Breast Reconstruction Use and Impact on Surgical and Oncologic Outcomes Amongst Inflammatory Breast Cancer Patients—A Systematic Review †
Abstract
1. Introduction
2. Methods
2.1. Study Strategy
2.2. Eligibility Criteria
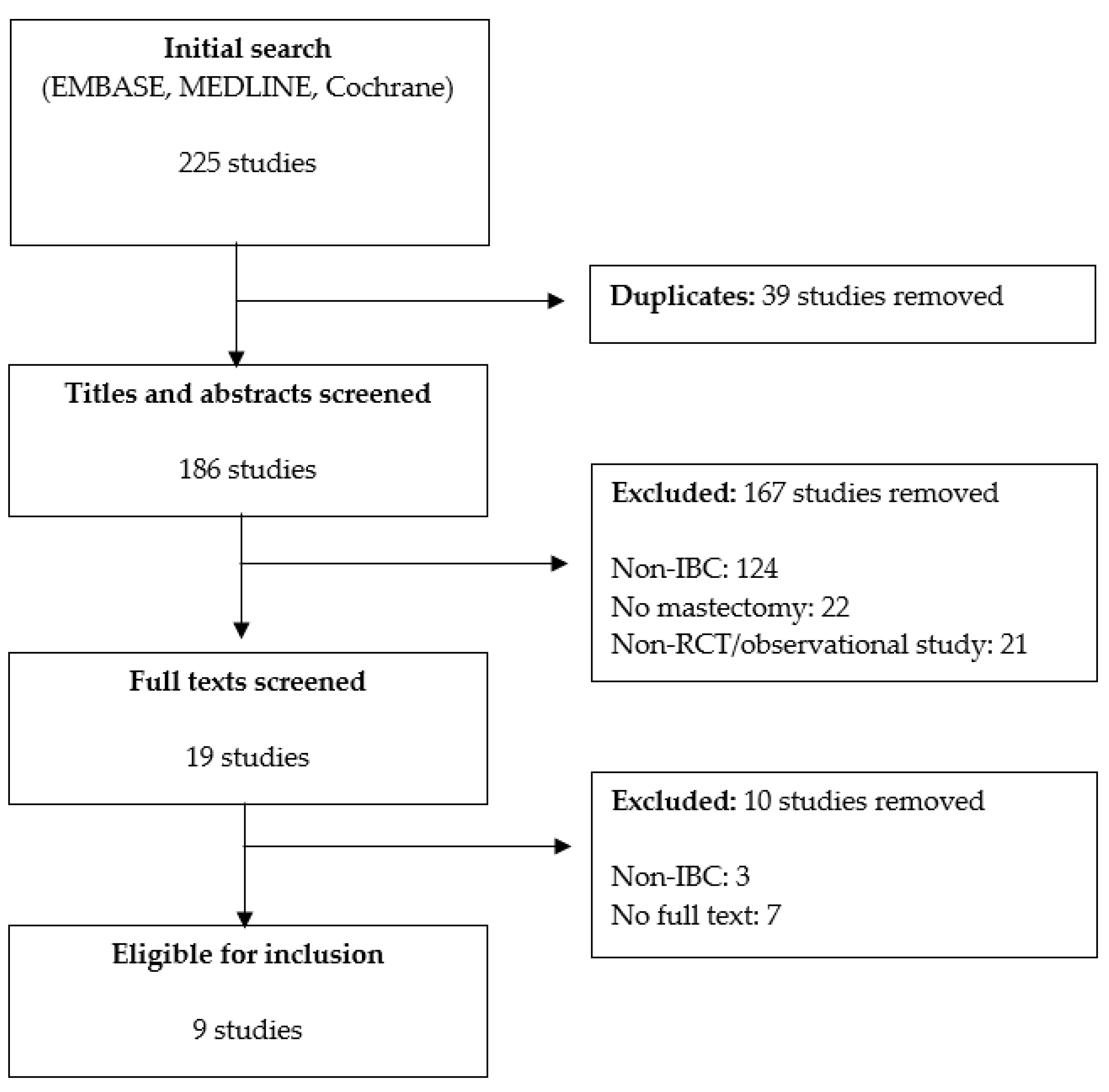
2.3. Study Selection
2.4. Data Extraction
2.5. Definitions of Outcomes of Interest
2.6. Quality Assessment
2.7. Data Synthesis and Analysis
3. Results
3.1. Methodological Quality
3.2. Patient Population
3.3. Contemporary Trends in Breast Reconstruction Use and Contralateral Prophylactic Mastectomy
3.4. Factors Associated with Breast Reconstruction Use
3.5. Breast Reconstruction and Oncologic Outcomes
Post-Operative Complications
3.6. Time to Adjuvant Therapy
3.7. Overall Survival and Mortality
3.8. Breast-Cancer Specific Survival (BCSS)
3.9. Recurrence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menta, A.; Fouad, T.M.; Lucci, A.; Le-Petross, H.; Stauder, M.C.; Woodward, W.A.; Ueno, N.T.; Lim, B. Inflammatory breast cancer: What to know about this unique, aggressive breast cancer. Surg. Clin. N. Am. 2018, 98, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Hester, R.H.; Hortobagyi, G.N.; Lim, B. Inflammatory breast cancer: Early recognition and diagnosis is critical. Am. J. Obstet. Gynecol. 2021, 225, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Palangie, T.; Mosseri, V.; Mihura, J.; Campana, F.; Beuzeboc, P.; Dorval, T.; Garcia-Giralt, E.; Jouve, M.; Scholl, S.; Asselain, B.; et al. Prognostic factors in inflammatory breast cancer and therapeutic implications. Eur. J. Cancer 1994, 30A, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Cristofanilli, M. Systemic treatments for inflammatory breast cancer. Breast Dis. 2006, 22, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Valero, V.; Buzdar, A.U.; Kau, S.W.; Broglio, K.R.; Gonzalez-Angulo, A.M.; Sneige, N.; Islam, R.; Ueno, N.T.; Buchholz, T.A.; et al. Inflammatory breast cancer (IBC) and patterns of recurrence: Understanding the biology of a unique disease. Cancer 2007, 110, 1436–1444. [Google Scholar] [CrossRef]
- Adesoye, T.; Lucci, A. Current surgical management of inflammatory breast cancer. Ann. Surg. Oncol. 2021, 28, 5461–5467. [Google Scholar] [CrossRef]
- Ueno, N.T.; Espinosa Fernandez, J.R.; Cristofanilli, M.; Overmoyer, B.; Rea, D.; Berdichevski, F.; El-Shinawi, M.; Bellon, J.; Le-Petross, H.T.; Lucci, A.; et al. International consensus on the clinical management of inflammatory breast cancer from the Morgan Welch Inflammatory Breast Cancer Research Program 10th anniversary conference. J. Cancer 2018, 9, 1437–1447. [Google Scholar] [CrossRef]
- Thoms, W.W., Jr.; McNeese, M.D.; Fletcher, G.H.; Buzdar, A.U.; Singletary, S.E.; Oswald, M.J. Multimodal treatment for inflammatory breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 739–745. [Google Scholar] [CrossRef]
- Baldini, E.; Gardin, G.; Evangeslita, G.; Prcohilo, T.; Collecchi, P.; Lionetto, R. Long-term results of combined-modality therapy for inflammatory breast carcinoma. Clin. Breast Cancer 2004, 5, 358–363. [Google Scholar] [CrossRef]
- Rehman, S.; Reddy, C.A.; Tendulkar, R.D. Modern outcomes of inflammatory breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 619–624. [Google Scholar] [CrossRef]
