Imaging Spectrum of the Developing Glioblastoma: A Cross-Sectional Observation Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Collection
2.2. Imaging Review
2.2.1. Imaging Categorization
- Those with TRA GBM on imaging at presentation and subsequently confirmed with tissue diagnosis,
- Those with a tissue diagnosis of GBM obtained when imaging showed TRA GBM but that had imaging preceding TRA GBM,
- Those with a tissue diagnosis of GBM obtained when imaging showed non-typical GBM; that is, imaging at the time of tissue acquisition did not show a centrally necrotic, peripherally enhancing tumor with adjacent low CT density or high T2 MRI signal.
2.2.2. Incipient Image Patterns
- CT density—low or high density relative to cerebral grey matter; Hounsfield units were also acquired as outlined above;
- T2 signal—relative to cerebral grey matter;
- The presence of reduced diffusivity on diffusion-weighted imaging, as outlined above;
- Enhancement pattern including none, solid (complete homogeneous enhancement), nodular (small foci of internal enhancement), complete peripheral and patchy peripheral (incomplete ring enhancement).
2.3. Statistical Analysis
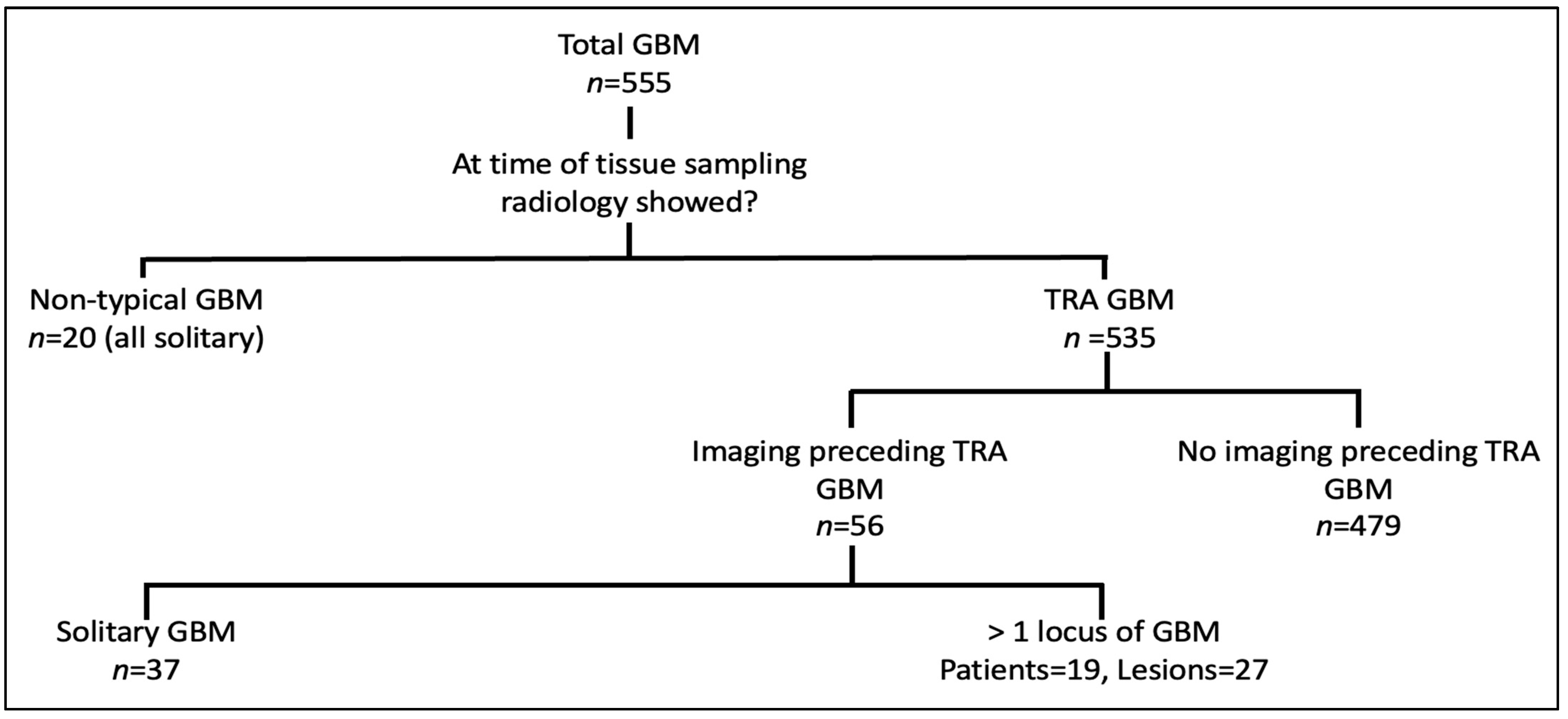
3. Results
- Non-typical GBM—patients who had tissue sampling and never showed TRA GBM, n = 20 (3.6%);
- TRA GBM at the time of tissue sampling with preceding imaging, n = 56 (10.1%);
- TRA GBM at the time of tissue sampling with no preceding imaging n = 479 (86.3%).
