID-19: Clinical Experience from the US Epicenter of the Pandemic. Blood 2020, 136, 17–18. [Google Scholar]
- Zeidan, A.M.; Divino, V.; DeKoven, M.; Wang, E.; Chen, J.; Salimi, T.; Epstein, R.S. Treatment Patterns and Characteristics Among Patients with Myelodysplastic Syndromes Initiating Oral Decitabine and Cedazuridine or Intravenous/Subcutaneous Hypomethylating Agents in a Real-World Setting. Blood 2022, 140, 4047–4049. [Google Scholar] [CrossRef]
- Silverman, L.R.; Demakos, E.P.; Peterson, B.L.; Kornblith, A.B.; Holland, J.C.; Odchimar-Reissig, R.; Stone, R.M.; Nelson, D.; Powell, B.L.; DeCastro, C.M.; et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group B. J. Clin. Oncol. 2002, 20, 2429–2440. [Google Scholar]
- Kantarjian, H.; Issa, J.P.; Rosenfeld, C.S.; Bennett, J.M.; Albitar, M.; DiPersio, J.; Klimek, V.; Slack, J.; de Castro, C.; Ravandi, F.; et al. Decitabine improves patient outcomes in myelodysplastic syndromes: Results of a phase III randomized study. Cancer 2006, 106, 1794–1803. [Google Scholar]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar]
- List, A.F.; Fenaux, P.; Mufti, G.J.; Hellström-Lindberg, E.; Gore, S.; Bennett, J.M.; Silverman, L.R.; Backstrom, J.; Allen, A.R.; Beach, C.L. Effect of azacitidine (AZA) on overall survival in higher-risk myelodysplastic syndromes (MDS) without complete remission. J. Clin. Oncol. 2008, 26, 7006. [Google Scholar]
- Kim, D.J.; Lee, H.S.; Moon, J.H.; Sohn, S.K.; Kim, H.J.; Cheong, J.W.; Jo, D.-Y.; Kim, H.; Lee, H.; Bang, S.-M.; et al. Can we consider discontinuation of hypomethylating agents in patients with myelodysplastic syndrome: A retrospective study from The Korean Society of Hematology AML/MDS Working Party. Oncotarget 2017, 8, 79414–79424. [Google Scholar]
- Silverman, L.R.; Fenaux, P.; Mufti, G.J.; Santini, V.; Hellström-Lindberg, E.; Gattermann, N.; Sanz, G.; List, A.F.; Gore, S.D.; Seymour, J.F. Continued azacitidine therapy beyond time of first response improves quality of response in patients with higher-risk myelodysplastic syndromes. Cancer 2011, 117, 2697–2702. [Google Scholar]
- Zeidan, A.M.; Salimi, T.; Epstein, R.S. Real-world use and outcomes of hypomethylating agent therapy in higher-risk myelodysplastic syndromes: Why are we not achieving the promise of clinical trials? Future Oncol. 2021, 17, 5163–5175. [Google Scholar] [PubMed]
- Khan, C.; Pathe, N.; Fazal, S.; Lister, J.; Rossetti, J.M. Azacitidine in the management of patients with myelodysplastic syndromes. Ther. Adv. Hematol. 2012, 3, 355–373. [Google Scholar] [PubMed]
- Silverman, L.R.; McKenzie, D.R.; Peterson, B.L.; Holland, J.F.; Backstrom, J.T.; Beach, C.L.; Larson, R.A. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: Studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J. Clin. Oncol. 2006, 24, 3895–3903. [Google Scholar] [PubMed]
- Wan, B.O.; Wells, R.A.; Chodirker, L.; Rockwood, K.; Geddes, M.; Zhu, N.; Christou, G.; Sabloff, M.M.; Keating, M.-M.; Leber, B.; et al. Prognostic Performance of Frailty Measures in MDS Patients Treated with Hypomethylating Agents. Blood 2019, 134 (Suppl. S1), 4245. [Google Scholar]
- Garcia-Manero, G.; Fenaux, P. Hypomethylating agents and other novel strategies in myelodysplastic syndromes. J. Clin. Oncol. 2011, 29, 516–523. [Google Scholar]