- Bristol, I.J.; Woodward, W.A.; Strom, E.A.; Cristofanilli, M.; Domain, D.; Singletary, S.E.; Paerkins, G.H.; Oh, J.L.; Terrefe, W.; Sahin, A.A. Locoregional treatment outcomes after multimodality management of inflammatory breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Mortenson, M.M.; Schneider, P.D.; Khatri, V.P.; Stevenson, T.R.; Whetzel, T.P.; Sommerhaug, E.J.; Goodnight, J.E., Jr.; Bold, R.J. Immediate breast reconstruction after mastectomy increases wound complications: However, initiation of adjuvant chemotherapy is not delayed. Arch. Surg. 2004, 139, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Karadsheh, M.J.; Katsnelson, J.Y.; Ruth, K.J.; Weiss, E.S.; Krupp, J.C.; Sigurdson, E.R.; Bleicher, R.J.; Ng, M.; Shafqat, M.S.; Patel, S.A. Breast reconstruction in inflammatory breast cancer: An analysis of predictors, trends, and survival from the National Cancer Database. Plast Reconstr. Surg. Glob. Open 2021, 9, e3528. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, K.; Wang, M.; Wang, F.; Zhang, M.; Zhang, P. A standard mastectomy should not be the only recommended breast surgical treatment for non-metastatic inflammatory breast cancer: A large population-based study in the Surveillance, Epidemiology, and End Results database 18. Breast 2017, 35, 48–54. [Google Scholar] [CrossRef]
- Adesoye, T.; Sun, S.X.; Scheaverien, M.V.; Woodard, W.A.; Lucci, A. Immediate breast reconstruction in inflammatory breast cancer: Are we there yet? Ann. Surg. Oncol. 2022, 29, 4019–4021. [Google Scholar] [CrossRef]
- Peterson, J.; Welch, V.; Losos, M.; Tugwell, P.J. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. Ott. Hosp. Res. Inst. 2011, 2, 1–2. [Google Scholar]
- Chang, E.I.; Chang, E.I.; Ito, R.; Zhang, H.; Nguyen, A.T.; Skoracki, R.J.; Hanasono, M.M.; Crosby, M.A.; Ueno, N.T.; Hunt, K.K. Challenging a traditional paradigm: 12-year experience with autologous free flap breast reconstruction for inflammatory breast cancer. Plast Reconstr. Surg. 2015, 135, 262e–269e. [Google Scholar] [CrossRef]
- Chin, P.L.; Andersen, J.S.; Somlo, G.; Chu, D.Z.; Schwarz, R.E.; Ellenhorn, J.D. Esthetic reconstruction after mastectomy for inflammatory breast cancer: Is it worthwhile? J. Am. Coll. Surg. 2000, 190, 304–309. [Google Scholar] [CrossRef]
- Hoffman, D.I.; Santos, P.M.; Goldbach, M.; Keele, L.J.; Taunk, N.K.; Bogen, H.S.; Burkbauer, L.; Jankowitz, R.C.; Fosnot, J.; Wu, L.C.; et al. Immediate breast reconstruction for inflammatory breast cancer: Trends in use and clinical outcomes 2004–2016. Ann. Surg. Oncol. 2021, 28, 8789–8801. [Google Scholar] [CrossRef]
- Nakhlis, F.; Regan, M.M.; Chun, Y.S.; Dominici, L.S.; Caterson, S.; Bellon, J.R.; Warren, L.; Yeh, E.D.; Jacene, H.A.; King, T.A.; et al. Patterns of breast reconstruction in patients diagnosed with inflammatory breast cancer: The Dana-Farber Cancer Institute’s Inflammatory Breast Cancer Program experience. Breast J. 2020, 26, 384–390. [Google Scholar] [CrossRef]
- Patel, S.A.; Ng, M.; Nardello, S.M.; Ruth, K.; Bleicher, R.J. Immediate breast reconstruction for women having inflammatory breast cancer in the United States. Cancer Med. 2018, 7, 2887–2902. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.B.; McCray, D.; Wengler, C.; Crowe, J.P.; Djohan, R.; Tendulkar, R.; O’Rourke, C.; Grobmyer, S.R.; Valente, S.A. Immediate reconstruction in inflammatory breast cancer: Challenging current care. Ann. Surg. Oncol. 2016, 23, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.G.; Giannakeas, V.; Semple, J.L.; Narod, S.A.; Lim, D.W. Contemporary trends in breast reconstruction use and impact on survival among women with inflammatory breast cancer. Ann. Surg. Oncol. 2022, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Romanoff, A.; Zabor, E.C.; Petruolo, O.; Stempel, M.; El-Tamer, M.; Morrow, M.; Barrio, A.V. Does nonmetastatic inflammatory breast cancer have a worse prognosis than other nonmetastatic T4 cancers? Cancer 2018, 124, 4314–4321. [Google Scholar] [CrossRef]