3.1. Common Imaging Patterns
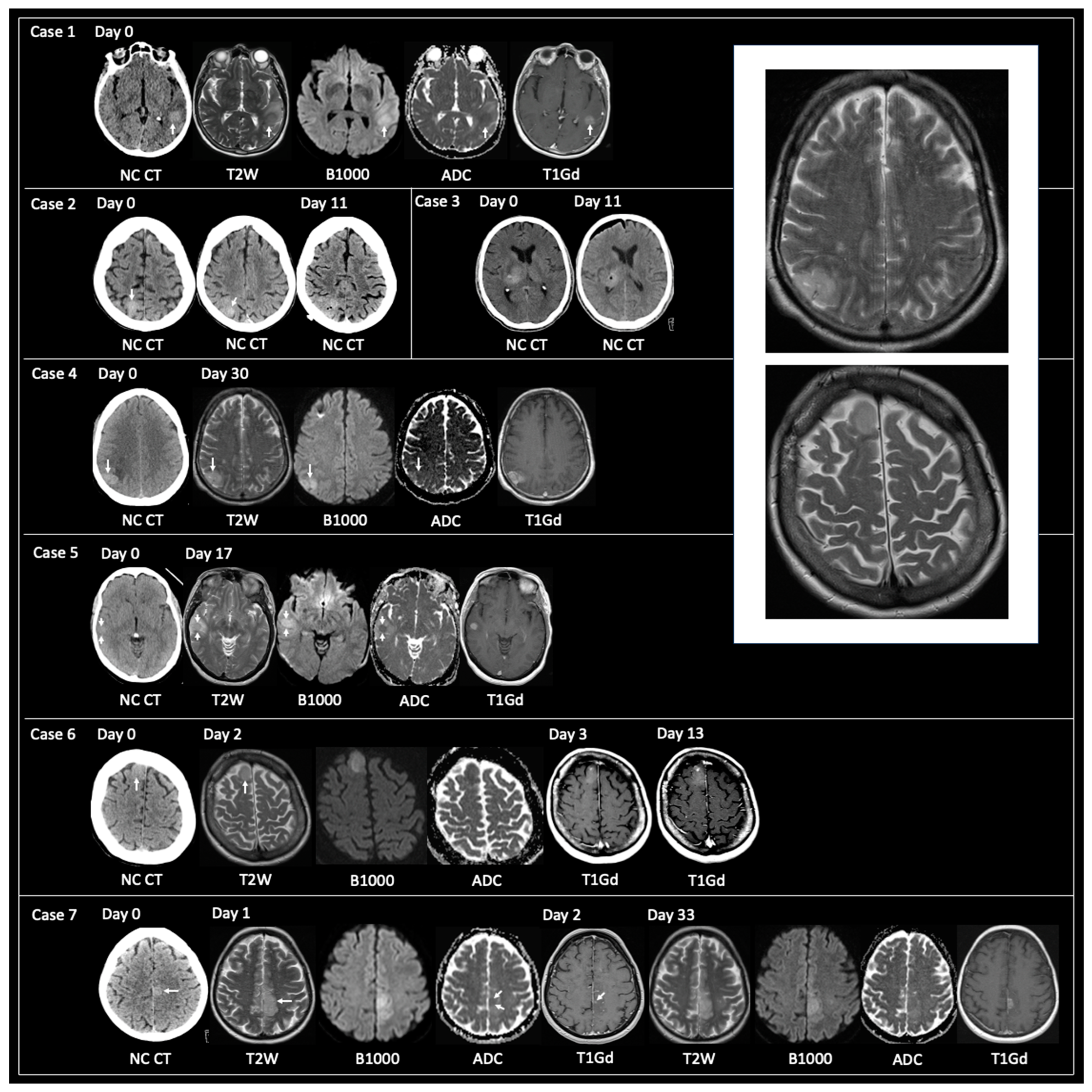
3.1.1. Non-Typical GBM at Time of Tissue Sampling
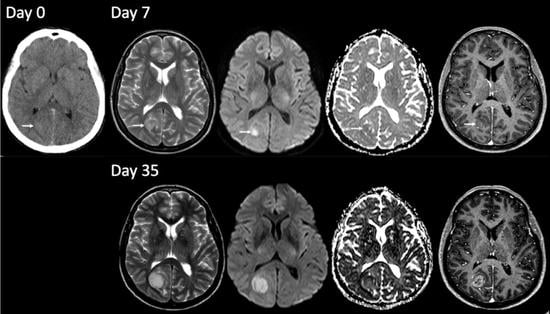
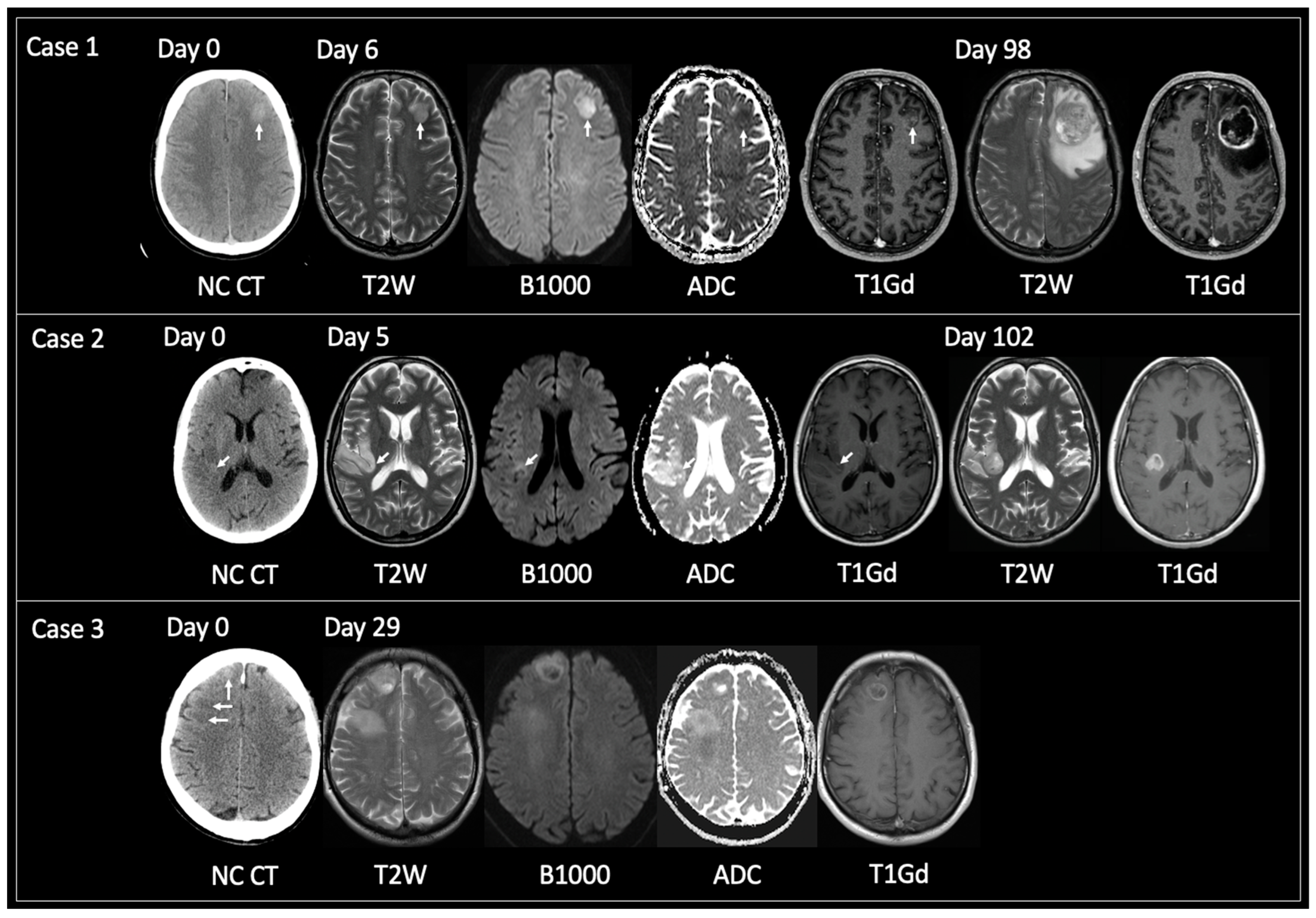
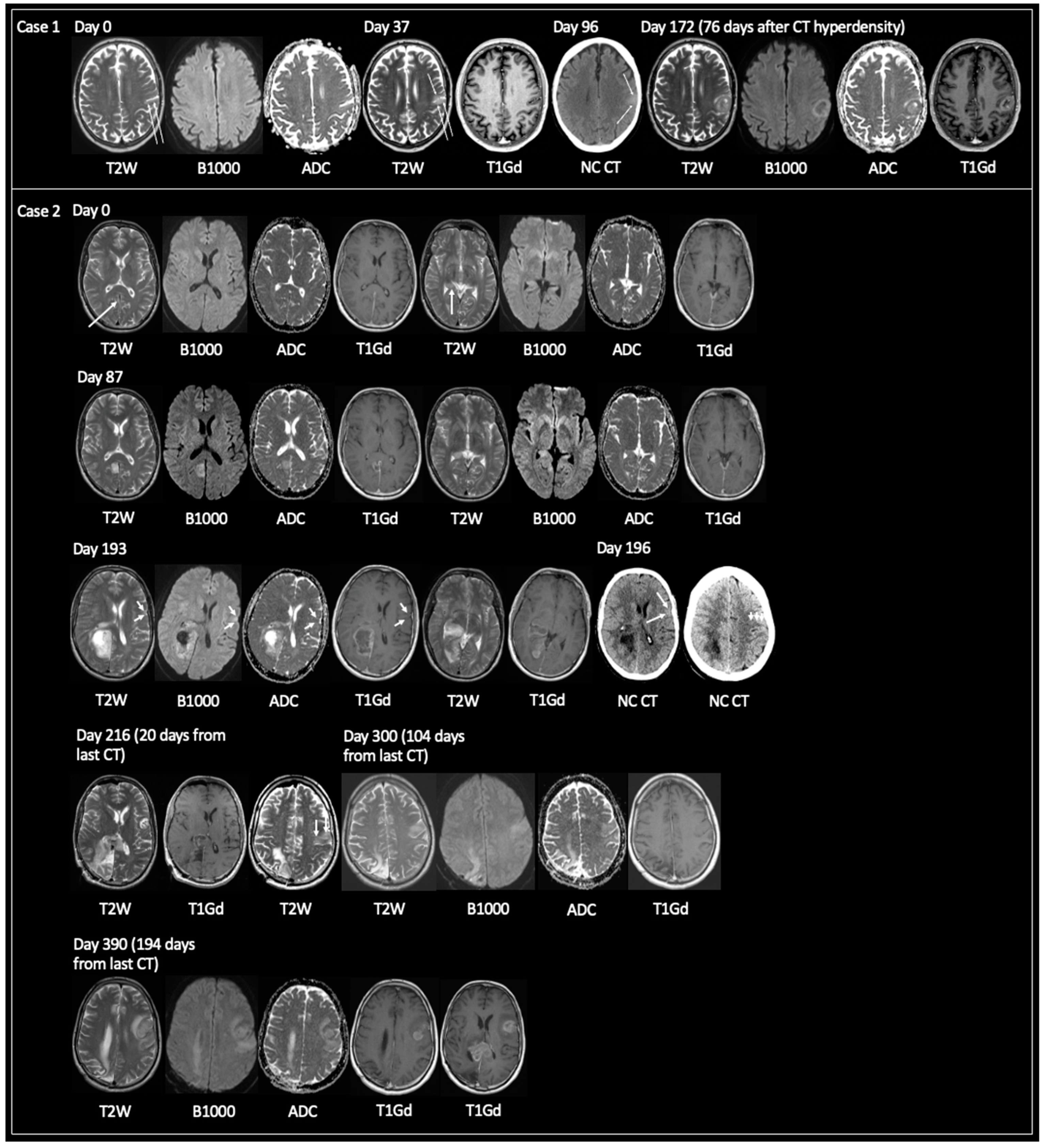
3.1.2. TRA GBM with Preceding Imaging
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Molinaro, A.M.; Taylor, J.W.; Wiencke, J.K.; Wrensch, M.R. Genetic and molecular epidemiology of adult diffuse glioma. Nat. Rev. Neurol. 2019, 15, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.R.; Wen, P.Y.; Lang-Orsini, M.; Chukwueke, U.N. World Health Organization 2021 Classification of Central Nervous System Tumors and Implications for Therapy for Adult-Type Gliomas: A Review. JAMA Oncol. 2022, 8, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Currie, S.; Fatania, K.; Matthew, R.; Wurdak, H.; Chakrabarty, A.; Murray, L.; Short, S. A comprehensive clinical review of adult-type diffuse glioma incorporating the 2021 World Health Organisation Classification. Neurographics 2022, 12, 43–70. [Google Scholar] [CrossRef]
- Iliadis, G.; Kotoula, V.; Chatzisotiriou, A.; Televantou, D.; Eleftheraki, A.G.; Lambaki, S.; Misailidou, D.; Selviaridis, P.; Fountzilas, G. Volumetric and MGMT parameters in glioblastoma patients: Survival analysis. BMC Cancer 2012, 12, 3. [Google Scholar] [CrossRef]
- Wangaryattawanich, P.; Hatami, M.; Wang, J.; Thomas, G.; Flanders, A.; Kirby, J.; Wintermark, M.; Huang, E.S.; Bakhtiari, A.S.; Luedi, M.M.; et al. Multicenter imaging outcomes study of The Cancer Genome Atlas glioblastoma patient cohort: Imaging predictors of overall and progression-free survival. Neuro-Oncology 2015, 17, 1525–1537. [Google Scholar] [CrossRef]
- Gregory, T.; Henson, J. Cortical T2-hyperintense lesions as the initial MRI finding in glioblastoma. Interdiscip. Neurosurg. Adv. Tech. Case Manag. 2021, 23, 100993. [Google Scholar] [CrossRef]
- Nam, T.-S.; Choi, K.-H.; Kim, M.-K.; Cho, K.-H. Glioblastoma Mimicking Herpes Simplex Encephalitis. J. Korean Neurosurg. Soc. 2011, 50, 119–122. [Google Scholar] [CrossRef]