- Dhillon, S. Decitabine/Cedazuridine: First Approval. Drugs 2020, 80, 1373–1378. [Google Scholar]
- Tendas, A.; Lissia, M.F.; Piccioni, D.; Tirimbelli, L.; Scaramucci, L.; Giovannini, M.; Dentamaro, T.; Perrotti, A.; de Fabritiis, P.; Niscola, P. Obstacles to adherence to azacitidine administration schedule in outpatient myelodysplastic syndrome and related disorders. Support. Care Cancer 2015, 23, 303–305. [Google Scholar]
- Wood, E.M.; McQuilten, Z.K. Outpatient transfusions for myelodysplastic syndromes. Hematol. Am. Soc. Hematol. Educ. Program. 2020, 2020, 167–174. [Google Scholar]
- Wan, Z.; Han, B. High-dose regimens of hypomethylating agents promote transfusion independence in IPSS lower-risk myelodysplastic syndromes: A meta-analysis of prospective studies. Aging 2021, 13, 11120–11134. [Google Scholar]
- Bell, J.A.; Galaznik, A.; Blazer, M.; Farrelly, E.; Ogbonnaya, A.; Raju, A.; Eaddy, M.; Fram, R.J.; Faller, D.V. Transfusion-free interval is associated with improved survival in patients with higher-risk myelodysplastic syndromes engaged in routine care. Leuk. Lymphoma 2019, 60, 49–59. [Google Scholar] [PubMed]
- Taplitz, R.A.; Kennedy, E.B.; Bow, E.J.; Crews, J.; Gleason, C.; Hawley, D.K.; Langston, A.A.; Nastoupil, L.J.; Rajotte, M.; Rolston, K.V.; et al. Antimicrobial Prophylaxis for Adult Patients With Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Weisel, D.; Gao, F.; Uy, G.L.; Cashen, A.F.; Jacoby, M.A.; Wartman, L.D.; Ghobadi, A.; Pusic, I.; Romee, R.; et al. Patterns of infectious complications in acute myeloid leukemia and myelodysplastic syndromes patients treated with 10-day decitabine regimen. Cancer Med. 2017, 6, 2814–2821. [Google Scholar] [PubMed]
- Livio, P.; Alessandro, B.; Anna, C.; Marianna, C.; Matteo, G.D.P.; Luana, F.; Federica, L.; Francesco, M.; Maria, T.V. Risk of infection in elderly patients with AML and MDS treated with hypomethylating agents. Acta Biomed. 2018, 89, 5–39. [Google Scholar]
- Davidoff, A.J.; Hu, X.; Bewersdorf, J.P.; Wang, R.; Podoltsev, N.A.; Huntington, S.F.; Gore, S.D.; Ma, X.; Zeidan, A.M. Hypomethylating agent (HMA) therapy use and survival in older adults with Refractory Anemia with Excess Blasts (RAEB) in the United States (USA): A large propensity score-matched population-based study†. Leuk. Lymphoma 2020, 61, 1178–1187. [Google Scholar] [CrossRef]
- Sekeres, M.A.; Schoonen, W.M.; Kantarjian, H.; List, A.; Fryzek, J.; Paquette, R.; Maciejewski, J.P. Characteristics of US patients with myelodysplastic syndromes: Results of six cross-sectional physician surveys. J. Natl. Cancer Inst. 2008, 100, 1542–1551. [Google Scholar] [PubMed]
- Steensma, D.P.; Komrokji, R.S.; Stone, R.M.; List, A.F.; Garcia-Manero, G.; Huber, J.M.; Dennison, B.; Sekeres, M.A. Disparity in perceptions of disease characteristics, treatment effectiveness, and factors influencing treatment adherence between physicians and patients with myelodysplastic syndromes. Cancer 2014, 120, 1670–1676. [Google Scholar]
- Ma, X.; Steensma, D.P.; Scott, B.L.; Kiselev, P.; Sugrue, M.M.; Swern, A.S. Selection of patients with myelodysplastic syndromes from a large electronic medical records database and a study of the use of disease-modifying therapy in the United States. BMJ Open 2018, 8, e019955. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).