- Rosso, K.J.; Tadros, A.B.; Weiss, A.; Watneke, C.L.; DeSnyder, S.; Kuerer, H.; Ueno, N.T.L.; Stecklein, S.R.; Woodward, W.A.; Lucci, A. Improved locoregional control in a contemporary cohort of nonmetastatic inflammatory breast cancer patients undergoing surgery. Ann. Surg. Oncol. 2017, 24, 2981–2988. [Google Scholar] [CrossRef]
- Warren, L.E.; Guo, H.; Regan, M.M.; Nakhlis, F.; Yeh, E.D.; Jacene, H.A.; Hirshfield-Bartek, J.; Overmoyer, B.A.; Bellon, J.R. Inflammatory breast cancer: Patterns of failure and the case for aggressive locoregional management. Ann. Surg. Oncol. 2015, 22, 2483–2491. [Google Scholar] [CrossRef]
- Van Uden, D.J.; van Maaren, M.C.; Bult, P.; Strobbe, L.J.; van der Hoeven, J.J.; Blanken-Peeters, C.F.; Siesling, S.; de Wilt, J.H. Pathologic complete response and overall survival in breast cancer subtypes in stage III inflammatory breast cancer. Breast Cancer Res. Treat. 2019, 176, 217–226. [Google Scholar] [CrossRef]
- Lee, R.X.; Yogeswaran, G.; Wilson, E.; Oni, G. Barriers and facilitators to breast reconstruction in ethnic minority women—A systematic review. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 463–474. [Google Scholar] [CrossRef]
- Jagsi, R.; Jiang, J.; Momoh, A.O.; Alderman, A.; Giordano, S.H.; Buchholz, T.A.; Kronowitz, S.J.; Smith, B.D. Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J. Clin. Oncol. 2014, 32, 919. [Google Scholar] [CrossRef]
- Morrow, M.; Scott, S.K.; Menck, H.R.; Mustoe, T.A.; Winchester, D.P. Factors influencing the use of breast reconstruction postmastectomy: A National Cancer Database study. J. Am. Coll. Surg. 2001, 192, 1–8. [Google Scholar] [CrossRef]
- Karunanayake, M.; Bortoluzzi, P.; Chollet, A.; Lin, J.C. Factors influencing the rate of post-mastectomy breast reconstruction in a Canadian teaching hospital. Plast. Surg. 2017, 25, 242–248. [Google Scholar] [CrossRef]
- Paterson, C.; Lengacher, C.A.; Donovan, K.A.; Kip, K.E.; Tofthagen, C.S. Body image in younger breast cancer survivors: A systematic review. Cancer Nurs. 2016, 39, E39. [Google Scholar] [CrossRef]
- Koçan, S.; Gürsoy, A. Body image of women with breast cancer after mastectomy: A qualitative research. J. Breast Cancer 2016, 12, 145. [Google Scholar]
- Oh, D.D.; Flitcroft, K.; Brenna, M.E.; Spillane, A.J. Patterns and outcomes of breast reconstruction in older women–a systematic review of the literature. Eur. J. Surg. Oncol. 2016, 42, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Droulias, C.A.; Sewell, C.W.; McSweeney, M.B.; Powell, R.W. Inflammatory carcinoma of the breast: A correlation of clinical, radiologic and pathologic findings. Ann. Surg. 1976, 184, 217. [Google Scholar] [CrossRef] [PubMed]
- Tracy, M.S.; Rosenberg, S.M.; Dominici, L.; Partridge, A.H. Contralateral prophylactic mastectomy in women with breast cancer: Trends, predictors, and areas for future research. Breast Cancer Res. Treat. 2013, 140, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, L.L.; Tran, K.N.; Heelan, M.C.; Van Zee, K.J.; Massie, M.J.; Payne, D.K.; Borgen, P.I. Issues of regret in women with contralateral prophylactic mastectomies. Ann. Surg. Oncol. 1999, 6, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.W.; Metcalfe, K.A.; Narod, S.A. Bilateral mastectomy in women with unilateral breast cancer: A review. JAMA Surg. 2021, 6, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.A.; Fischer, J.P.; Chung, C.; Wu, L.C.; Sertelli, J.M.; Kovach, S.J. Risk of readmission following immediate breast reconstruction: Results from the 2011 American College of Surgeons National Surgical Quality Improvement Program data sets. Plast. Reconstr. Surg. 2014, 134, 193e–201e. [Google Scholar] [CrossRef]