- Thaler, P.B.; Li, J.Y.; Isakov, Y.; Black, K.S.; Schulder, M.; Demopoulos, A. Normal or non-diagnostic neuroimaging studies prior to the detection of malignant primary brain tumors. J. Clin. Neurosci. 2012, 19, 411–414. [Google Scholar] [CrossRef]
- Ideguchi, M.; Kajiwara, K.; Goto, H.; Sugimoto, K.; Nomura, S.; Ikeda, E.; Suzuki, M. MRI findings and pathological features in early-stage glioblastoma. J. Neuro-Oncol. 2015, 123, 289–297. [Google Scholar] [CrossRef]
- Takagi, I.; Shakur, S.F.; Lukas, R.V.; Eller, T.W. Spontaneous radiographic resolution and subsequent redemonstration of an untreated glioblastoma. J. Neurosurg. 2011, 115, 24–29. [Google Scholar] [CrossRef]
- Nishi, N.; Kawai, S.; Yonezawa, T.; Fujimoto, K.; Masui, K. Early Appearance of High Grade Glioma on Magnetic Resonance Imaging. Neurol. Med.-Chir. 2009, 49, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Simonet Redondo, M.; Auger Acosta, C.; Rovira-Gols, A.; Toledo Argany, M.; Sarria Estrada, S.; Rovira Canellas, A. Neuroimaging findings in the initial phase of development of high grade cortical astrocytomas. Radiologia 2012, 54, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Figus, A.; Corraine, S. Early presentation of de novo high grade glioma with epileptic seizures: Electroclinical and neuroimaging findings. Seizure 2010, 19, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Oyama, H.; Ando, Y.; Aoki, S.; Kito, A.; Maki, H.; Hattori, K.; Tanahashi, K. Glioblastoma detected at the initial stage in its developmental process -case report. Neurol. Med. Chir. 2010, 50, 414–417. [Google Scholar] [CrossRef]
- Cohen-Gadol, A.A.; DiLuna, M.L.; Bannykh, S.I.; Piepmeier, J.M.; Spencer, D.D. Non-enhancing de novo glioblastoma: Report of two cases. Neurosurg. Rev. 2004, 27, 281–285. [Google Scholar] [CrossRef]
- Okamoto, K.; Ito, J.; Takahashi, N.; Ishikawa, K.; Furusawa, T.; Tokiguchi, S.; Sakai, K. MRI of high-grade astrocytic tumors: Early appearance and evolution. Neuroradiology 2002, 44, 395–402. [Google Scholar] [CrossRef]
- Jung, T.Y.; Jung, S. Early neuroimaging findings of glioblastoma mimicking non-neoplastic cerebral lesion. Neurol. Med. Chir. 2007, 47, 424–427. [Google Scholar] [CrossRef]
- Toh, C.; Castillo, M. Early-Stage Glioblastomas: MR Imaging–Based Classification and Imaging Evidence of Progressive Growth. Am. J. Neuroradiol. 2017, 38, 288–293. [Google Scholar] [CrossRef]
- Ceravolo, I.; Barchetti, G.; Biraschi, F.; Gerace, C.; Pampana, E.; Pingi, A.; Stasolla, A. Early stage glioblastoma: Retrospective multicentric analysis of clinical and radiological features. Radiol. Medica 2021, 126, 1468–1476. [Google Scholar] [CrossRef]
- Perry, J.R.; Laperriere, N.; O’Callaghan, C.J.; Brandes, A.A.; Menten, J.; Phillips, C.; Fay, M.; Nishikawa, R.; Cairncross, G.; Roa, W.; et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N. Engl. J. Med. 2017, 376, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.R.; Pope, W.B.; Horvath, S.; Braunstein, J.G.; Nghiemphu, P.; Tso, C.-L.; Mellinghoff, I.; Lai, A.; Liau, L.M.; Mischel, P.S.; et al. Relationship between Survival and Edema in Malignant Gliomas: Role of Vascular Endothelial Growth Factor and Neuronal Pentraxin 2. Clin. Cancer Res. 2007, 13, 2592–2598. [Google Scholar] [CrossRef] [PubMed]
- Madhugiri, V.S.; Sreenivasan, S.A.; Sasidharan, G.M.; Kumar, R.V. Measuring glioma volumes: A comparison of linear measurement based formulae with the manual image segmentation technique. J. Cancer Res. Ther. 2016, 12, 161–168. [Google Scholar] [CrossRef]
- Kamalian, S.; Lev, M.H.; Gupta, R. Neuroimaging Part 1. In Handbook of Clinical Neurology, 1st ed.; Masdeu, J.C., Gonzalez, R.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Pramanik, P.P.; Parmar, H.A.; Mammoser, A.G.; Junck, L.R.; Kim, M.M.; Tsien, C.I.; Lawrence, T.S.; Cao, Y. Hypercellularity Components of Glioblastoma Identified by High b-Value Diffusion-Weighted Imaging. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 811–819. [Google Scholar] [CrossRef]
- Ishikura, R.; Ando, K.; Tominaga, S.; Nakao, N.; Ikeda, J.; Takemura, Y.; Morikawa, T. CT diagnosis of hyperdense intracranial neoplasms: Review of the literature. Nihon Igaku Hoshasen Gakkai Zasshi. 1999, 59, 105–112. [Google Scholar]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Markwell, S.M.; Ross, J.L.; Olson, C.L.; Brat, D.J. Necrotic reshaping of the glioma microenvironment drives disease progression. Acta Neuropathol. 