- Jagsi, R.; Jiang, J.; Momoh, A.O.; Alderman, A.; Giordano, S.H.; Buchholaz, T.A.; Pierce, L.J.; Kronowitz, S.J.; Smith, B.D. Complications after mastectomy and immediate breast reconstruction for breast cancer: A claims-based analysis. Ann. Surg. 2016, 263, 219. [Google Scholar] [CrossRef]
- Siegel, E.L.; Whiting, J.; Kim, Y.; Sun, W.; Laronga, C.; Lee, M.C. Effect of surgical complications on outcomes in breast cancer patients treated with mastectomy and immediate reconstruction. Breast Cancer Res. Treat. 2021, 188, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Magno-Padron, D.A.; Collier, W.; Kim, J.; Agarwal, J.P.; Kwok, A.C. A nationwide analysis of early and late readmissions following free tissue transfer for breast reconstruction. J. Reconstr. Microsurg. 2020, 36, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Masoomi, H.; Paydar, K.Z.; Evans, G.R.; Tan, E.; Lane, K.T.; Wirth, G. Does immediate tissue expander placement increase immediate postoperative complications in patients with breast cancer? Am. Surg. 2015, 81, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Tsoutsou, P.G.; Koukourakis, M.I.; Azria, D.; Belkacemi, Y. Optimal timing for adjuvant radiation therapy in breast cancer: A comprehensive review and perspectives. Crit. Rev. Oncol. Hematol. 2009, 71, 102–116. [Google Scholar] [CrossRef]
- Tsoutsou, P.G.; Belkacemi, Y.; Gligorov, J.; Kuten, A.; Boussen, H.; Bese, N.; Koukourakis, M.I. Association of Radiotherapy and Oncology in the Mediterranean area (AROME). Optimal sequence of implied modalities in the adjuvant setting of breast cancer treatment: An update on issues to consider. Oncologist 2010, 15, 1169–1178. [Google Scholar] [CrossRef]
- Cao, L.; Xu, C.; Cai, G.; Qi, W.X.; Cai, R.; Wang, S.B.; Ou, D.; Li, M.; Shen, K.W.; Chen, J.Y. How does the interval between completion of adjuvant chemotherapy and initiation of radiotherapy impact clinical outcomes in operable breast cancer patients? Ann. Surg. Oncol. 2021, 28, 2155–2168. [Google Scholar] [CrossRef]
- Baek, S.H.; Bae, S.J.; Yoon, C.I.; Park, S.E.; Cha, C.H.; Ahn, S.G.; Kim, Y.S.; Roh, T.S.; Jeong, J. Immediate breast reconstruction does not have a clinically significant impact on adjuvant treatment delay and subsequent survival outcomes. J. Breast Cancer 2019, 22, 109–119. [Google Scholar] [CrossRef]
- Shammas, R.L.; Ren, Y.; Thomas, S.M.; Hollenback, S.T.; Greenup, R.A.; Blitzblau, R.C. Immediate breast reconstruction allows for the timely initiation of post-mastectomy radiation therapy. Plast. Reconstr. Surg. 2019, 144, 347e. [Google Scholar] [CrossRef]
- Muzaffar, M.; Johnson, H.M.; Vohra, N.A.; Liles, D.; Wong, J.H. The impact of locoregional therapy in nonmetastatic inflammatory breast cancer: A population-based study. Int. J. Breast Cancer 2018, 2018, 6438635. [Google Scholar] [CrossRef]
- Nakhlis, F. Inflammatory breast cancer: Is there a role for de-escalation of surgery? Ann. Surg. Oncol. 2022, 29, 6106–6611. [Google Scholar] [CrossRef]