2022, 143, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Z.; Zhang, Y.; Hou, F.; Fu, W.; Lin, J.; Liu, Y.; Liu, X. Additional Diagnostic Value of Unenhanced Computed Tomography plus Diffusion-Weighted Imaging Combined with Routine Magnetic Resonance Imaging Findings of Early-Stage Gliblastoma. BioMed Res. Int. 2020, 2020, 1672736. [Google Scholar] [CrossRef] [PubMed]
- Swanton, C. Intratumor Heterogeneity: Evolution through Space and Time. Cancer Res 2012, 72, 4875–4882. [Google Scholar] [CrossRef]
- Apostoli, A.J.; Ailles, L. Clonal evolution and tumor-initiating cells: New dimensions in cancer patient treatment. Crit. Rev. Clin. Lab. Sci. 2016, 53, 40–51. [Google Scholar] [CrossRef]
- Liu, C.; Sage, J.C.; Miller, M.R.; Verhaak, R.G.; Hippenmeyer, S.; Vogel, H.; Foreman, O.; Bronson, R.T.; Nishiyama, A.; Luo, L.; et al. Mosaic Analysis with Double Markers Reveals Tumor Cell of Origin in Glioma. Cell 2011, 146, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Koeller, K.K.; Henry, J.M. From the archives of the AFIP: Superficial gliomas: Radiologic-pathologic correlation. Armed Forces Institute of Pathology. Radiographics 2001, 21, 1533–1556. [Google Scholar] [CrossRef]
- Das, S.; Srikanth, M.; Kessler, J.A. Cancer stem cells and glioma. Nat. Clin. Pract. Neurol. 2008, 4, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Roman, N.I.S.; Quinones-Hinojosa, A. Gliomagenesis and the Use of Neural Stem Cells in Brain Tumor Treatment. Anti-Cancer Agents Med. Chem. 2010, 10, 121–130. [Google Scholar]
- Kroonen, J.; Nassen, J.; Boulanger, Y.-G.; Provenzano, F.; Capraro, V.; Bours, V.; Martin, D.; Deprez, M.; Robe, P.; Rogister, B. Human glioblastoma-initiating cells invade specifically the subventricular zones and olfactory bulbs of mice after striatal injection. Int. J. Cancer 2011, 129, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Timme, C.R.; Degorre-Kerbaul, C.; McAbee, J.H.; Rath, B.H.; Wu, X.; Camphausen, K.; Tofilon, P.J. The Olfactory Bulb Provides a Radioresistant Niche for Glioblastoma Cells. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 194–201. [Google Scholar] [CrossRef]