| Study Author, (Publication Year), Article Country of Origin | Methodology (Years Analyzed) | Sample Size | Median Age of Diagnosis, Years (Range) | Race | Insurance Status | Median Income (USD) | Patient Location |
|---|---|---|---|---|---|---|---|
| Chang et al. [17], (2015) USA | Retrospective (2000–2012) | 830 | 48.0 (27–65) | NR | NR | NR | NR |
| Chen et al. [14], (2017) China | Retrospective SEER (1998–2013) | 3224 | 53.0 (22–90) | White: 2723 Black: 434 Asian/Indian: 209 Unk: 8 | NR | NR | NR |
| Chin et al. [18], (2000) USA | Retrospective (1987–1997) | 23 | 49 (35–62) | NR | NR | NR | NR |
| Hoffman et al. [19], (2021) USA | Retrospective NCDB (2004–2016) | 6589 | 54.9 ± 12.1 (mean ± SD) | White: 5358 Black: 960 Asian: 152 Other/Unk: 119 | Uninsured: 304 Private: 3827 Medicaid: 836 Medicare: 1444 Other govt: 85 Unk: 93 | <40,227: 1250 40,227–50,353: 1469 50,354–63,332: 1542 >63,333: 2217 Other/Unk: 111 | Metro: 5356 Urban: 948 Rural: 136 Other/Unk: 149 |
| Karadsheh et al. [13], (2021) USA | Retrospective NCDB (2004–2016) | 12,544 | 56.9 mean | NR | Uninsured: 521 Private: 6665 Medicaid: 1569 Medicare: 3484 Other govt: 143 Unk: 162 | <38,000: 2309 38,000–47,999: 2989 48,000–62,999: 3422 >63,000: 3748 Unk: 76 | Large metro: 6382 Metro: 3810 Urban: 746 Rural: 1293 Unk: 313 |
| Nair et al. [23], (2022) Canada | Retrospective SEER (2004–2015) | 4076 | 55.1 ± 12.5 (mean ± SD) | White: 3242 Black: 569 Southeast Asian: 112 East Asian: 58 Other/Unk: 95 | Uninsured: 304 Private: 3827 Medicaid: 836 Medicare: 1444 Other govt: 85 Unk: 93 | <55,000: 1032 55,000–74,999: 1993 >75,000: 1050 Unk: 1 | Metro: 3209 Non-metro: 856 Unk: 11 |
| Nakhlis et al. [20], (2019) USA | Retrospective (1997–2016) | 240 | 51.0 (28–78) | NR | NR | NR | NR |
| Patel et al. [21], (2018) USA | Retrospective SEER-Medicare (1991–2009) | 1472 | 75.5 (65–103) | White: 1235 Black: 153 Other: 84 | Medicare: 1472 | <25,000: 155 25,000–50,000: 844 50,000–75,000: 348 >75,000: 125 | Large metro: 750 Metro: 480 Urban: 84 Rural: 158 |
| Simpson et al. [22], (2016) USA | Retrospective (2006–2014) | 60 | 55 (33–67) | NR | NR | NR | NR |
| Study | Selection | Comparability | Outcome | Score | Overall Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Representative Exposed Cohort | Selection of Non-Exposed Cohort | Ascertainment of Exposure | Prospective | Control for Age, Stage | Other Controls | Outcome Assessment | Length Follow-Up | Adequate Follow-Up | |||
| Chang et al., 2015 [17] | (A) * | (A) * | (A) * | (B) | (C) | (C) | (B) * | (A) * | (A) * | 6 | Poor |
| Chen et al., 2017 [14] | (A) * | (A) * | (A) * | (B) | (A) * | (B) * | (B) * | (A) * | (A) * | 8 | Good |
| Chin et al., 2000 [18] | (A) * | (C) | (A) * | (B) | (C) | (C) | (B) * | (A) * | (A) * | 5 | Poor |
| Hoffman et al., 2021 [19] | (A) * | (A) * | (A) * | (B) | (A) * | (B) * | (B) * | (A) * | (A) * | 8 | Good |
| Karadsheh et al., 2021 [13] | (A) * | (A) * | (A) * | (B) | (A) * | (B) * | (B) * | (A) * | (A) * | 8 | Good |
| Nair et al., 2022 [23] | (A) * | (A) * | (A) * | (B) | (A) * | (B) * | (B) * | (A) * | (A) * | 8 | Good |
| Nakhlis et al., 2020 [20] | (A) * | (A) * | (A) * | (B) | (C) | (C) | (A) * | (A) * | (A) * | 6 | Poor |
| Patel et al., 2018 [21] | (A) * | (A) * | (A) * | (B) | (A) * | (B) * | (A) * | (A) * | (A) * | 8 | Good |
| Simpson et al., 2016 [22] | (A) * | (A) * | (A) * | (B) | (C) | (B) * | (A) * | (A) * | (A) * | 7 | Good |
| Study | BRC Surgery | BRC Type | Treatment History |
|---|---|---|---|