- Casalbore, P.; Budoni, M.; Ricci-Vitiani, L.; Cenciarelli, C.; Petrucci, G.; Milazzo, L.; Montano, N.; Tabolacci, E.; Maira, G.; Larocca, L.M.; et al. Tumorigenic Potential of Olfactory Bulb-Derived Human Adult Neural Stem Cells Associates with Activation of TERT and NOTCH1. PLoS ONE 2009, 4, e4434. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Kobayashi, H.; Terasaka, S.; Ishii, N.; Ikeda, J.; Kanno, H.; Nishihara, H.; Tanaka, S.; Houkin, K. The Impact of Extent of Resection and Histological Subtype on the Outcome of Adult Patients with High-grade Gliomas. Jpn. J. Clin. Oncol. 2012, 42, 270–277. [Google Scholar] [CrossRef]
- Stark, A.M.; van de Bergh, J.; Hedderich, J.; Mehdorn, H.M.; Nabavi, A. Glioblastoma: Clinical characteristics, prognostic factors and survival in 492 patients. Clin. Neurol. Neurosurg. 2012, 114, 840–845. [Google Scholar] [CrossRef]
- Jovčevska, I. Genetic secrets of long-term glioblastoma survivors. Bosn. J. Basic Med. Sci. 2019, 19, 116–124. [Google Scholar] [CrossRef]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef]
- Sanai, N.; Polley, M.-Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, M.A.; Sawaya, R.; Shi, W.; Thall, P.F.; Leeds, N.E. Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J. Neuro-Oncol. 1996, 27, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Shui, L.; Ren, H.; Yang, X.; Li, J.; Chen, Z.; Yi, C.; Zhu, H.; Shui, P. The Era of Radiogenomics in Precision Medicine: An Emerging Approach to Support Diagnosis, Treatment Decisions, and Prognostication in Oncology. Front. Oncol. 2020, 10, 570465. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Quantity |
|---|---|
| Mean age (range, years) | |
| Entire cohort (n = 76) | 61 (18–81) |
| Solitary (n = 57) | 61 (18–81) |
| >1 locus (n = 19) | 60 (42–80) |
| M:F | |
| Entire cohort (n = 76) | 45:31 |
| Solitary (n = 57) | 30:27 |
| >1 locus (n = 19) | 15:04 |
| Overall survival (months, range) | |
| Entire cohort (n = 55 diseased at censor date) | 15 (1–57) |
| Solitary (n = 37) | 16 (1–57) |
| >1 locus (n = 18) | 13 (3–26) |
| Presenting complaint (%) * | |
| Seizure | 36 (63.0) |
| Dysphasia | 8 (14.0) |