| Chang et al., (2015) [17] | NBRC: 771 (92.9%) IBRC: 7 (0.8%) DBRC: 52 (6.3%) | 59 ATL (100.0%) | 59 CTX, XRT (100%) 45 SMX (5.4%) 12 SMX + CPM (1.4%) 1 DMX (for bilateral breast cancer) (0.1%) 1 SMX + CL (0.1%) |
| Chen et al., (2017) [14] | NBRC: 2960 (91.8%) IBRC: 264 (8.2%) | 112 ATL (42.4%) 68 ALP (25.8%) 30 CMB (11.4%) 54 unspecified (20.4%) | 3224 CTX, XRT (100%) 2632 SMX (81.6%) 592 SMX + CPM (18.4%) |
| Chin et al., (2000) [18] | IBRC: 14 (60.9%) DBRC: 9 (39.1%) | 20 ATL (87.0%) 3 ALP (13.0%) | 22 CTX (95.7%) 14 XRT (60.9%) 23 SMX (100%) |
| Hoffman et al., (2021) [19] | NBRC: 5954 (90.4%) IBRC: 635 (9.6%) | 250 ATL (39.4%) 171 ALP (26.9%) 64 CMB (10.1%) 150 unspecified (23.6%) | 6589 CTX, XRT (100%) 4031 SMX (61.2%) 1705 SMX + CPM (25.9%) 853 unknown if CPM performed (12.9%) |
| Karadsheh et al., (2021) [13] | NBRC: 11 237 (89.6%) IBRC:1307 (10.4%) | 491 ATL (37.6%) 374 ALP (28.6%) 142 CMB (10.9%) 300 unspecified (22.9%) | 12,252 CTX, XRT (97.7%) 9579 SMX (76.4%) 2965 SMX + CPM (23.6%) |
| Nair et al., (2022) [23] | NBRC: 3688 (90.5%) IBRC: 388 (9.5%) | NR | 3181 SMX (78.0%) 895 SMX + CPM (22.0%) |
| Nakhlis et al., (2019) [20] | NBRC: 200 (83.3%) IBRC: 13 (5.4%) DBRC: 27 (11.3%) | 29 ATL (72.5%) 9 ALP (22.5%) 1 CMB (2.5%) 1 unspecified (2.5%) | 240 ptx CTX (100%) 240 SMX (100%) |
| Patel et al., (2018) [21] | NBRC: 1428 (97.0%) IBRC: 44 (3.0%) | NR | 957 CTX (65.0%) 893 XRT (60.7%) 1472 SMX (100%) |
| Simpson et al., (2016) [22] | NBRC: 39 (65.0%) IBRC: 16 (26.7%) DBRC: 5 (8.3%) | 8 ATL (38.1%) 13 ALP (61.9%) | 60 CTX, XRT (100%) 43 SMX (71.7%) 17 SMX + CPM (28.3%) |
| Study | BRC Rate | CPM Rate | Demographic Factors | Tumor/Pathology Factors | Socioeconomic Factors |
|---|---|---|---|---|---|
| Chen et al., (2017) [14] | 6.5% to 10.9% (p = 0.011) | 6.1% to 29.4% (p < 0.001) | NR | DMX or SMX + CPM increased BRC (p < 0.001) | NR |
| Hoffman et al., (2021) [19] | 61% increase from 6.3% to 10.1% (p < 0.001) | NR | Younger age—BRC (49.5 ± 10.4) NBRC (55.4 ± 12.1) [OR 1.83, 95% CI 1.39–2.41] p < 0.01 No differences in race or comorbidity status | Bilateral mastectomy [OR 1.67, 95% CI 1.28–2.17] p < 0.01 No differences in tumor characteristics, immunohistochemical subtype, clinical, pathologic stage, tumor grade, margin status, lymphovascular invasion. | Private insurance—BRC (74.3%) vs. NBRC (56.3%) [OR 2.49, 95% CI 1.65–3.77]; p < 0.01 Not on Medicare—BRC (9.6%) vs. NBRC (23.2%); p < 0.01 Higher median income (>USD 63,333)—BRC (49.0%) vs. NBRC (32.0%); p < 0.01 Metropolitan region—BRC (90.4%) vs. NBRC (80.3%) [OR 2.34, 95% CI 1.36–4.03]; p < 0.01 Higher education; p < 0.01 |
| Karadsheh et al., (2021) [13] | 7.3% to 12.3% (p < 0.001) | 11.7% to 26.3% (p < 0.001) | Younger age—BRC (50.8 yrs) vs. NBRC (57.2 yrs); p < 0.001 Lower CCI; p < 0.001 | Lower pathologic stage; p < 0.001 Lower pathologic nodal status; p < 0.001 CPM; p < 0.001 | Private insurance—BRC (71.8%) vs. NBRC (51.0%); p < 0.001 Higher median income (>USD 63,000)—BRC (44.1%) vs. NBRC (28.2%); p < 0.001 Metropolitan region—BRC (65.3%) vs. NBRC (49.2%); p < 0.001 |
| Nair et al., (2022) [23] | 6.2% to 15.3% (p < 0.001) | 12.9% to 29.6% (p < 0.001) | Younger age (p < 0.0001) No difference in race or ethnicity | CPM, p < 0.0001 No differences in tumor size, grade, nodal burden, or receptor status. | High income (>USD 75,000); p = 0.027 Metropolitan living; p < 0.02 |
| Patel et al., (2018) [21] | NR | NR | Younger age—BRC (72.6 yrs) vs. NBRC (75.6 yrs); p = 0.008 Lower CCI; p = 0.29 | NR | Median income (USD 25,000–75,000) (p = 0.024) In MV analysis, income was an independent predictor (p = 0.047) |
| Study | Post-Operative Complication | Time to Adjuvant XRT |
|---|---|---|
| Chang et al., (2015) [17] | 21 complications out of 59 patients (7 delayed flap healing, 5 abdominal donor site healing, 1 abdominal donor site hernia, 1 total flap loss, 4 fat necrosis, 4 pedicle thrombosis, 2 medical complications) | NR |
| Hoffman et al., (2021) [19] | BRC longer length of stay than NBRC 2.4 (±8.0) days vs. 1.4 (±3.8) days; p < 0.01 No difference in 30-day readmission rates; p = 0.94 No difference in 30-day or 90-day mortality; p = 0.12 | BRC: 8 weeks (6–10 weeks) NBRC: 7 weeks (5–10 weeks) No difference in time to XRT between BRC and NBRC group p = 0.93 |
| Nakhlis et al., (2019) [20] | 1/13 IBRC (TRAM flap necrosis) 7/27 DBRC (1 abdominal donor site dehiscence, 1 chronic hematoma, 1 infection requiring hospitalization, 1 donor site hernia, 3 fat necrosis/capsular contracture) | NBRC: 3 months (1–10 mos) IBRC: 56.5 days (23–123 days) DBRC: 3 months (2–10 mos) |
| Patel et al., (2018) [21] | BRC: 3/44 implant-related complications (7%, p < 0.003 versus 0/1428 NBRC)) | NR |
| Simpson et al., (2016) [22] | NBRC: 1/39 (2.6%)—hematoma IBRC: 6/16 (37.5%)—2 infection, 1 expander removal, 2 tissue loss DBRC: 0/5 (0.0%) p = 0.006 | NBRC: 42 days IBRC: 52.5 days DBRC: 45 days BRC not associated with delay to XRT p = 0.86 |
| Study | Median Follow-Up (Months) | OS | OM | BCSS | Recurrence |
|---|---|---|---|---|---|
| Chang et al., (2015) [17] | 43.9 (5.1–140) | Median survival: 44.0 mos (38.6–48.6 mos) | BRC: 13.6% (8/59 deaths—7 IBC related, 1 other cause) NBRC: NR | 11.9% (7/59 patients) | 1 LRR at 7 mos |
| Chen et al., (2017) [14] | 47.0 (4–203) | 5-year OS: 55.9% 3-year OS improved over time (62.8% to 78.5%; p < 0.0001) | NR | 5-year BCSS: 59% 3-year BCSS improved over time (64.9% to 80.7%; p < 0.0001) | NR |
| Chin et al., (2000) [18] | 44.0 (14–120) | Median survival: 22.0 mos (1–120 mos) | BRC: 52% (12 IBC-related deaths) NBRC: NR | 52% (12/23 patients) | Median DFS: 19.0 mos (1.0–120 mos) 16 ptx: 6 LRR, 10 distant |
| Hoffman et al., (2021) [19] | IBRC 42.9 (24.4–76.3) NBRC 45.4 (23.7–80.9) | 5-year OS 64.3% BRC vs. 57.2% NBRC IBRC vs. NBRC Adjusted OS (HR 0.63, 95% CI 0.44–0.90, p = 0.01) PM cohort OS (0.60, 95% CI 0.40–0.92, p = 0.02) | NR | NR | NR |
| Karadsheh et al., (2021) [13] | NR | Median unadjusted OS BRC—93.7 mos (95% CI 72.2–117.5) NBRC—68.1 mos (95% CI 65.5–71.7) p < 0.001 | NR | NR | NR |
| Nair et al., (2022) [23] | Mean, 68.4 (±46.8) | NR | NR | Crude (10-year survival): IBRC (62.9%), NBRC (47.6%) PM analysis (10-year survival): BRC (56.6%) and NBRC (62.2%), not significant | NR |
| Nakhlis et al., (2019) [20] | 66.0 | Median survival: 87 mos (<1–212 mos) | NR | NR | Median DFS: 35 mos (<1–212 mos) 105 NBRC ptx after median follow-up: 66 mos (<1–212 mos) −6 LRR only −44 LRR + distant −55 Distant only 22/40 BRC ptx (12 IBRC, 10 DBRC) with median follow-up: 78 mos (7–191 mos) −3 LRR only −5 LRR + distant −14 distant |
| Patel et al., (2018) [21] | NR | NR | Cumulative incidence of OM lower amongst IR patients (p = 0.013) | No difference between IBR status in BCSM (sHR 1.04; CI 0.71–1.54; p = 0.83) or adjusted BCSM (sHR 1.13; CI 0.84–1.93; p = 0.058) IBR did not influence cumulative incidence of BCSM | NR |