| Unilateral weakness or sensory change | 6 (10.5) |
| Memory impairment | 2 (3.5) |
| Homonymous hemianopia | 3 (5.0) |
| Change in behavior | 1 (2.0) |
| Atremulous Parkinson’s | 1 (2.0) |
| Lesion location n = 84 (%) | |
| R:L cerebral hemisphere | 43:41 |
| Grey–white matter location | |
| Cortical | 60 (71.5) |
| Cortical/subcortical | 10 (12.0) |
| Subcortical | 10 (12.0) |
| Deep white matter | 3 (3.5) |
| Deep grey matter | 1 (1.0) |
| Cerebral location (%) | |
| Temporal | 27 (32.0) |
| Frontal | 24 (28.5) |
| Parietal | 10 (12.0) |
| Occipital | 8 (9.5) |
| Olfactory grove | 4 (5.0) ** |
| Paracentral lobule | 2 (2.5) |
| Insula | 2 (2.5) |
| Parieto–temporal | 2 (2.5) |
| Parieto–occipital | 2 (2.5) |
| Subcentral gyrus | 1 (1.0) |
| Centrum semiovale | 1 (1.0) |
| Thalamus | 1 (1.0) |
| Variableat Time of Tissue Sampling | Non-Typical GBM (n = 20) | TRA GBM but with Imaging Preceding (n = 37) | TRA GBM with No Imaging Preceding (n = 479) | Statistical Significance (TRA GBM vs. Non-Typical GBM) |
|---|---|---|---|---|
| Mean age, years (range) | 61 (27–81) | 61 (18–77) | 61 (25–86) | p = 0.94 |
| M:F proportion | 9:11 | 21:16 | 307:172 | p = 0.13 |
| OS, months (range) * | 17 (2–49) | 16 (1–57) | 10 (0–80) | 0.67 |
| Oedema grade with initial lesion (%) | Overall p < 0.001 | |||
| No edema | 15 (75.0) | 25 (68.0) | 10 (2.0) | <0.001 |
| 0–2 cm edema | 5 (25.0) | 12 (32.0) | 125 (26.0) | 1 |
| >2 cm edema | 0 (0.0) | 0 (0.0) | 344 (72.0) | <0.001 |
| Mean tumor volume with initial lesion (cm3) | 5.5 (range 0.5–21.9) | 4.1 (0.2–18.8) | 34.4 (0.1–185.9) | p < 0.001 |
| Surgical resection: biopsy | 9:11 | 30:7 | 336:143 | p = 0.09 |
| % BRAF wild type (fraction of samples with a known result) | 100% (14/14) | 95% (21/22) | 98% (287/294) | p = 1 |
| TERT mutation (fraction of samples with a known result) | 93% (13/14) | 87% (20/23) | 89% (259/291) | p = 0.67 |
| EGFR amplified (fraction of samples with a known result) | 67% (8/12) | 60% (9/15) | 42% (30/72) | p = 0.12 |
| Chromosome 7 polysomy (fraction of samples with a known result) | 25% (1/4) | 60% (3/5) | 85% (22/26) | p = 0.03 |