| Simpson et al., (2016) [22] | 2.3 years (1.4–4.6 yrs) | 1-year OS 94.7% (95% CI 30.0–56.9) 2-year OS 76.5% (95% CI 65.6–89.2) | 22 ptx NBRC: 14 IBRC: 7 DBRC: 1 Median time to death 21.9 mos | NR | Median DFS: 9.9 mos 26 ptx overall NBRC: 18 ptx —LRR: 4 —Distant: 14 IBRC: 7 ptx —LRR: 0 —Distant: 7 DBRC: 1 ptx —LRR: 0 —distant: 1 Recurrence rate 1-year 30.9% (95% CI 17.9–48.1) 2-year 45.1% (95% CI 30.0–56.9) |
| Study | OS | OM | BCSS | Recurrence |
|---|---|---|---|---|
| Chang et al., (2015) [17] | BRC improved OS compared to NBRC (p = 0.004) | NR | NR | NR |
| Chen et al., (2017) [14] | In UV and MV analysis: younger age, increasing year of diagnosis, non-black race, married status, low histologic grade, lower N stage, positive hormonal status, 10+ lymph node removal | NR | In UV and MV analysis: younger age, increasing year of diagnosis, non-black race, married status, low histologic grade, lower N stage, positive hormonal status, 10+ lymph node removal | NR |
| Chin et al., (2000) [18] | Positive surgical margin decreased OS (p = 0.02) | NR | NR | Positive surgical margin increased local recurrence (p = 0.02) |
| Karadsheh et al., (2021) [13] | BRC vs. NBRC Unadjusted HR 0.79 (95% CI 0.72–0.88, p < 0.001) Adjusted HR 0.95 (95% CI 0.85–1.06; p = 0.35) | NR | NR | NR |
| Nair et al., (2022) [23] | NR | NR | Crude: BCSS higher in NBRC (HR 0.72, 95% CI 0.60–0.86; p < 0.001) PM analysis: No difference in BCSS HR 0.96, 95% CI 0.79–1.16; p = 0.65 | NR |
| Patel et al., (2018) [21] | NR | No difference in OM by race, US region, poverty, median income, year of IBC diagnosis. UV and MV analysis: BRC not associated with lower OM (HR = 0.82, CI 0.55–1.21; p = 0.319). Independent predictors of worse OM: older age, higher CCI, single or widowed, negative or unknown hormone receptor status, no or unknown # LN examined, and increasing # positive LN (p < 0.0001) Poor histologic grade (p = 0.0318) No radiation received (p = 0.0066), No chemotherapy received (p = 0.0343). | BCSS not associated with age of diagnosis, race, marital status, US region, SES factors, CCI, or XRT UV and MV analysis: BRC not associated with increased BCSS (HR = 1.14, CI 0.71–1.76; p = 0.55). Independent predictors of worse BCSS: Earlier diagnosis year (p = 0.0003) Poor or intermediate histologic grade (p = 0.0005) ER/PR negative or unknown, increasing # positive LN (p < 0.0001) Receiving CTX (p = 0.0006). | NR |
| Simpson et al., (2016) [22] | NR | BRC not associated with increased mortality p = 0.91 | NR | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, A.G.; Ko, G.T.Y.; Semple, J.L.; Lim, D.W. Breast Reconstruction Use and Impact on Surgical and Oncologic Outcomes Amongst Inflammatory Breast Cancer Patients—A Systematic Review. Curr. Oncol. 2023, 30, 6666-6681. https://doi.org/10.3390/curroncol30070489
Nair AG, Ko GTY, Semple JL, Lim DW. Breast Reconstruction Use and Impact on Surgical and Oncologic Outcomes Amongst Inflammatory Breast Cancer Patients—A Systematic Review. Current Oncology. 2023; 30(7):6666-6681. https://doi.org/10.3390/curroncol30070489
Chicago/Turabian StyleNair, Ananya Gopika, Gary Tsun Yin Ko, John Laurie Semple, and David Wai Lim. 2023. "Breast Reconstruction Use and Impact on Surgical and Oncologic Outcomes Amongst Inflammatory Breast Cancer Patients—A Systematic Review" Current Oncology 30, no. 7: 6666-6681. https://doi.org/10.3390/curroncol30070489
APA StyleNair, A. G., Ko, G. T. Y., Semple, J. L., & Lim, D. W. (2023). Breast Reconstruction Use and Impact on Surgical and Oncologic Outcomes Amongst Inflammatory Breast Cancer Patients—A Systematic Review. Current Oncology, 30(7), 6666-6681. https://doi.org/10.3390/curroncol30070489