| Chromosome 10 monosomy (fraction of samples with a known result) | 33% (1/3) | 100% (5/5) | 83% (19/23) | p = 0.12 |
| MGMT hypermethylation (fraction of samples with a known result) | 59% (10/17) | 38% (12/32) | 38% (155/403) | p = 0.08 |
| Treatment received | Overall p = 0.94 | |||
| Full Stupp | 5 (25.0) | 15 (40.5) | 87 (18.0) | p = 0.56 |
| Partial Stupp | 7 (35.0) | 7 (18.95) | 126 (26.0) | p = 0.44 |
| Full Perry | 0 (0.0) | 3 (8.1) | 9 (2.0) | p = 1 |
| Partial Perry | 0 (0.0) | 3 (8.1) | 23 (5.0) | p = 1 |
| Short course RT only | 4 (20.0) | 7 (18.95) | 103 (22.0) | p = 1 |
| Long RT only | 0 (0.0) | 0 (0.0) | 21 (4.0) | p = 1 |
| Other (chemo)radiotherapy | 0 (0.0) | 1 (2.7) | 17 (4.0) | p = 1 |
| Primary chemotherapy (no RT) | 1 (5.0) | 0 (0.0) | 20 (4.0) | p = 0.42 |
| No RT or chemo | 3 (15.0) | 1 (2.7) | 73 (15.0) | p = 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Currie, S.; Fatania, K.; Frood, R.; Whitehead, R.; Start, J.; Lee, M.-T.; McDonald, B.; Rankeillor, K.; Roberts, P.; Chakrabarty, A.; et al. Imaging Spectrum of the Developing Glioblastoma: A Cross-Sectional Observation Study. Curr. Oncol. 2023, 30, 6682-6698. https://doi.org/10.3390/curroncol30070490
Currie S, Fatania K, Frood R, Whitehead R, Start J, Lee M-T, McDonald B, Rankeillor K, Roberts P, Chakrabarty A, et al. Imaging Spectrum of the Developing Glioblastoma: A Cross-Sectional Observation Study. Current Oncology. 2023; 30(7):6682-6698. https://doi.org/10.3390/curroncol30070490
Chicago/Turabian StyleCurrie, Stuart, Kavi Fatania, Russell Frood, Ruth Whitehead, Joanna Start, Ming-Te Lee, Benjamin McDonald, Kate Rankeillor, Paul Roberts, Aruna Chakrabarty, and et al. 2023. "Imaging Spectrum of the Developing Glioblastoma: A Cross-Sectional Observation Study" Current Oncology 30, no. 7: 6682-6698. https://doi.org/10.3390/curroncol30070490
APA StyleCurrie, S., Fatania, K., Frood, R., Whitehead, R., Start, J., Lee, M.-T., McDonald, B., Rankeillor, K., Roberts, P., Chakrabarty, A., Mathew, R. K., Murray, L., Short, S., & Scarsbrook, A. (2023). Imaging Spectrum of the Developing Glioblastoma: A Cross-Sectional Observation Study. Current Oncology, 30(7), 6682-6698. https://doi.org/10.3390/curroncol30070490